Inflammatory joint diseases cause pain and disability. The objective of this study was to measure the quality of life of patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis treated with certolizumab pegol and compare the results with those of the general population.
MethodUsing a cross-sectional design and sociodemographic and clinical variables, adherence to treatment and quality of life data were collected using the Euroqol-5d-5L (EQ-5D) questionnaire. The quality of life of the general population was obtained from the Spanish National Health Survey. Answers to the EQ-5D questionnaire were analyzed in both groups using two-part models, which measure the probability of having perfect health as well as the disutility caused by the disease.
ResultsThe sample included 59 patients with high adherence (92.3%). The mean utility value was 0.78 and pain was the most affected dimension. The reduction in utility (EQ-5D index) of patients with inflammatory joint disease as compared to the general population was 0.127.
ConclusionsThe subjects of the study showed a significantly lower quality of life than the general population despite effective control of the disease. Two-part models facilitate the interpretation of quality-of-life studies using the EQ-5D.
Las enfermedades articulares inflamatorias causan dolor y discapacidad. El objetivo fue medir la calidad de vida de los pacientes con artritis reumatoide, artritis psoriásica, espondilitis anquilosante en tratamiento con certolizumab pegol y compararla con la de la población general.
MétodoCon un diseño transversal se recogieron variables sociodemográficas y clínicas, adherencia al tratamiento y calidad de vida mediante el cuestionario Euroqol-5d-5L (EQ-5D). La calidad de vida de la población general se obtuvo de la Encuesta Nacional de Salud. El EQ-5D se analizó en ambos grupos mediante modelos de dos partes que miden la probabilidad de tener una salud perfecta y la disutilidad causada por la enfermedad.
ResultadosLa muestra incluyó 59 pacientes con una adherencia alta (92,3%). La utilidad media fue de 0,78 y el dolor resultó la dimensión más afectada. La reducción de utilidad (índice EQ-5D) de los pacientes con enfermedades articulares inflamatorias respecto a la población general fue de 0,127.
ConclusionesLos pacientes muestran una calidad de vida significativamente menor que la población general a pesar del buen control de la enfermedad. Los modelos de dos partes facilitan la interpretación de los estudios de calidad de vida mediante EQ-5D.
Rheumatoid arthritis (RA), psoriatic arthritis (PA), and ankylosing spondylitis (AS) are joint diseases known to significantly reduce patients’ quality of life1–3. The goal the medications used to treat those conditions is therefore to improve patients’ quality of life by inducing remission of disease. Of all the different drugs developed, biologic anti-tumor necrosis factor alpha agents have been the most disruptive4. Certolizumab pegol was approved in 2009 by the European Medicines Agency (EMA) for the treatment of RA, PA and AS5. However, no studies have been published in Spain analyzing health-related quality of life (HRQOL) in patients treated according to the clinical guidelines. The goal of this study was to measure HRQOL in patients diagnosed with RA, PA and AS treated with certolizumab pegol and compare the results with those of the general population.
MethodsA cross-sectional study was conducted to measure HRQOL in a sample of patients served by a hospital pharmacy department and to subsequently compare the results with those of the general population. Inclusion criteria were: age over 18 years; having a confirmed diagnosis of RA, PA or AS; having been on treatment with certolizumab pegol for at least 3 months before initiation of the study; and voluntary participation as ratified by signing an informed consent form. The study protocol was approved by Navarre's Clinical Research Ethics Committee (code: Pyto-2018/24).
Patient data was collected in an anonymized way and included the following variables: age, sex, condition (RA, PA, AS), age at diagnosis, duration of disease (in months), glomerular filtration rate, duration of treatment with certolizumab pegol, number of previous biological treatments, use of nonsteroid anti-inflammatory drugs (NSAIDs) in the last 48 hours, use of corticosteroids in the last 48 hours, use of other concomitant disease-modifying drugs (DMDs), discontinuation caused by adverse events, date of discontinuation, type of adverse event, body mass index, smoking, level of educational attainment, Euroqol-5D-5L questionnaire (EQ-5D).
The EQ-5D questionnaire is a standardized instrument that measures patients’ self-reported health status. It comprises two parts: a descriptive system and a visual analog scale. The descriptive system comprises five dimensions (mobility, self-care, usual activities, pain/discomfort, anxiety/ depression). Each dimension has five levels: no problems, slight problems, moderate problems, severe problems, and total disability. Health-related quality of life is represented by utility values (EQ-5D index) measured from 0 to 1 on the basis of the preferences obtained from a sample of the Spanish population6. Adherence to treatment was measured based on the information in the patients’ dispensation7 records at baseline and at 6 months as per the following formula: % adherence = [(units dispensed – n° units returned) / (units prescribed)] x 100.
