Tyrosine kinase inhibitors (TKIs) have been successful in changing the course of chronic myeloid leukaemia (CML) due to their high efficacy. However, their effectiveness is conditioned by adherence to treatment. The aim of this study was to analyse the adherence of CML patients treated with TKIs and to evaluate the impact of pharmaceutical care on adherence in a prospective and interventional manner.
MethodsMulticentre, prospective study including CML patients on treatment with TKIs attending the outpatient units of the Pharmacy Services. Adherence was assessed using a combination of two methods: the Simplified Adherence Problems Scale and the treatment dispensing register (a patient with a percentage <90% being considered “non-adherent”); patients who demonstrated a non-adherence in either of these two methods were classified as “non-adherent patients”. In individuals with inadequate adherence, pharmaceutical care was reinforced for 8 months by means of a specific programme.
ResultsA total of 130 patients were included, 56.9% had optimal adherence to treatment. Pharmaceutical care in the oncohaematology-specific outpatient units of the Pharmacy Services improved adherence (from 67.1% to 90.9%; p < 0.001) while the generalist outpatient units kept it constant (from 70.2% to 72.4%; p = 0.509).
ConclusionsAdherence is one of the most relevant parameters in the effectiveness of chronic treatments. Approximately half of our CML patients showed inadequate adherence to TKIs. This is the first prospective study to determine that the pharmacist's actions in oncohaematology-specific outpatient units of the Pharmacy Services are capable of influencing adherence and improving it.
los inhibidores de la tirosin-cinasa (TKI) han logrado cambiar el curso de la leucemia mieloide crónica (LMC) gracias a su elevada eficacia. Sin embargo, su efectividad está condicionada por la adherencia al tratamiento. El objetivo del presente trabajo ha consistido en analizar la adherencia de los pacientes con LMC en tratamiento con TKI y evaluar de forma prospectiva e intervencionista el impacto de la atención farmacéutica en la adherencia.
Métodosestudio multicéntrico y prospectivo en el que se incluyeron a los pacientes con LMC en tratamiento con TKI que acudían a las unidades de pacientes externos de los servicios de farmacia. Se evaluó la adherencia mediante la combinación de 2 métodos: la Escala Simplificada de Problemas de Adherencia y el registro de dispensaciones del tratamiento (considerándose como paciente «no adherente» aquel con un porcentaje menor que el 90%); los individuos que demostraron una falta de adherencia en cualquiera de estos 2 métodos fueron clasificados como «pacientes no adherentes». En los sujetos con adherencia inadecuada se reforzó la atención farmacéutica durante 8 meses mediante un programa específico.
Resultadosfueron incluidos 130 pacientes, de los cuales el 56,9% tuvo una adherencia óptima al tratamiento. La atención farmacéutica en las unidades de pacientes externos específicas oncohematológicas logró mejorar la adherencia (de 67,1% a 90,9%; p < 0,001) mientras que las unidades de pacientes externos generalistas la mantuvieron constante (de 70,2% a 72,4%; p = 0,509).
Conclusionesla adherencia es uno de los parámetros más relevantes en la efectividad de los tratamientos crónicos. Aproximadamente la mitad de nuestros pacientes con LMC mostró una adherencia inadecuada a los TKI. Este es el primer estudio que, de forma prospectiva, determina que la actuación del farmacéutico desde las unidades de pacientes externos específicas oncohematológicas es capaz de influir en la adherencia y mejorarla.
Chronic myeloid leukaemia (CML) is a myeloproliferative disorder that accounts for 15% of all leukaemias.1 The proliferating cells of this haematological neoplasm are characterised by the presence of the so-called Philadelphia chromosome, the result of a reciprocal translocation between chromosomes 9 and 22 with a rearrangement of the BCR-ABL gene, which encodes a protein with high constitutive tyrosine kinase activity.1
Until the development of tyrosine kinase inhibitors (TKIs), CML was a disease with a low survival rate. However, the advent of these drugs has changed the outlook for this disease, achieving responses and survival rates that often allow CML to become a chronic process.2 TKIs are oral antineoplastic drugs that give patients freedom and autonomy compared to intravenous drugs, thus improving their quality of life.2 The high efficacy of TKIs means that they are prescribed for long periods (years in most cases), which can compromise adherence and therefore the therapeutic success of this type of drug.
