The aim of this study was to stratify medications used in hospital care according to their potential risk.
MethodThe RAND/UCLA Appropriateness Method was used. Anatomical Therapeutic Chemical subgroups were classified according to their potential risk. A literature search, bulletins, and alerts issued by patient safety organizations were used to identify the potential safety risk of these subgroups. Nine experts in patient/medication safety were selected to score the subgroups for their appropriateness in the classification. Two evaluation rounds were conducted: the first by email and the second by a panel meeting.
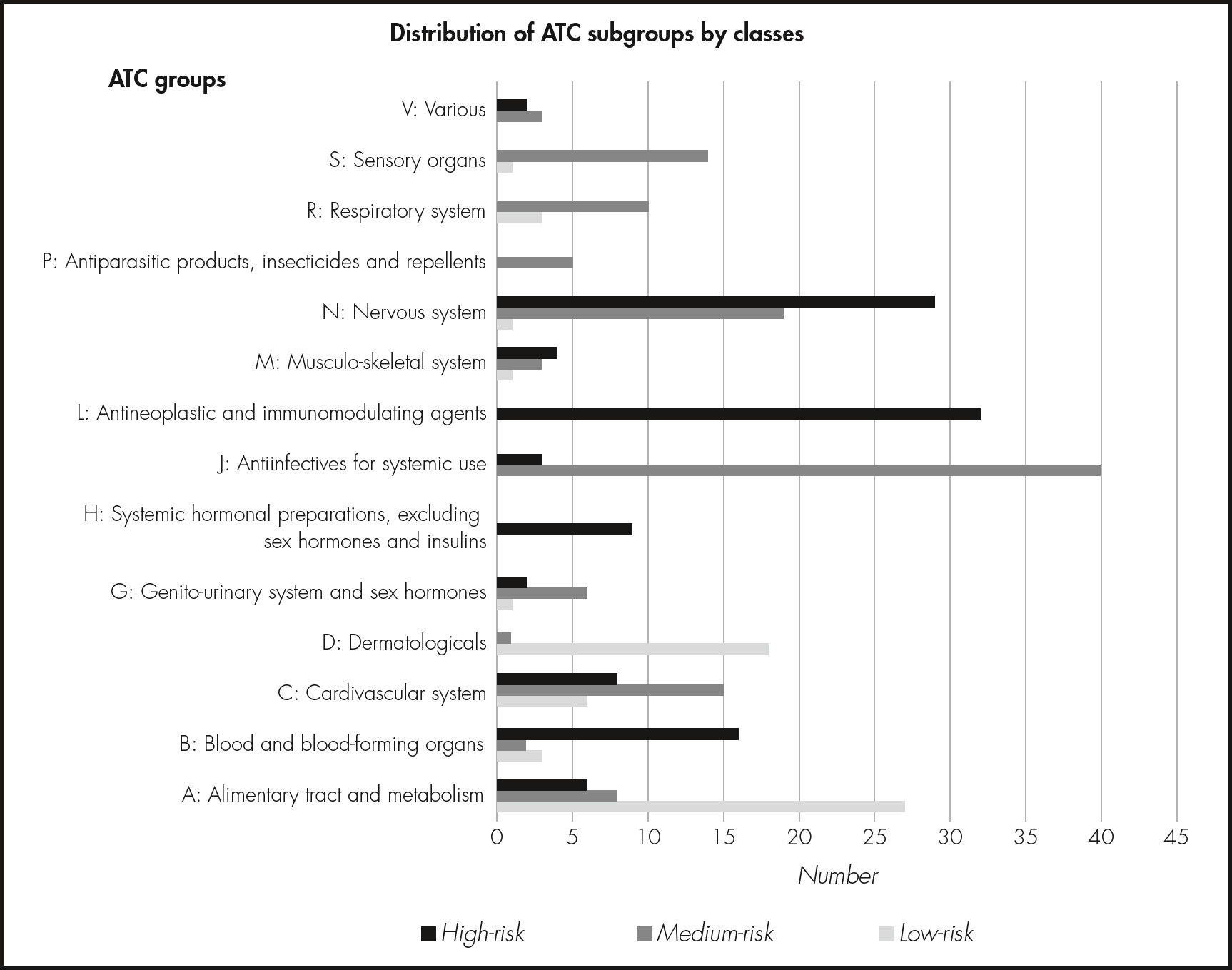
ResultsA total of 298 Anatomical Therapeutic Chemical subgroups were evaluated. They were classified into three scenarios (low, medium, and high risk). In the first round, 266 subgroups were classified as appropriate to the assigned scenario, 32 were classified as uncertain, and none were classified as inappropriate. In the second round, all subgroups were classified as appropriate. The most frequent subgroups in the low-risk scenario belonged to group A “Alimentary tract and metabolism” (44%); the most frequent in the medium-risk scenario belonged to group J “Antiin- fectives for systemic use” (32%); and the most frequent in the high-risk scenario belonged to group L “Antineoplastic and immunomodulating agents” (29%) and group N “Nervous system” (26%).