A two-step regression analysis was carried out of the quality-of-life values estimated by the EQ-5D-5L as a function of the characteristics of each patient8. This procedure made it possible to calculate the utility score as the difference between perfect utility and the loss of utility resulting from disease. The first step was to estimate the adjusted probability [p(x)] of being in perfect health using logistic regression models. Secondly, generalized linear models were used to estimate mean disutility values [w(x)] in a population that does not enjoy perfect health [utility lower than 1 or 1 – w(x)]. Thus, disutilities are defined as 1 – utilities [w(x) = 1 – u(x)]. Lastly, both models were used together to calculate utility values by means of the following formula:
The utility of the subgroups analyzed in terms of their clinical characteristics was compared with that of the general population in groups that were equivalent in terms of age, sex and other variables such as level of educational attainment or smoking. The equivalent population was determined by applying Arrospide et al.’s two-part model to data from the 2017 Spanish National Health Survey8,9. This model allows estimation of the mean utility and of its confidence intervals for a subsample of the general Spanish population with a specified age and sex structure.
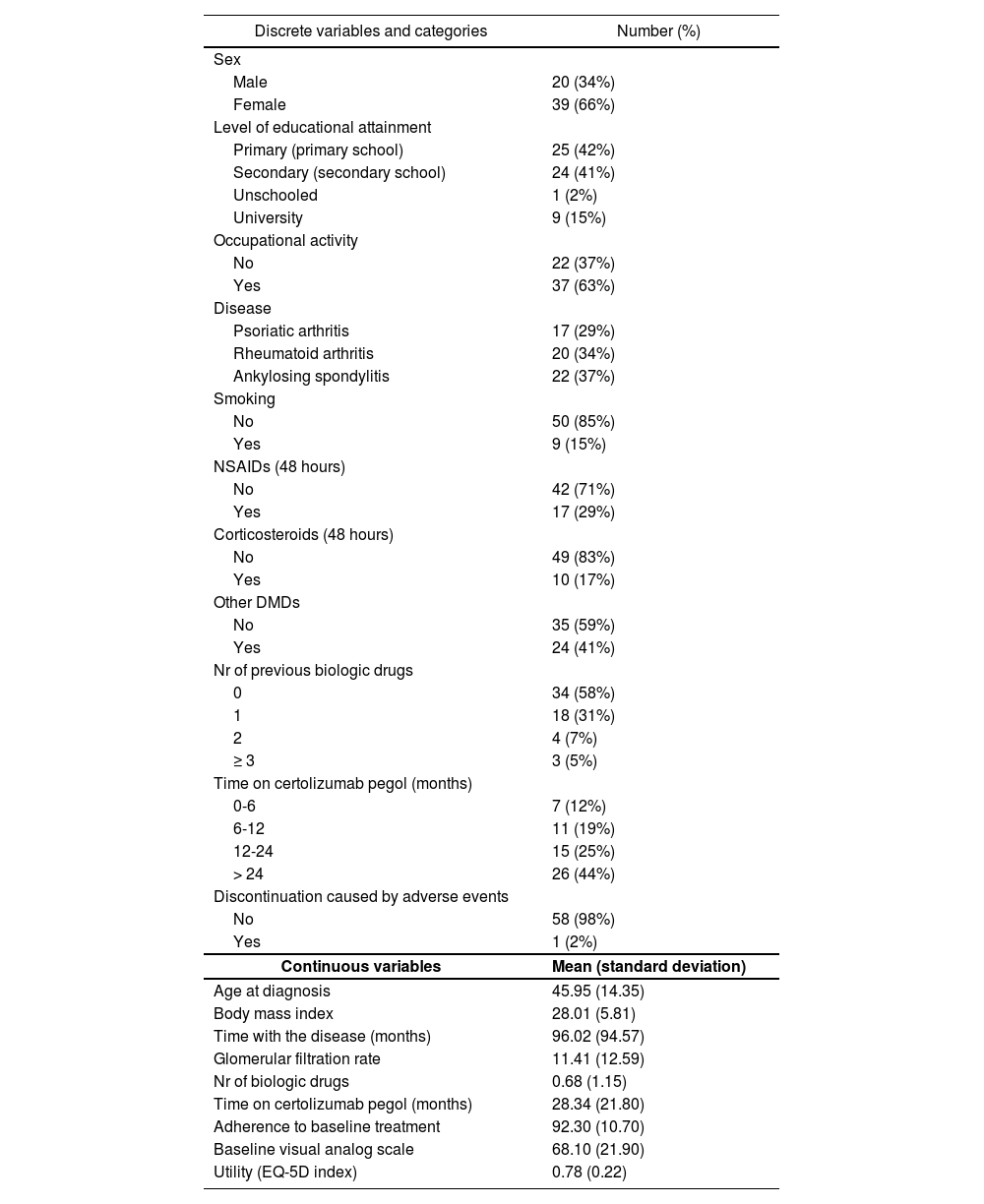
ResultsAlthough the number of eligible patients was 62, the study ended up recruiting 59 patients of a mean age of 46 years; 29% of them had PA, 34% RA and 37% AS (Table 1). The three other patients refused to participate. Mean duration of the disease was 7.6 years, of which certolizumab pegol was administered for a mean of 2.3 years. This is indicative of a significantly developed disease profile. The study sample was made up mainly of women (66%) and patients in employment (63%). Twenty-nine percent of subjects took concomitant NSAIDs and 17% took corticosteroids for 48 hours prior to the intervention. Forty-one percent of subjects had been prescribed other DMDs too. Mean adherence was 92.3%, with 87% of subjects above 85.0%.