Therapeutic nonadherence is now recognised as a global public health problem with negative consequences for both patients and healthcare systems, as it is one of the main causes of therapeutic failure. In recent years, nonadherence has been exacerbated by the increase in the number of individuals with chronic diseases, those taking multiple medications, and the increase in life expectancy.3
Therapeutic nonadherence often goes unnoticed by the patient's clinical team, limiting the effectiveness of treatment.3 Therefore, it is necessary to assess the areas in which interventions can be made so that once the factors that have the greatest impact on adherence have been identified, a set of strategies and recommendations can be developed in an individualised manner to improve the patient's adherence. Within multidisciplinary teams in hospitals, pharmacists are healthcare professionals with very close contact with patients and can play a fundamental role in assessing the degree of adherence to treatment and establishing the appropriate interventions to improve it.
The aim of this study was to analyse the adherence of CML patients to TKI treatment and to assess the impact of pharmaceutical care (PC) on adherence. We also compared the differences in results according to the degree of specialisation of the pharmacist in charge of the outpatient units (OPUs).
MethodsStudy design and populationA 20-month multicentre prospective study divided into two stages: an initial observational stage aimed at assessing and analysing adherence, involving the use of different methods, such as surveys and medication dispensed records. The second stage—pharmaceutical intervention—lasted 8 months, during which the pharmacist sent reminders and provided written information about the medication to patients identified as nonadherent during the initial stage.
This study included all patients diagnosed with CML who collected their oral treatment from the OPUs of the hospital pharmacy services.
Exclusion criteria were individuals less than 18 years of age, who were participating in clinical trials or who had been receiving TKI treatment for less than 3 months.
Data collection and assessment of resultsAdherence was analysed using a combination of two measures:
The Simplified Adherence Problems Scale (ESPA): this tool was originally validated to assess therapeutic adherence in patients with human immunodeficiency virus (HIV), although it has also been used to analyse adherence in other chronic conditions, categorising patients as adherent or nonadherent.4 A nonadherent patient was defined as an individual who scored less than 5 points (a positive answer = 1 point, a negative answer = 0 points) in relation to the following points:
- •
The patient attends for medication according to the scheduled appointments or for justifiable reasons.
- •
The patient knows how to take their medication.
- •
The patient knows the name (brand name or active ingredient) of the medication they are taking.
- •
The patient takes their medication in an appropriate manner, taking into account their habits, diet, etc.
- •
Appropriate clinical evolution and good subjective assessment.
- •
The amount of medication the patient keeps at home does not exceed the amount needed for 15 days of treatment.
The record of medication dispensed over the last 6 months by the different OPUs: for this purpose, the percentage of dispensed medication was determined using the following formula: ([total prescribed dose – undispensed dose] X 100/total prescribed dose). A patient was considered nonadherent if the percentage was less than 90%.
Individuals who demonstrated a lack of adherence using either of these 2 methods were classified as nonadherent patients. Pharmaceutical care was reinforced for 8 months in participants who showed poor adherence during the first stage of the study. To this end, a specific PC programme was developed to provide patients with individualised verbal and written information about their pharmacotherapy plan (Annex 1, see supplementary material). After this period, patients' adherence was reassessed using the combination of these 2 methods.
Clinical data were obtained from the patients' electronic medical records, including age at diagnosis, gender, years of treatment, TKI used, and dosage regimen.
Statistical analysisThe data were assessed using descriptive statistics and stratified by patient adherence (adherent versus nonadherent).
A chi-squared test was used to analyse the significance of the percentage change in baseline adherence compared with adherence after the pharmaceutical intervention. A P-value ≤.05 was used as a cutoff for statistical significance.
ResultsA total of 130 patients participated in this study; 55.4% (n = 72) were men. Mean age was 58.9 years (range 20–90 years), and mean duration of TKI treatment was 4.7 years (0.6–13 years).
