To compare the relative efficacy of infliximab, adalimumab and golimumab through adjusted indirect treatment comparisons (ITCs).
MethodsAn exhaustive search was performed until October 2013. Databases consulted were MEDLINE, EMBASE, the Cochrane Library, the Centre for Reviews and Dissemination and the Web of Science. Randomized control trials (RCTs) comparing the efficacy of infliximab, adalimumab or golimumab versus placebo, in terms of clinical remission, clinical response and mucosal healing, were included. In the case that more than one RCT fulfilled the inclusion criteria for the same drug, a metanalysis was undertaken using a fixed effects model. ITCs were carried out using the method proposed by Bucher et al.
Results6 RCTs published in 5 papers were included: 2 for infliximab (ACT 1 and ACT 2), 2 for adalimumab (ULTRA 1 y ULTRA 2) and 2 for golimumab (PURSUIT-SC y PURSUIT-M).In these RTCs, each biological agent was superior in efficacy to placebo. The results of the adjusted ITC are the following. In relation to the clinical remission, in the induction and maintenance period, there are no statistically significant differences between the three anti-TNF drugs. In relation to the clinical response and mucosal healing, in the induction period, there are statistically significant differences between infliximab and adalimumab.
ConclusionIn view of the results obtained, infliximab, adalimumab and golimumab appear to be similarly effective therapeutic alternatives. Therefore, other considerations such as safety, tolerance and cost-effectiveness should be taken into account in order to select the most appropriate treatment.
Comparar la eficacia relativa de infliximab, adalimumab y golimumab mediante comparaciones indirectas (CI) ajustadas.
MétodosSe realizó una búsqueda bibliográfica que abarcó hasta Octubre 2013. Las bases de datos consultadas fueron: MEDLINE, EMBASE, the Cochrane Library, the Centre for Reviews and Dissemination y the Web of Science. Se incluyeron ensayos clínicos aleatorizados (ECA) que compararan la eficacia de infliximab, adalimumab o golimumab frente a placebo en términos de remisión clínica, respuesta clínica y curación de la mucosa. En el caso de que se incluyera más de un ECA para un mismo fármaco se llevó a cabo un metanálisis utilizado el modelo de efectos fijos. Las CI se realizaron utilizando el método de Butcher et al.
ResultadosSe incluyeron 6 ECA publicados en 5 artículos: 2 para infliximab (ACT 1 y ACT 2), 2 para adalimumab (ULTRA 1 y ULTRA 2) y 2 para golimumab (PURSUIT-SC y PURSUIT-M). Los tres agentes biológicos presentaron mayor eficacia que placebo. Los resultados de las CI fueron los siguientes: en relación a la remisión clínica, en el período de inducción y en el período de mantenimiento, no hubo diferencias estadísticamente significativas entre los tres fármacos anti-TNF. En relación a la respuesta clínica y a la curación de la mucosa, en el período de inducción hay diferencias estadísticamente significativas entre infliximab y adalimumab.
ConclusionesEn base a los resultados obtenidos (eficacia similar), infliximab, adalimumab y golimumab parecen ser alternativas terapéuticas. Así, otras consideraciones como la seguridad, la tolerancia y el coste-efectividad deben considerarse a la hora de seleccionar el tratamiento más adecuado.
Ulcerative colitis (UC) is a chronic inflammatory bowel disease of multifactorial aetiology that mainly affects the colon. It has a relapsing-remitting pattern. It could be classified in function of its extension in ulcerative proctitis, left sided colitis or extensive colitis; and in function of its severity in colitis in remission, mild, moderate or severe colitis1.
Symptoms of active disease or relapse include bloody diarrhoea, an urgent need to defecate and abdominal pain2.
The incidence in Europe is estimated at 1.5 to 20.3 cases per 100,000 person-years3. Disease onset can occur at any age, with a peak incidence between 15 and 25 years and a second smaller between 55 and 65 years2.
Current medical approaches focus on treating active disease to address symptoms, to improve quality of life, and thereafter to maintain remission. The treatment chosen for active disease is likely to depend on clinical severity, extent of disease and the patient’s preference, and may include the use of aminosalicylates, corticosteroids or biological drugs. Surgery may be considered as emergency treatment for severe ulcerative colitis that does not respond to drug treat-ment2,4.
Currently, three anti-TNF (tumour necrosis factor) drugs have been authorized by the European Medicines Agency (EMA) with the following indication: treatment of moderately to severely active ulcerative colitis in adult patients who have had an inadequate response to conventional therapy including corticosteroids and 6-mercaptopurine or azathioprine, or who are intolerant to or have medical contraindications for such therapies5,6,7.
To ensure the rational use of these drugs in clinical practice, aspects such as the efficacy, safety and cost-effectiveness of each drug must be evaluated. No direct head-to-head clinical trials have evaluated the superiority or non-inferiority of these drugs. Given the lack of head-to-head trials comparing biologic agents, indirect treatment comparisons (ITCs) were carried out to explore the relative efficacy of these drugs.