ConclusionsBased on the RAND/UCLA appropriateness method, Anatomical Therapeutic Chemical subgroups used in hospital care were classified according to their potential risk (low, medium, or high). These lists can be incorporated into a risk-scoring tool for future patient/medica- tion safety studies.
Estratificar los medicamentos utilizados en el ámbito hospitalario según el riesgo de provocar daño al paciente.
MétodoSe utilizó la metodología RAND/UCLA para clasificar los subgrupos terapéuticos del código Anatómica, Terapéutica, Química según el riesgo de provocar daño al paciente. Para ello se realizó una revisión de la evidencia disponible en publicaciones, boletines y alertas de organismos de seguridad del paciente. A continuación se seleccionaron nueve expertos en seguridad del paciente/medicamento para evaluar la clasificación de los subgrupos terapéuticos: una primera ronda de evaluación por vía telemática y una segunda ronda en una reunión presencial en la que se presentaron y discutieron los resultados de la primera.
ResultadosSe evaluaron 298 subgrupos terapéuticos. Se clasificaron en tres escenarios (riesgo bajo, medio y alto). En la primera ronda se clasificaron 266 subgrupos como adecuados al escenario asignado, 32 subgrupos fueron clasificados como inciertos y ninguno fue clasificado como inapropiado. En la segunda ronda, todos los subgrupos fueron clasificados como adecuados. Los subgrupos más frecuentes en el escenario de riesgo bajo pertenecieron al Grupo A: “Tracto alimentario y metabolismo” (44%), en el de riesgo medio al Grupo J: “Antiinfecciosos para uso sistémico” (32%), y en el de riesgo alto al Grupo L: “Agentes antineoplásicos e inmu- nomoduladores” (29%) y al Grupo N: “Sistema nervioso” (26%).
ConclusionesLa metodología RAND/UCLA ha permitido estratificar los subgrupos utilizados en el ámbito hospitalario según el riesgo potencial de provocar daño al paciente. Esta estratificación puede servir como herramienta para futuros estudios de seguridad en la utilización de medicamentos.
Medication errors (ME) are important contributors to patient morbidity and mortality, and are associated with inadequate patient safety measures1. The severity of an ME can be graded according to its impact on the patient and/or its potential future risk to patients and the healthcare organization. This approach has the advantage that it can classify and analyse the severity of MEs that pass unnoticed because they have no effect on the patient. Moreover, this type of assessment is useful for prioritizing cases that require special monitoring, analysis, or urgent solutions2.
The National Patient Safety Agency (NPSA) designed a risk matrix for grading MEs according to their potential future risk to patients and the healthcare organization. This matrix has two categories: likelihood of recurrence; and most likely consequences. However, details were not provided on the criteria by which a specific type of ME is classified according to its likelihood of recurrence and consequences3. Thus, the lack of definition allows room for subjectivity and researchers will interpret the risk matrix according to their knowledge and expertise4.
Subjectivity can be reduced by standardizing the classification of the potential risk of an ME. In a previous article, we adapted the NPSA risk matrix to medication errors in medication administration records (ME-MAR). The definition of each grade of the likelihood of ME-MAR recurrence was based on the incidence of ME-MAR in our hospital, and that of the most likely consequences was based on the type of ME-MAR and the medication involved. We found that this adaptation was reliable. However, during this process, the degree of agreement differed according to the medication involved in the error. The highest degree of agreement was achieved on high-risk medications5.
All medications can cause adverse events if they are incorrectly used. Nonetheless, certain medications are more dangerous than others and can have very severe or even catastrophic effects on patient health6. The Institute of Safe Medication Practices (ISMP) has provided a list of high-risk medications in hospitals7,8. However, lists of low- and medium-risk medications are not available. The hospital pharmacotherapeutic guide (HPG) not only includes high-risk medications but also unclassified medications, which may range from low to high risk. Therefore, the aim of the present study was to stratify medications in the HPG according to their potential risk.
MethodsThe study was conducted between October 2015 and March 2016 in a 947-bed teaching hospital. The RAND/UCLA Appropriateness Method (RAM)9,10 was used to stratify medications in the HPG according to their potential risk. The medications included in the HPG are classified according to the Anatomical Therapeutic Chemical (ATC) classification system11, and so the medications were evaluated per ATC subgroup.