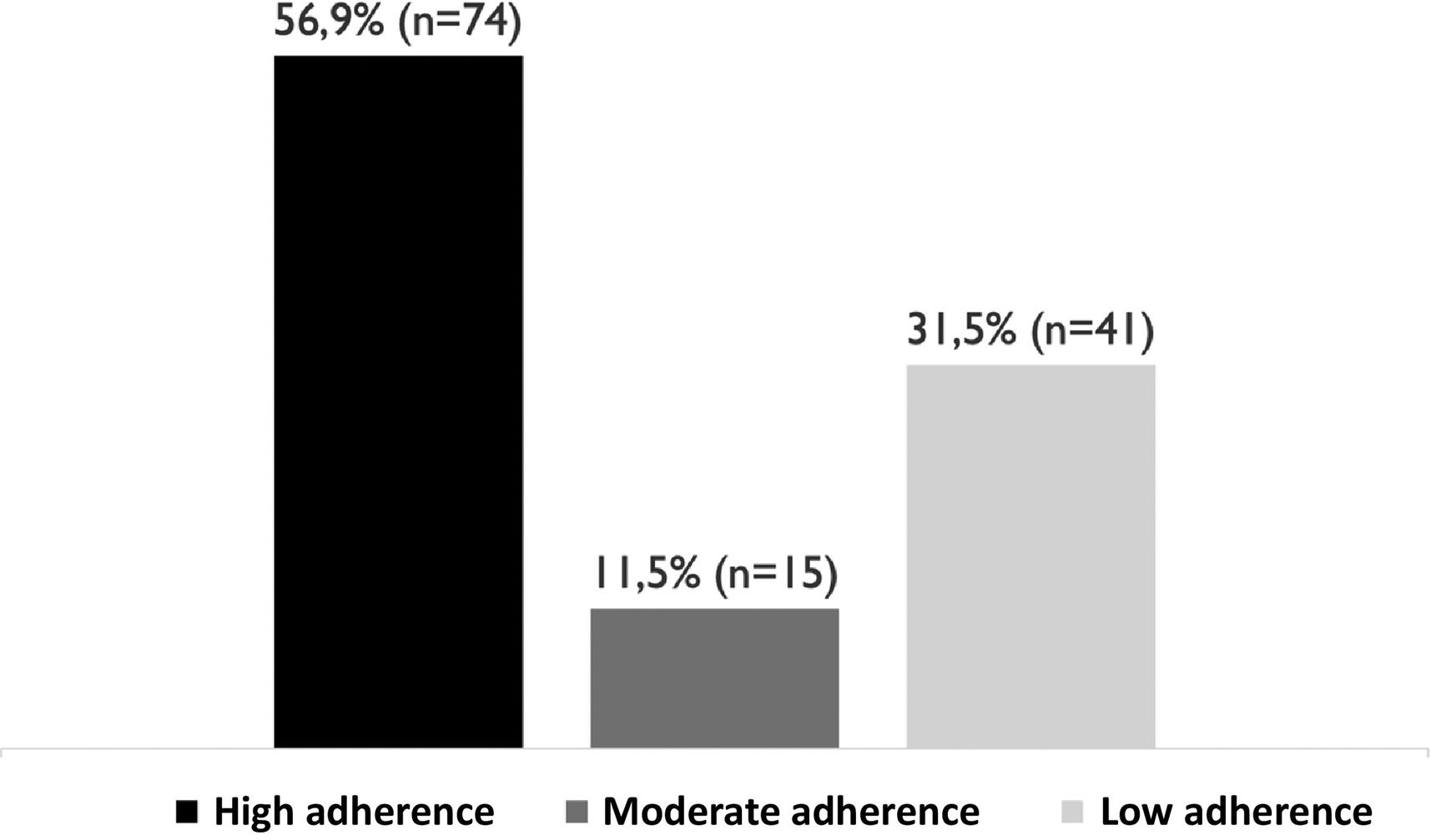
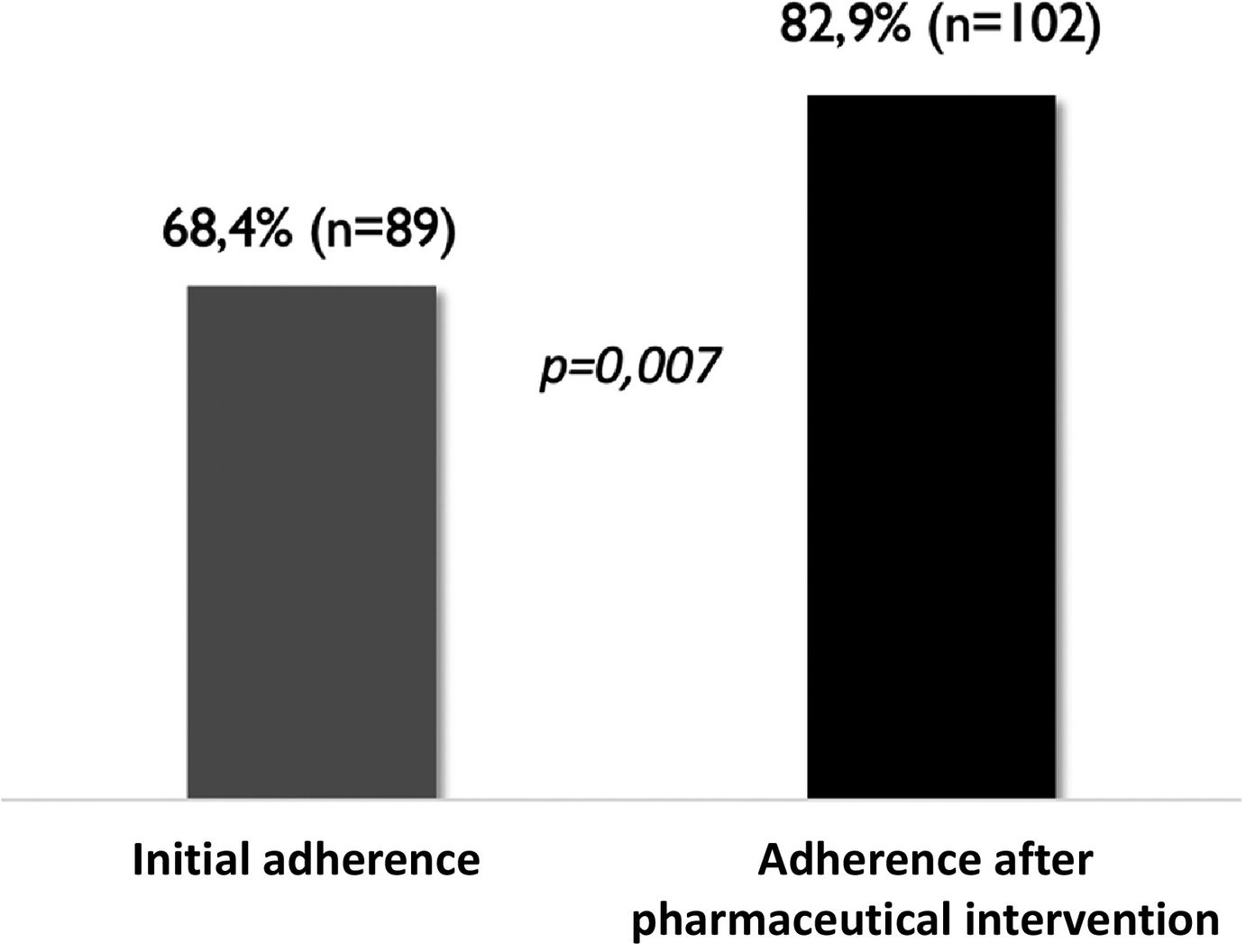
Adherence in this patient population was 68.4% (n = 89). When the patients were stratified according to the degree of therapeutic adherence, it was found that 56.9% (n = 74) had high adherence, 11.5% (n = 15) medium adherence, and 31.5% (n = 41) were nonadherent (Fig. 1).
At the time of the study, 63.1% (n = 82) of patients were being treated with imatinib, 25.4% (n = 33) with nilotinib, and 11.5% (n = 15) with dasatinib. It was found that adherence did not vary according to the drug used: imatinib (54.8%), nilotinib (63.6%), and dasatinib (54.3%) (P = .67).
During the second stage, 7 patients (5.38%) were lost to follow-up, leaving a final total of 123 participants: 55.4% male, mean age 58.9 years (20–90 years), mean duration of treatment 5.5 years (0.6–13 years). Of the 5 hospitals participating in this study, 3 had dedicated oncohaematology OPUs and 2 had general OPUs, which were attended by all patients receiving hospital-dispensed treatment, regardless of disease type. The baseline characteristics of the populations included in both groups were found to be homogeneous (Table 1).