Descriptive characteristics of the patients in the study
| Discrete variables and categories | Number (%) |
|---|---|
| Sex | |
| Male | 20 (34%) |
| Female | 39 (66%) |
| Level of educational attainment | |
| Primary (primary school) | 25 (42%) |
| Secondary (secondary school) | 24 (41%) |
| Unschooled | 1 (2%) |
| University | 9 (15%) |
| Occupational activity | |
| No | 22 (37%) |
| Yes | 37 (63%) |
| Disease | |
| Psoriatic arthritis | 17 (29%) |
| Rheumatoid arthritis | 20 (34%) |
| Ankylosing spondylitis | 22 (37%) |
| Smoking | |
| No | 50 (85%) |
| Yes | 9 (15%) |
| NSAIDs (48 hours) | |
| No | 42 (71%) |
| Yes | 17 (29%) |
| Corticosteroids (48 hours) | |
| No | 49 (83%) |
| Yes | 10 (17%) |
| Other DMDs | |
| No | 35 (59%) |
| Yes | 24 (41%) |
| Nr of previous biologic drugs | |
| 0 | 34 (58%) |
| 1 | 18 (31%) |
| 2 | 4 (7%) |
| ≥ 3 | 3 (5%) |
| Time on certolizumab pegol (months) | |
| 0-6 | 7 (12%) |
| 6-12 | 11 (19%) |
| 12-24 | 15 (25%) |
| > 24 | 26 (44%) |
| Discontinuation caused by adverse events | |
| No | 58 (98%) |
| Yes | 1 (2%) |
| Continuous variables | Mean (standard deviation) |
| Age at diagnosis | 45.95 (14.35) |
| Body mass index | 28.01 (5.81) |
| Time with the disease (months) | 96.02 (94.57) |
| Glomerular filtration rate | 11.41 (12.59) |
| Nr of biologic drugs | 0.68 (1.15) |
| Time on certolizumab pegol (months) | 28.34 (21.80) |
| Adherence to baseline treatment | 92.30 (10.70) |
| Baseline visual analog scale | 68.10 (21.90) |
| Utility (EQ-5D index) | 0.78 (0.22) |
DMDs: disease-modifying drugs; EQ-5D: Euroqol-5d-5L questionnaire; NSAIDs: nonsteroid anti-inflammatory drugs.
Mean baseline utility was 0.78 (Table 1). The pain/discomfort dimension was the most affected of the five dimensions in the EQ-5D questionnaire, with only 22% of patients scoring 1 point (perfect health). Pain/discomfort was followed by mobility (53%), usual activities (54%), anxiety/depression (68%) and self-care (78%). Only eight patients (13.56%) reported perfect health or utility equal to 1.