The first step in the RAM was to identify scenarios, which were subsequently assessed by an expert panel in 2 consecutive rounds.
Information search and development of scenariosIn order to develop the scenarios (i.e., the stratification of the ATC subgroups according their potential risk), we conducted a review of MedLine publications (October 2005 to October 2015) on medications and their potential risk to inpatients. The search was restricted to the English and Spanish languages (see search strategy in Table 1). We selected studies that stratify medication risk or those that meet the following criteria: a) contain information on incidents caused by the clinical use of medications; b) report the number or percentage of incidents associated with each different medication /medication class, or provide sufficient information to calculate the number or percentage; and c) report the severity or the potential risk of these incidents.
Search strategy used to search MedLine
| SEARCH TERMS | |
|---|---|
| NO MESH: Medication/drug Medication error/drug error /adverse event/adverse reaction/incident Stratification/classification/list/scoring method Potential Risk/harm/severity High-risk drugs/ high-alert medication/risk profile Hospital | MESH: Risk management Drug-related side effects and adverse reactions Medication errors Hospital |
Search strategy:
# 1 «(medication OR drug) AND (medication error OR drug error OR adverse event or adverse reaction or incident) AND (stratification OR classification OR list OR scoring method) AND ((potential AND (risk OR harm OR severity)) OR high-risk drugs OR high-alert medication OR risk profile) AND hospital» [All fields]
# 2 ((medication errors [MeSH Terms]) OR (“Drug-Related Side Effects and Adverse Reactions”[Mesh])) AND (risk management [MeSH Terms]) AND (hospital [MeSH Terms]) # 1 OR #2
This information was supplemented by searching the websites of safety organizations for bulletins and alerts referring to severe MEs12–15, by consulting recent drug information16,17, and by reviewing high-alert medications lists published for hospitals by the ISMP 8.
Expert panel selectionThe panel was selected according to the following criteria: a) expertise in medication and patient safety and management; b) expertise in medication use process (physicians, pharmacists, and nurses).
The panel comprised 9 experts: 3 physicians (a geriatrician, an internist, and a pharmacologist); 3 hospital pharmacists with clinical experience in geriatrics, paediatrics and rheumatology, and intensive medicine, respectively; and 3 nurses (the inpatient care chief nurse, the emergency department nurse manager, and the traumatology department nurse manager).
Expert panel evaluationThe experts participated in two consecutive evaluation rounds. In the first round, they received the following documents by email: the identified scenarios, the evidence-based summary, the definitions of terms, and instructions for rating.
The experts were asked to assess the appropriateness of the ATC subgroup to the assigned scenario. Their appropriateness was rated on a 9-point scale, where 1 indicated “completely inappropriate” and 9 indicated “completely appropriate”. Agreement was defined as no more than 2 panel members rating the indicator as being outside the same 3-point region as the observed median (i.e., 1-3, 4—6, 7—9). The median panel rating and interquartile range were calculated. Any median ratings that fell exactly between the 3-point boundaries (3.5 and 6.5) were included in the higher appropriateness category.
ATC subgroups with a median rating in the top third of the scale (7-9) without disagreement were classified as appropriate, those with intermediate median ratings (4-6) or any median with disagreement were classified as uncertain, and those with median ratings in the bottom third (1-3) without disagreement were classified as inappropriate.
The second round comprised a face-to-face meeting during which the results of the first round were presented. Each panel member received an individualized evaluation questionnaire with the panellist's own rating from round one, the overall panel median rating from round one, and the anonymised frequency distribution of the ratings for purposes of comparison. During the meeting, the moderator introduced the ATC subgroups that had been classified as inappropriate or uncertain during round one. The experts discussed each of these ATC subgroups with the option of changing the assigned scenario. Changes were made by panel consensus. Finally, the members individually and anonymously re-evaluated the ATC subgroups. The results obtained from the second round were analysed and classified using the same methods as those used in the first round.
ResultsReview of information and definition of scenariosA total of 593 articles were reviewed, of which 38 were initially selected based on the title and abstract screening. After reviewing the full text of the articles, 19 were finally selected. The main reasons for exclusion were not reporting the number or percentage of incidents associated with each medication (n = 8), not reporting the severity or the potential risk of the incidents associated with each medication /medication class (n = 7), or not including in-hospital events (n = 4).
The scenarios comprised three lists: low-risk (scenario 1), medium-risk (scenario 2), and high-risk medications (scenario 3). The low-risk list contained the ATC subgroups unlikely to cause patient discomfort or clinical deterioration; medium-risk list contained the ATC subgroups with the potential to cause moderate discomfort or clinical deterioration; and high-risk list contained the ATC subgroups with the potential to cause severe discomfort or clinical deterioration.