Stratification and baseline characteristics of the participants according to the outpatient units they attended.
| Specialised OPU (n = 66) | General OPU (n = 57) | P | |
|---|---|---|---|
| Mean age, y | 58.9 | 59.1 | 0.748 |
| Male, % | 58.5 | 50 | 0.327 |
| Mean time on TKI, y | 5.1 | 5.7 | 0.187 |
TKI, tyrosine kinase inhibitors; OPU, outpatient unit.
After 8 months of pharmaceutical intervention, a significant improvement in adherence was observed (Fig. 2).
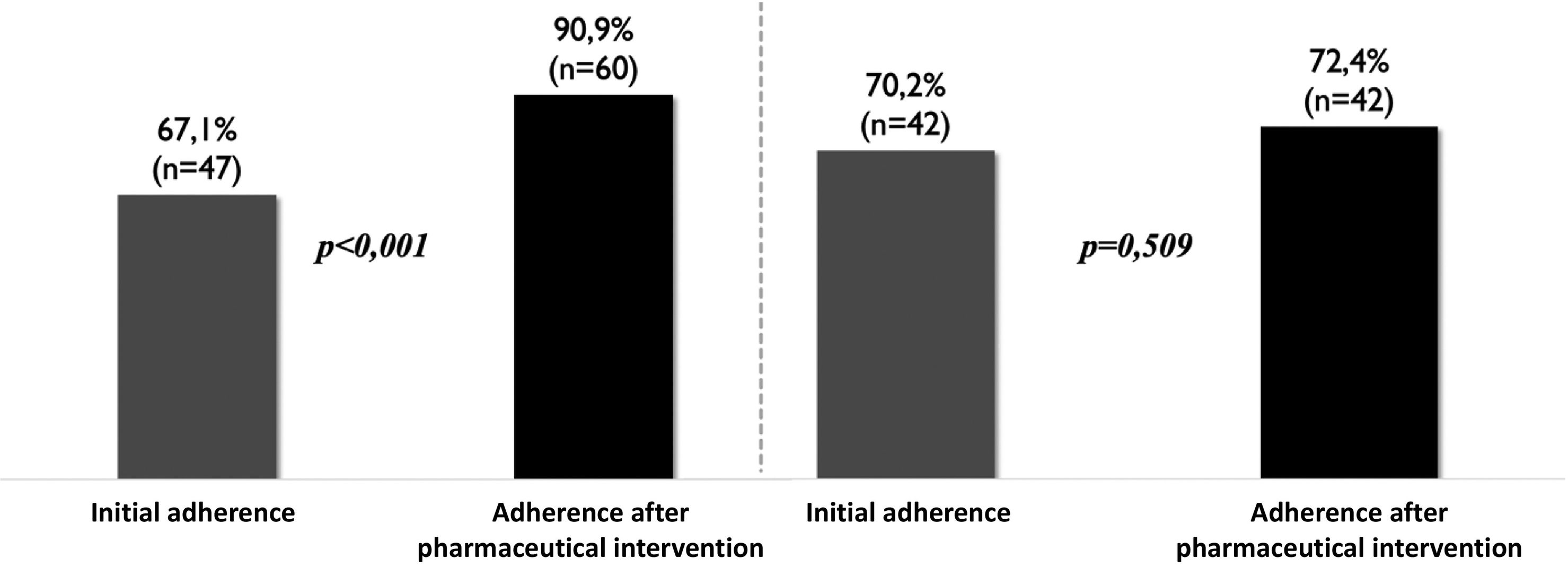
Analysis of improvement in adherence by type of OPU after the pharmaceutical intervention showed a significant improvement in the adherence rate of patients attending dedicated oncohaematology OPUs (P < .001) (Fig. 3). However, the degree of adherence remained unchanged in patients who collected their medications from general OPUs (Fig. 3).