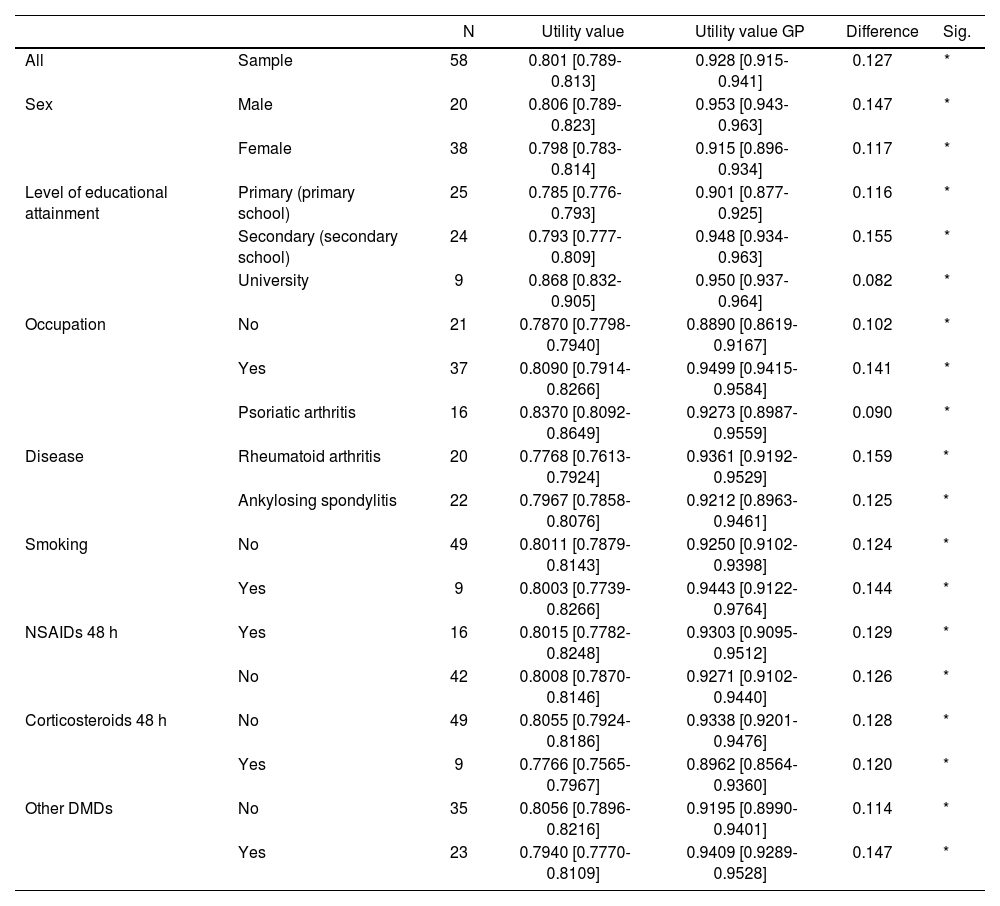
Table 2 shows the utility values for each group according to their clinical characteristics, and provides a comparison of each group with an equivalent sample in terms of age and sex from the Spanish general population, as reported by the 2017 Spanish National Health Survey. The difference for the whole sample was 0.127, with higher values for males (0.147), and far lower ones for subjects with a university degree (0.082). When the values for the general population were compared with utility values as broken down by different etiologies, the greatest difference was obtained for RA (0.159) and the smallest for PA (0.090). AS values were in the middle (0.125).