The literature review and web search yielded 47 subgroups that were classified as low-risk, 136 subgroups as medium-risk, and 115 subgroups as high-risk.
Results of the evaluation roundsA total of 298 ATC groups were evaluated and rated. Sixty-one (21%) of the ATC subgroups included in the HPG were classified as low-risk, 126 (42%) as medium-risk, and 111 (37%) as high-risk. The most frequent ATC subgroups in the low-risk list belonged to group A “Alimentary tract and metabolism” (44%, n = 27), the most frequent in the medium-risk list belonged to group J “Antiinfectives for systemic use” (32%, n = 40), and the most frequent in the high-risk list belonged to groups L “Antineoplastic and immunomodulating agents” (29%, n = 32) and N “Nervous system” (26%, n = 29) (see Figure 1).
Nine experts were selected to serve on the panel. All 9 completed the first round and 8 completed the second.
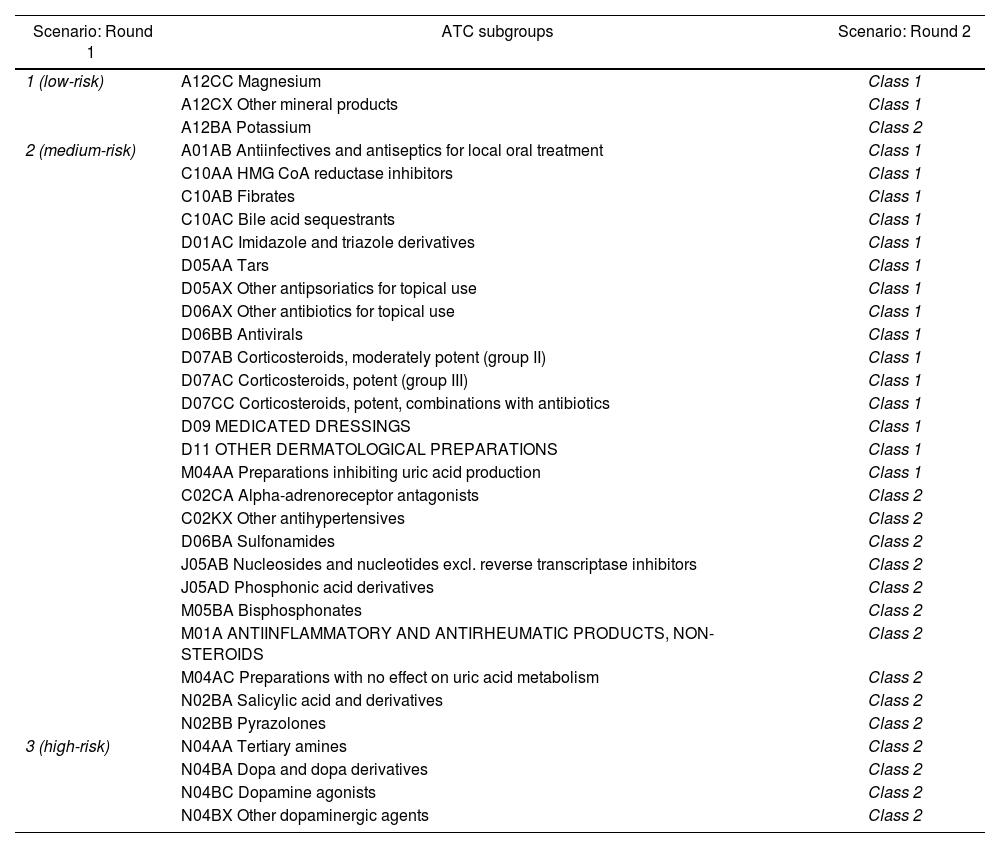
In the first round, 266 ATC subgroups were classified as appropriate, 32 were classified as uncertain, and none were classified as inappropriate. In the second round, the experts met face-to-face to re-evaluate the ATC subgroups classified as uncertain. After discussion, 12 subgroups remained in the same class, whereas 20 subgroups changed class by consensus (Table 2). The final rating panel classified all subgroups as appropriate.