DiscussionOral antineoplastic drugs have given patients a degree of autonomy and quality of life that other treatments cannot match. However, a disadvantage is that patients may not adhere to them, which can reduce their effectiveness.5,6 Therapeutic nonadherence is currently considered one of the most important public health problems affecting both patients and healthcare systems. It is recognised as one of the most relevant factors affecting pharmacological effectiveness and safety, reducing patient quality of life, increasing morbidity and mortality, and increasing the use and cost of healthcare resources.3
Therapeutic adherence has been studied extensively in different healthcare fields. However, in the field of oncohaematology, the available data on adherence to oral therapy are scarce. In theory, cancer patients would be expected to be highly adherent due to the perceived seriousness of their disease. However, adherence to oral antineoplastic treatment can range from 16% to 100%, depending on the type of therapy and the method used to determine it.7 A study of a large cohort of CML patients on TKIs found that only 49% had high adherence.8 This study also assessed the clinical impact of therapeutic adherence in this patient population, finding that participants with high adherence had better overall survival than those with low or moderate adherence.8 On the other hand, a study of breast cancer patients receiving anastrozole as adjuvant treatment found that therapeutic adherence decreased over time.9 During the first year of therapy, around 75% of patients achieved optimal adherence, which decreased to around 60% from the third year onwards.9 Similarly, our study shows that with an average treatment duration of approximately 5 years, the proportion of individuals with high adherence was 56.9%, which was in principle independent of the TKI used (imatinib [54.8%], nilotinib [63.6%], and dasatinib [54.3%]) (P = .67).
We analysed adherence to TKIs in CML and determined the impact of PC on improving therapeutic adherence. The first step was to assess each participant's degree of adherence at the time of inclusion, which allowed us to identify individuals with low and moderate therapeutic adherence, and thus candidates for a more in-depth pharmaceutical intervention. Over a period of 8 months, each of these patients was monitored more closely for therapeutic adherence, with both verbal and written information reinforced and tailored to their individual characteristics. The importance of individualising PC in this way is to ensure that patients are able to internalise their own therapeutic plan in their daily lives. In a previous study, we found that certain socioeconomic characteristics of patients, such as their educational level and employment status, had a significant impact on adherence to TKIs. For this reason, it was essential to take these factors into account when developing corrective measures for therapeutic adherence.10
In the second stage, we aimed to validate the role of pharmacists in encouraging correct therapeutic adherence through appropriate PC. To this end, healthcare professionals must provide patients with the necessary information and make them aware of all aspects of their condition and medication, thus promoting continuity of treatment and ensuring its effectiveness and safety. Given this need, pharmacists play a fundamental role in assessing adherence and in implementing measures to optimise and maintain it. However, to date, no single intervention has been found to improve adherence globally in all patients and for all diseases.11 Therefore, given the diversity of factors that influence adherence, a multifactorial approach is required to improve it, as well as the use of multiple combined strategies implemented by the OPUs. In our case, we designed a specific PC plan (see Annex 1) to improve therapeutic adherence, mainly based on 2 types of interventions: behavioural strategies and educational strategies.
We used behavioural interventions to change or reinforce patients' attitudes, thereby increasing their involvement in self-care and in solving their problems. These strategies included reminder systems such as pill boxes, calendars, and electronic devices. On the other hand, motivational clinical interviewing is one of the most valuable tools available to health professionals to promote behavioural change in patients. This strategy not only improves the professional-patient relationship but also allows us to obtain accurate information about the individuals and identify the barriers to proper adherence. As educational interventions are mainly based on the provision of verbal and written information, we designed a patient information leaflet containing the essential aspects that individuals need to know about their treatment, which could be easily adapted to each participant's therapeutic plan. In addition, the contents of this leaflet were discussed and reviewed with the patients during their visits to the OPUs.
This study is the first to show that PC improves adherence outcomes in CML patients on TKI treatment. However, as our data show, although general OPUs applied the same measures as dedicated oncohaematology OPUs, only the latter achieved a percentage increase in adherence, highlighting the need for specialised clinical work with professionals trained in the disease. It is well known that a targeted, specific, and deliberate intervention is required to improve adherence. Over the last decade, this understanding has formed the basis of one of the most ambitious projects of the Spanish Society of Hospital Pharmacy (SEFH): the Strategic Map of Pharmaceutical Care for Outpatients (MAPEX).12 Thanks to this project, a set of models for stratifying patient care, documents on integration into multidisciplinary teams, and clinical practice guidelines for PC adapted to different diseases have been developed.12 This new PC model offers more targeted care and can help improve clinical outcomes and quality of life for individuals, while promoting maximum efficiency for the healthcare system. One of the key requirements of this model is that pharmacists must be highly specialised in each type of disease. However, we still have a long way to go in this regard, as there are still pharmacy services that do not have dedicated oncohaematology services.