Utility values calculated using the two-part model for different patient groups and comparison with the values for members of the general population of equivalent age and sex
| N | Utility value | Utility value GP | Difference | Sig. | ||
|---|---|---|---|---|---|---|
| All | Sample | 58 | 0.801 [0.789-0.813] | 0.928 [0.915-0.941] | 0.127 | * |
| Sex | Male | 20 | 0.806 [0.789-0.823] | 0.953 [0.943-0.963] | 0.147 | * |
| Female | 38 | 0.798 [0.783-0.814] | 0.915 [0.896-0.934] | 0.117 | * | |
| Level of educational attainment | Primary (primary school) | 25 | 0.785 [0.776-0.793] | 0.901 [0.877-0.925] | 0.116 | * |
| Secondary (secondary school) | 24 | 0.793 [0.777-0.809] | 0.948 [0.934-0.963] | 0.155 | * | |
| University | 9 | 0.868 [0.832-0.905] | 0.950 [0.937-0.964] | 0.082 | * | |
| Occupation | No | 21 | 0.7870 [0.7798-0.7940] | 0.8890 [0.8619-0.9167] | 0.102 | * |
| Yes | 37 | 0.8090 [0.7914-0.8266] | 0.9499 [0.9415-0.9584] | 0.141 | * | |
| Psoriatic arthritis | 16 | 0.8370 [0.8092-0.8649] | 0.9273 [0.8987-0.9559] | 0.090 | * | |
| Disease | Rheumatoid arthritis | 20 | 0.7768 [0.7613-0.7924] | 0.9361 [0.9192-0.9529] | 0.159 | * |
| Ankylosing spondylitis | 22 | 0.7967 [0.7858-0.8076] | 0.9212 [0.8963-0.9461] | 0.125 | * | |
| Smoking | No | 49 | 0.8011 [0.7879-0.8143] | 0.9250 [0.9102-0.9398] | 0.124 | * |
| Yes | 9 | 0.8003 [0.7739-0.8266] | 0.9443 [0.9122-0.9764] | 0.144 | * | |
| NSAIDs 48 h | Yes | 16 | 0.8015 [0.7782-0.8248] | 0.9303 [0.9095-0.9512] | 0.129 | * |
| No | 42 | 0.8008 [0.7870-0.8146] | 0.9271 [0.9102-0.9440] | 0.126 | * | |
| Corticosteroids 48 h | No | 49 | 0.8055 [0.7924-0.8186] | 0.9338 [0.9201-0.9476] | 0.128 | * |
| Yes | 9 | 0.7766 [0.7565-0.7967] | 0.8962 [0.8564-0.9360] | 0.120 | * | |
| Other DMDs | No | 35 | 0.8056 [0.7896-0.8216] | 0.9195 [0.8990-0.9401] | 0.114 | * |
| Yes | 23 | 0.7940 [0.7770-0.8109] | 0.9409 [0.9289-0.9528] | 0.147 | * |
DMDs: disease-modifying drugs; GP: general population; NSAIDs: nonsteroid anti-inflammatory drugs; Sig.: statistical significance.
Deterioration of HRQOL in patients with RA, PA and AS, as compared with equivalent age and sex samples from the general population, was significant despite high adherence to treatment7. The high rate of adherence achieved makes it possible for improvements to be made across other treatment-related problems.
The most affected HRQOL dimension was pain/discomfort. This is in line with the findings of other studies related of the loss of quality of life experienced by patients with RA10. Even if loss of HRQOL in this patient population is significant as compared with that of the general population, it can be at least partly mitigated by recourse to a series of compensatory functional skills and/or an appropriate emotional environment that reduces the negative impact of pain and disability11. The percentage of patients on concomitant treatment with DMDs was high (60%), which may be related with an increase in the efficacy and the persistence of the drug. The incidence of concomitant use of DMDs with anti-TNF medicines tends to high during the first year of treatment and gradually diminishes over time, although the persistence of anti-TNF treatment remained high all along12.
One of the strengths of this study is the method used to calculate utilities, which allowed an estimation of mean values and confidence intervals as a function of the subjects’ individual characteristics, ensuring that the estimated value would always be within the interval (-“, 1]89. The proportion of subjects who find themselves in perfect health is a key factor for choosing the most efficient method to- estimate utilities by means of the EQ-5D questionnaire. Two-part models allow proper management of that proportion. The two-part procedure is founded on the principle that the choice of the model that is best adjusted during the second step determines the final result. The main limitations of this study include the use of one single medication for the analysis and the small size of the sample.
Two important conclusions can be drawn from this study. Firstly, patients exhibited a significantly lower quality of life than the general population despite proper control of their disease; secondly, use of a two-part model facilitated interpretation of an EQ-5D-based quality-of-life study.
FundingThis study has been undertaken with resources contributed by the two participating departments and by a small grant from UCB Pharma that was used to fund the statistical analysis.
Conflict of interestNo conflict of interests.
Contribution to the scientific literature
Evaluation of the quality of life of rheumatologic patients constitutes a useful tool for clinical decision making. The methodology applied here complies with the standard criteria propounded in the most recent literature including, for the first time in the history of rheumatology research, two-part models based on the EuroQuol 5D quality of life questionnaire.. The conclusion drawn by comparing the outcomes obtained with those of the general population was clinically relevant given the significant quality of life impairment resulting from rheumatic conditions, even in highly adherent patients.
Early Access date (12/08/2021).