ATC subgroups classified as uncertain in the first round and changes after the second round
| Scenario: Round 1 | ATC subgroups | Scenario: Round 2 |
|---|---|---|
| 1 (low-risk) | A12CC Magnesium | Class 1 |
| A12CX Other mineral products | Class 1 | |
| A12BA Potassium | Class 2 | |
| 2 (medium-risk) | A01AB Antiinfectives and antiseptics for local oral treatment | Class 1 |
| C10AA HMG CoA reductase inhibitors | Class 1 | |
| C10AB Fibrates | Class 1 | |
| C10AC Bile acid sequestrants | Class 1 | |
| D01AC Imidazole and triazole derivatives | Class 1 | |
| D05AA Tars | Class 1 | |
| D05AX Other antipsoriatics for topical use | Class 1 | |
| D06AX Other antibiotics for topical use | Class 1 | |
| D06BB Antivirals | Class 1 | |
| D07AB Corticosteroids, moderately potent (group II) | Class 1 | |
| D07AC Corticosteroids, potent (group III) | Class 1 | |
| D07CC Corticosteroids, potent, combinations with antibiotics | Class 1 | |
| D09 MEDICATED DRESSINGS | Class 1 | |
| D11 OTHER DERMATOLOGICAL PREPARATIONS | Class 1 | |
| M04AA Preparations inhibiting uric acid production | Class 1 | |
| C02CA Alpha-adrenoreceptor antagonists | Class 2 | |
| C02KX Other antihypertensives | Class 2 | |
| D06BA Sulfonamides | Class 2 | |
| J05AB Nucleosides and nucleotides excl. reverse transcriptase inhibitors | Class 2 | |
| J05AD Phosphonic acid derivatives | Class 2 | |
| M05BA Bisphosphonates | Class 2 | |
| M01A ANTIINFLAMMATORY AND ANTIRHEUMATIC PRODUCTS, NON-STEROIDS | Class 2 | |
| M04AC Preparations with no effect on uric acid metabolism | Class 2 | |
| N02BA Salicylic acid and derivatives | Class 2 | |
| N02BB Pyrazolones | Class 2 | |
| 3 (high-risk) | N04AA Tertiary amines | Class 2 |
| N04BA Dopa and dopa derivatives | Class 2 | |
| N04BC Dopamine agonists | Class 2 | |
| N04BX Other dopaminergic agents | Class 2 |
Table 3 shows the final lists of ATC subgroups according to their potential risk.
Final lists of ATC subgroups according to their potential safety risk
| Low-risk subgroups | Medium-risk subgroups | High-risk subgroups |
|---|---|---|
| A01AB Antiinfectives and antiseptics for local oral treatment | A03F PROPULSIVES | A03BA Belladonna alkaloids, tertiary amines |
| A02A ANTACIDS | A04AA Serotonin (5HT3) antagonists | A03BB Belladonna alkaloids, semisynthetic, quaternary ammonium compounds |
| A02BA H2-receptor antagonists | A04AD Other antiemetics | A10A INSULINS AND ANALOGUES |
| A02BC Proton pump inhibitors | A07AA Antibiotics | A10BA Biguanides |
| A02BX Other drugs for peptic ulcer and gastro-oesophageal reflux disease | A07DA Antipropulsives | A10BB Sulfonamides, urea derivatives |
| A03AX Other drugs for functional gastrointestinal disorders | A07EA Corticosteroids acting locally | A10BF Alpha glucosidase inhibitors |
| A05AA Bile acid preparations | A07EC Aminosalicylic acid and similar agents | B01AA Vitamin K antagonists |
| A06AA Softeners, emollients | A12BA Potassium | B01AB Heparin group |
| A06AB Contact laxatives | B02BC Local hemostatics | B01AC Platelet aggregation inhibitors excl. heparin |
| A06AC Bulk-forming laxatives | B03XA Other antianemic preparations | B01AD Enzymes |
| A06AD Osmotically acting laxatives | C02CA Alpha-adrenoreceptor antagonists | B01AE Direct thrombin inhibitors |
| A06AG Enemas | C02KX Other antihypertensives | B01AX Other antithrombotic agents |
| A07CA Oral rehydration salt formulations | C03AA Thiazides, plain | B02AA Amino acids |
| A09AA Enzyme preparations | C03BA Sulfonamides, plain | B02AB Proteinase inhibitors |
| A11AA Multivitamins with minerals | C03CA Sulfonamides, plain | B02BA Vitamin K |
| A11BA Multivitamins, plain | C03DA Aldosterone antagonists | B02BD Blood coagulation factors |
| A11CA Vitamin A, plain | C03EA Low-ceiling diuretics and potassium- sparing agents | B05AA Blood substitutes and plasma protein fractions |
| A11CC Vitamin D and analogues | C07AA Beta blocking agents, non-selective | B05BA Solutions for parenteral nutrition |
| A11DA Vitamin B1, plain | C07AB Beta blocking agents, selective | B05BB Solutions affecting the electrolyte balance |
| A11DB Vitamin B1 in combination with vitamin B6 and/or vitamin B1 | C07AG Alpha and beta blocking agents | B05BC Solutions producing osmotic diuresis |
| A11GA Ascorbic acid (vitamin C), plain | C08CA Dihydropyridine derivatives | B05X I.V. SOLUTION ADDITIVES |