This study may be limited by the inherent bias of this type of study: patients who are assessed repeatedly over time learn to respond in “right” way to the aspects that are being assessed, so the results obtained may be overestimated. However, to reduce such bias, adherence was determined by combining 2 measures: the ESPA scale and the record of medication dispensed by OPUs. However, although the ESPA scale was easily applicable to the CML patients on TKIs, it was originally validated to assess adherence to antiretroviral therapy in HIV patients, so we had no previous experience of its use in other types of population.4
Nevertheless, to the best of our knowledge, this is the first prospective interventional study to demonstrate how PC affects adherence to TKIs in CML patients. Given that adherence is one of the most important factors in the effectiveness of chronic treatments, our study offers several key insights that can help improve health outcomes by actively enhancing adherence in patients treated with oral antineoplastic drugs. Thus, the results show that dedicated oncohaematology OPUs provide more effective and higher quality PC, while offering better adaptation to the needs of individuals, which ultimately translates into better adherence outcomes for our patients.
Contribution to the scientific literatureAlthough therapeutic adherence has been studied extensively in various healthcare settings, this has not been the case in oncohaematology. We analysed adherence to tyrosine kinase inhibitors in patients with chronic myeloid leukaemia, while also assessing the impact of pharmaceutical care on adherence. Our results not only highlight the importance of pharmacists in the management of chronic treatment but also help to lay the groundwork for the implementation of more specific pharmaceutical care programmes, thus improving the health outcomes of oncohaematology patients.
This study shows that dedicated oncohaematology outpatient units provide more effective and higher quality pharmaceutical care, while offering better adaptation to the needs of individuals, which ultimately translates into better therapeutic adherence outcomes for our patients. To the best of our knowledge, this is the first prospective interventional study to demonstrate how pharmaceutical care affects adherence to tyrosine kinase inhibitors in patients with chronic myeloid leukaemia.
Ethical responsibilitiesIn order to guarantee confidentiality, the participants' data were anonymised, thus ensuring that the participants could not be identified. All patients received an information sheet and signed an informed consent form to participate in the study.
This study was approved by each of the clinical research ethics committees of the participating hospitals (code LOH-IMA-2013-01).
Statement of authorshipAll of the authors of this study contributed significantly to its preparation. Study design: Betel Del Rosario, María Micaela Viña Romero, Virginia González Rosa, Carolina Alarcón Payer, Leonor Oliva Oliva, Gloria Julia Nazco Casariego, Fernando Gutiérrez Nicolás.
Literature search: Betel Del Rosario, María Micaela Viña Romero, Virginia González Rosa, Carolina Alarcón Payer, Leonor Oliva Oliva.
Data collection and statistical analysis: Betel Del Rosario, Virginia González Rosa, Carolina Alarcón Payer, Fernando Gutiérrez Nicolás.
Manuscript preparation: Betel Del Rosario, María Micaela Viña Romero, Virginia González Rosa, Carolina Alarcón Payer, Leonor Oliva Oliva, Gloria Julia Nazco Casariego, Fernando Gutiérrez Nicolás.
Responsibility and transfer of rightsAll authors accept the responsibilities defined by the International Committee of Medical Journal Editors (available at: http://www.icmje.org/). In the event of publication, the authors grant exclusive rights of reproduction, distribution, translation, and public communication (by any means or medium, whether sound, audiovisual or electronic support) of their work to Farmacia Hospitalaria and, by extension, to the SEFH.
For this purpose, a letter of assignment of rights will be signed at the time of submitting the work through the online manuscript management system.
CRediT authorship contribution statementBetel Del Rosario García: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. María Micaela Viña Romero: Visualization, Supervision, Methodology, Investigation, Data curation, Conceptualization. Virginia González Rosa: Validation, Methodology, Investigation, Formal analysis, Data curation. Carolina Alarcón Payer: Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation. Leonor Oliva Oliva: Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation. Gloria Julia Nazco Casariego: Validation, Supervision, Investigation, Formal analysis. Fernando Gutiérrez Nicolás: Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
FundingNone declared.