| A11HA Other plain vitamin preparations | C08DA Phenylalkylamine derivatives | B06AB Other hem products |
| A11JA Combinations of vitamins | C08DB Benzothiazepine derivatives | C01A CARDIAC GLYCOSIDES |
| A12AA Calcium | C09A ACE INHIBITORS, PLAIN | C01B ANTIARRHYTHMICS, CLASS I AND III |
| A12AX Calcium, combinations with vitamin D and/or other drugs | C09C ANGIOTENSIN II ANTAGONISTS, PLAIN | C01CA Adrenergic and dopaminergic agents |
| A12CC Magnesium | D06BA Sulfonamides | C01CE Phosphodiesterase inhibitors |
| A12CX Other mineral products | G03A HORMONAL CONTRACEPTIVES FOR SYSTEMIC USE | C01CX Other cardiac stimulants |
| B03A IRON PREPARATIONS | G03H ANTIANDROGENS | C01D VASODILATORS USED IN CARDIAC DISEASES |
| B03BA Vitamin B12 (cyanocobalamin and analogues) | G03X OTHER SEX HORMONES AND MODULATORS OF THE GENITAL SYSTEM | C01EA Prostaglandins |
| B03BB Folic acid and derivatives | G04BD Drugs for urinary frequency and incontinence | C01EB Other cardiac preparations |
| C04A PERIPHERAL VASODILATORS | G04BE Drugs used in erectile dysfunction | G02A OXYTOCICS |
| C05AA Corticosteroids | G04CB Testosterone-5-alpha reductase inhibitors | G02CB Prolactine inhibitors |
| C05BA Heparins or heparinoids for topical use | J01AA Tetracyclines | H01A ANTERIOR PITUITARY LOBE HORMONES AND ANALOGUES |
| C10AA HMG CoA reductase inhibitors | J01CA Penicillins with extended spectrum | H01B POSTERIOR PITUITARY LOBE HORMONES |
| C10AB Fibrates | J01CE Beta-lactamase sensitive penicillins | H01C HYPOTHALAMIC HORMONES |
| C10AC Bile acid sequestrants | J01CF Beta-lactamase resistant penicillins | H02A CORTICOSTEROIDS FOR SYSTEMIC USE, PLAIN |
| Low-risk subgroups | Medium-risk subgroups | High-risk subgroups |
| D01AC Imidazole and triazole derivatives | J01CR Combinations of penicillins, incl. betalactamase inhibitors | H03A THYROID PREPARATIONS |
| D02AB Zinc products | J01DB First-generation cephalosporins | H03B ANTITHYROID PREPARATIONS |
| D02AC Soft paraffin and fat products | J01DC Second-generation cephalosporins | H04A GLYCOGENOLYTIC HORMONES |
| D03BA Proteolytic enzymes | J01DD Third-generation cephalosporins | H05BA Calcitonins |
| D05AA Tars | J01DE Fourth-generation cephalosporins | H05BX Other anti-parathyroid agents |
| D05AX Other antipsoriatics for topical use | J01DF Monobactams | J06AA Immune sera |
| D06AX Other antibiotics for topical use | J01DH Carbapenems | J06BA Immunoglobulins, normal human |
| D06BB Antivirals | J01EC Intermediate-acting sulfonamides | J06BB Specific immunoglobulins |
| D07AB Corticosteroids, moderately potent (group II) | J01EE Combinations of sulfonamides and trimethoprim, incl. derivatives | L01AA Nitrogen mustard analogues |
| D07AC Corticosteroids, potent (group III) | J01FA Macrolides | L01AB Alkyl sulfonates |
| D07CC Corticosteroids, potent, combinations with antibiotics | J01FF Lincosamides | L01AC Ethylene imines |
| D08AC Biguanides and amidines | J01GA Streptomycins | L01AD Nitrosoureas |
| D08AF Nitrofuran derivatives | J01GB Other aminoglycosides | L01AX Other alkylating agents |
| D08AG Iodine products | J01MA Fluoroquinolones | L01BA Folic acid analogues |
| D08AJ Quaternary ammonium compounds | J01XA Glycopeptide antibacterials | L01BB Purine analogues |
| D08AL Silver compounds | J01XB Polymyxins | L01BC Pyrimidine analogues |
| D09 MEDICATED DRESSINGS | J01XD Imidazole derivatives | L01CA Vinca alkaloids and analogues |
| D11 OTHER DERMATOLOGICAL PREPARATIONS | J01XE Nitrofuran derivatives | L01CB Podophyllotoxin derivatives |
| G01AX Other antiinfectives and antiseptics | J01XX Other antibacterials | L01CD Taxanes |
| M04AA Preparations inhibiting uric acid production | J02AA Antibiotics | L01CX Other plant alkaloids and natural products |
| N02BE Anilides | J02AB Imidazole derivatives | L01DA Actinomycines |
| R01AA Sympathomimetics, plain | J02AC Triazole derivatives | L01DB Anthracyclines and related substances |
| R01AD Corticosteroids | J02AX Other antimycotics for systemic use | L01DC Other cytotoxic antibiotics |
| R05CB Mucolytics | J04AB Antibiotics | L01XA Platinum compounds |
| S01XA Other ophthalmologicals | J04AC Hydrazides | L01XB Methylhydrazines |
| J04AK Other drugs for treatment of tuberculosis | L01XC Monoclonal antibodies | |
| J04AM Combinations of drugs for treatment of tuberculosis | L01XE Protein kinase inhibitors | |