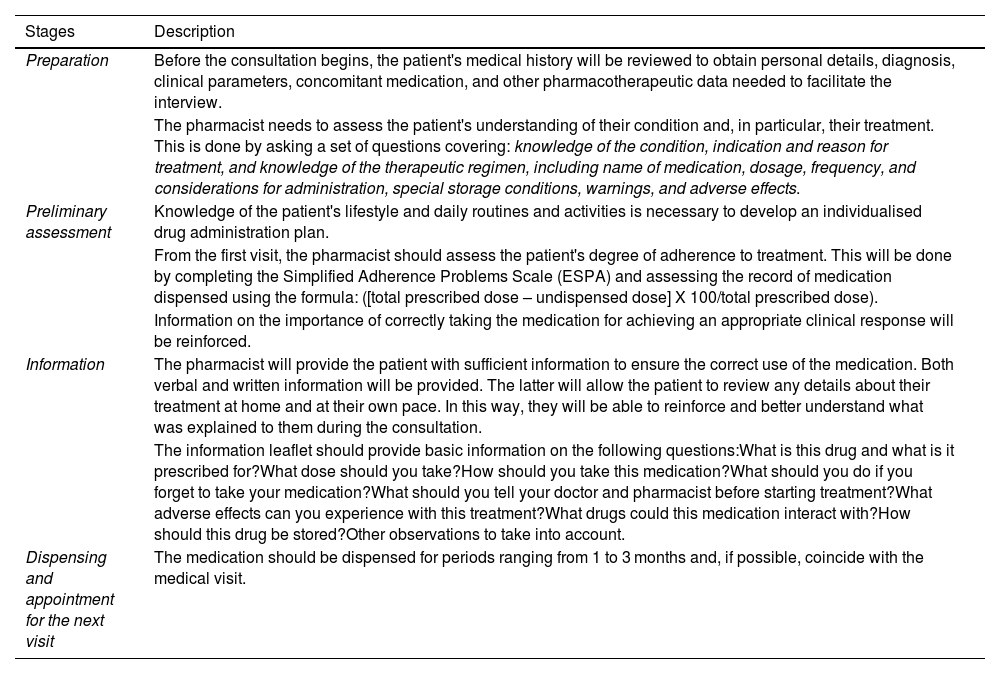
Specific Pharmaceutical Care Program.
| Stages | Description |
|---|---|
| Preparation | Before the consultation begins, the patient's medical history will be reviewed to obtain personal details, diagnosis, clinical parameters, concomitant medication, and other pharmacotherapeutic data needed to facilitate the interview. |
| The pharmacist needs to assess the patient's understanding of their condition and, in particular, their treatment. This is done by asking a set of questions covering: knowledge of the condition, indication and reason for treatment, and knowledge of the therapeutic regimen, including name of medication, dosage, frequency, and considerations for administration, special storage conditions, warnings, and adverse effects. | |
| Preliminary assessment | Knowledge of the patient's lifestyle and daily routines and activities is necessary to develop an individualised drug administration plan. |
| From the first visit, the pharmacist should assess the patient's degree of adherence to treatment. This will be done by completing the Simplified Adherence Problems Scale (ESPA) and assessing the record of medication dispensed using the formula: ([total prescribed dose – undispensed dose] X 100/total prescribed dose). | |
| Information on the importance of correctly taking the medication for achieving an appropriate clinical response will be reinforced. | |
| Information | The pharmacist will provide the patient with sufficient information to ensure the correct use of the medication. Both verbal and written information will be provided. The latter will allow the patient to review any details about their treatment at home and at their own pace. In this way, they will be able to reinforce and better understand what was explained to them during the consultation. |
| The information leaflet should provide basic information on the following questions:What is this drug and what is it prescribed for?What dose should you take?How should you take this medication?What should you do if you forget to take your medication?What should you tell your doctor and pharmacist before starting treatment?What adverse effects can you experience with this treatment?What drugs could this medication interact with?How should this drug be stored?Other observations to take into account. | |
| Dispensing and appointment for the next visit | The medication should be dispensed for periods ranging from 1 to 3 months and, if possible, coincide with the medical visit. |