| J05AB Nucleosides and nucleotides excl. reverse transcriptase inhibitors | L01XX Other antineoplastic agents | |
| J05AC Cyclic amines | L02AB Progestogens | |
| J05AD Phosphonic acid derivatives | L02AE Gonadotropin releasing hormone analogues | |
| J05AE Protease inhibitors | L02BA Anti-estrogens | |
| J05AF Nucleoside and nucleotide reverse transcriptase inhibitors | L02BB Anti-androgens | |
| J05AG Non-nucleoside reverse transcriptase inhibitors | L02BG Aromatase inhibitors | |
| J05AH Neuraminidase inhibitors | L02BX Other hormone antagonists and related agents | |
| J05AR Antivirals for treatment of HIV infections, combinations | L03AA Colony stimulating factors | |
| J05AX Other antivirals | L03AB Interferons | |
| M01A ANTIINFLAMMATORY AND ANTIRHEUMATIC PRODUCTS, NON-STEROIDS | L03AC Interleukins | |
| M04AC Preparations with no effect on uric acid metabolism | L03AX Other immunostimulants | |
| M05BA Bisphosphonates | L04A IMMUNOSUPPRESSANTS | |
| Low-risk subgroups | Medium-risk subgroups | High-risk subgroups |
| N02BA Salicylic acid and derivatives | L04AX Other immunosuppressants | |
| N02BB Pyrazolones | M03AB Choline derivatives | |
| N02CC Selective serotonin (5HT1) agonists | M03AC Other quaternary ammonium compounds | |
| N04AA Tertiary amines | M03AX Other muscle relaxants, peripherally acting agents | |
| N04BA Dopa and dopa derivatives | M03BX Other centrally acting agents | |
| N04BC Dopamine agonists | N01AB Halogenated hydrocarbons | |
| N04BX Other dopaminergic agents | N01AF Barbiturates, plain | |
| N05BA Benzodiazepine derivatives | N01AH Opioid anesthetics | |
| N05BB Diphenylmethane derivatives | N01AX Other general anesthetics | |
| N05CD Benzodiazepine derivatives | N01BA Esters of aminobenzoic acid | |
| N05CF Benzodiazepine related drugs | N01BB Amides | |
| N05CM Other hypnotics and sedatives | N01BX Other local anesthetics | |
| N06AA Non-selective monoamine reuptake inhibitors | N02AA Natural opium alkaloids | |
| N06AB Selective serotonin reuptake inhibitors | N02AB Phenylpiperidine derivatives | |
| N06AX Other antidepressants | N02AE Oripavine derivatives | |
| N06BA Centrally acting sympathomimetics | N02AX Other opioids | |
| N06BX Other psychostimulants and nootropics | N03AA Barbiturates and derivatives | |
| N06D ANTI-DEMENTIA DRUGS | N03AB Hydantoin derivatives | |
| N06DX Other anti-dementia drugs | N03AD Succinimide derivatives | |
| P01BB Biguanides | N03AE Benzodiazepine derivatives | |
| P01BD Diaminopyrimidines | N03AF Carboxamide derivatives | |
| P01CB Antimony compounds | N03AG Fatty acid derivatives | |
| P01CX Other agents against leishmaniasis and trypanosomiasis | N03AX Other antiepileptics | |
| P02CA Benzimidazole derivatives | N05AA Phenothiazines with aliphatic sidechain | |
| R03AC Selective beta-2-adrenoreceptor agonists | N05AD Butyrophenone derivatives | |
| R03AK Adrenergics and other drugs for obstructive airway diseases | N05AE Indole derivatives | |
| R03BA Glucocorticoids | N05AH Diazepines, oxazepines, thiazepines and oxepines | |
| R03BB Anticholinergics | N05AL Benzamides | |
| R03CC Selective beta-2-adrenoreceptor agonists | N05AN Lithium | |
| R03DA Xanthines | N05AX Other antipsychotics | |
| R05DA Opium alkaloids and derivatives | N07AA Anticholinesterases | |
| R06AB Substituted alkylamines | N07BB Drugs used in alcohol dependence | |
| R06AD Phenothiazine derivatives | N07BC Drugs used in opioid dependence | |
| R06AX Other antihistamines for systemic use | N07XX Other nervous system drugs | |
| S01AA Antibiotics | V03AB Antidotes | |
| S01AD Antivirals | V08A X-RAY CONTRAST MEDIA, IODINATED | |
| S01AE Fluoroquinolones | ||
| S01BA Corticosteroids, plain | ||
| S01BC Antiinflammatory agents, non-steroids | ||
| S01CA Corticosteroids and antiinfectives in combination | ||
| S01EA Sympathomimetics in glaucoma therapy | ||
| S01EB Parasympathomimetics | ||
| S01EC Carbonic anhydrase inhibitors | ||
| S01ED Beta blocking agents | ||
| S01EE Prostaglandin analogues | ||
| S01FA Anticholinergics | ||
| S01FB Sympathomimetics excl. antiglaucoma preparations | ||
| S01HA Local anesthetics | ||
| V03AC Iron chelating agents | ||
| V03AE Drugs for treatment of hyperkalemia and hyperphosphatemia | ||
| V03AF Detoxifying agents for antineoplastic treatment |
To the best of our knowledge, this is the first study to stratify medications used in hospital care according to their potential risk (low to high-risk). The RAM was used to classify the ATC subgroups included in the HPG into low, medium, and high potential risk. In the first evaluation round, 32 groups were classified as uncertain. Because the potential risk of a medication is driven by the clinical characteristics of the patient18, the majority of the disagreements between experts could have been due to their experience in attending and treating different types of patients. However, we believe that the final results were enriched by the different criteria applied by the experts.
Some subgroups classified as uncertain were subject to further discussion. These subgroups included some dermatological subgroups, some subgroups which belong to group C10 “Lipid-modifying agents”, and some anti-Parkinson drug subgroups. The dermatological subgroups were finally reclassified as low-risk. This classification is consistent with those reported by other studies that consider this group to have no association with patient harm19,20. The subgroups that belong to group C10 “Lipid-modifying agents” were also reclassified as low-risk. The expert panel considered that the potential risk for inpatients was low. Authors such as Saeder et al.21 have also classified fibrates as low risk. The anti-Parkinson drug subgroups were reclassified as medium-risk, although the nervous system group is associated with severe adverse events22. According to the clinical experience of the experts, severe adverse events are uncommon with anti-Parkinson drugs. This reclassification is consistent with the high-alert medication list for patients with chronic disease, which excluded anti-Parkinson drugs (see Otero et al.23).
The methodology used in this study has some limitations. Firstly, although the RAM has objective characteristics, it also has subjective ones because it measures opinions24. However, this method has advantages over other methods used to reach consensus, because it uses confidential ratings and group discussion. It has good reproducibility and is considered to be a rigorous method that can be used whenever a combination of scientific evidence and expert opinion is required9,23,25. Secondly, the results of the RAM always depend on the composition of the expert panel9. The RAM panel included physicians and nurses from different medical specialities, and pharmacists with different types of clinical expertise. Thus, several fields were covered by experts with deep knowledge of all medications assessed in this study.
The lists that were created provide an objective measure that could be used during routine data collection of MEs in order to reduce subjectivity and provide a standard by which the severity of an ME can be assessed and measured. These medication lists could be a useful tool for future pa- tient/medication safety studies, leading to better prevention measures and the improved management of follow-up activities after the detection of an ME.
Ideally, these lists could be integrated into an electronic tool to facilitate resource allocation for patients at high risk of severe MEs. It is relevant to individualize the risk assessment for each patient undergoing drug thera- py21,26. Given that resources are limited, the same intervention is currently provided to all patients in our hospital, even though they may receive medications with a higher risk of adverse events. The integration of these lists into an electronic tool would assist in patient stratification.
A RAM was used to classify ATC subgroups by their potential risk (low, medium, or high). The main contribution of this study is to make these reference lists available. These lists can be integrated into a risk-scoring tool for future patient/medication safety studies.
FundingNo funding.
Conflict of interestsNo conflict of interests.
Contribution to scientific literatureAll medications can cause adverse events if they are incorrectly used. Nonetheless, certain medications are more dangerous than others. A list of high-risk medications has been published, but lists of low- and medium-risk medications are not available. This study is the first to classify medications used in hospital settings according to their potential risk. This classification is of relevance to future patient/medication safety studies and for patient resource allocation according to treatment.