ITCs are relatively new approaches to evaluate the relative treatment effect when two or more interventions have not been compared directly.
An adjusted indirect comparison is an indirect comparison of different treatments adjusted according to the results of their direct comparison with a common control, so that the strength of the randomised trials is preserved. Empirical evidence indicates that results of adjusted indirect comparison are usually, but not always, consistent with the results of direct comparison. Basic assumptions underlying indirect comparisons include a homogeneity assumption for standard meta-analysis, and similarity assumption for adjusted indirect comparison8.
These approaches are being increasingly used by health technology assessmentl (HTA) agencies9 as new and existing drugs must be placed within the context of all available evidence for technology appraisals.
The main objective of this study was to compare the relative efficacy of infliximab, adalimumab and golimumab through adjusted indirect comparisons.
Material and methodsA systematic review was carried out to identify relevant studies published between 2005 (when the first trial about the first anti-TNF drug, infliximab, was published) and October 2013. The electronic search was performed by an information specialist in referential sources. Databases consulted were MEDLINE (through OVID), EMBASE, the Cochrane Library, the databases of the Center for Reviews and Dissemination (CRD) and the Web of Science (WOS). Also, Pubmed was revised in order to detect papers not included in MEDLINE (OVID) yet. The search strategies used in the main databases are shown in table 1.
Search strategy.
| MEDLINE | EMBASE | WOS |
|---|---|---|
| 1. *inflammatory bowel diseases/ or *colitis, | 1. ‘ulcerative colitis’/mj OR | 1. TI=(colitis AND (ulcerati* |
| ulcerative/ | ‘enteritis’/de | OR ulcero* OR mucosal) OR |
| 2. ((colitis and (ulcerati* or ulcero* or mucosal)) | 2. colitis:ab,ti AND | (procto$colitis OR colorectitis AND |
| or ((procto?colitis or colorectitis) and ulcerati*) or | (ulcerati*:ab,ti OR ulcero*:ab,ti | ulcerati*) OR ((chronic NEAR/3 |
| ((chronic adj3 colon) and (ulcerati* or ulcero*))).ti,ab. | OR mucosal:ab,ti) OR | colon) AND (ulcerati* OR ulcero*)) |
| 3. (chronic and colon and inflammat*).ti,ab. | (procto$colitis:ab,ti OR | OR (chronic AND colon AND |
| 4. 1 or 2 or 3 | colorectitis:ab,ti AND | inflammat*)) OR TS=(colitis AND |
| 5. *Tumor Necrosis Factor-alpha/ad, ae, ag, ai, ct, | ulcerati*:ab,ti) OR ((chronic | (ulcerati* OR ulcero* OR mucosal) |
| de, im, tu | NEAR/3 colon):ab,ti | OR (procto$colitis OR colorectitis |
| 6. exp Antibodies, Monoclonal/ad, ae, ct, de, im, tu | AND (ulcerati*:ab,ti OR | AND ulcerati*) OR ((chronic NEAR/3 |
| 7. Anti-Inflammatory Agents/tu | ulcero*:ab,ti)) OR (chronic:ab,ti | colon) AND (ulcerati* OR ulcero*)) |
| 8. Gastrointestinal Agents/tu | AND colon:ab,ti AND | OR (chronic AND colon AND |
| 9. 5 or 6 or 7 or 8 | inflammat*:ab,ti) | inflammat*)) |
| 10. ((anti?bod* adj3 monoclonal) or (anti?bod* | 3. #1 OR #2 | 2. TI=(infliximab OR adalimumab |
| adj3 single?done) or (inmunologic adj2 factor?) | 4. ‘infliximab’/exp OR | OR golimumab) OR TS=(infliximab |
| or (digestant* or ((gastric or gastrointestin*) | ‘adalimumab’/exp OR | OR adalimumab OR golimumab) |
| adj2 (agent? or Drug?))) or (inflammation or | ‘golimumab’/exp | 3. TI=(((drug$ OR pharmaco*) |
| anti?inflammator*) or ((tumo?r adj3 necros*) or | 5. infliximab OR adalimumab | NEAR/3 (treatments OR therap*)) |
| tnf?alpha or “tnf”)).ti,ab. | OR golimumab | OR (pharmaco* NEAR/3 |
| 11. (((drug? or pharmaco*) adj3 (treatment? or | 6. #4 OR #5 | management)) OR |
| therap*)) or (pharmaco* adj3 management)).ti,ab. | 7. #3 AND #6 | TS=(((drug$ OR pharmaco*) NEAR/3 |
| 12. ((anti?bod* adj3 monoclonal) or (anti?bod* | 8. 7 NOT [medline]/lim | (treatments OR therap*)) OR |
| adj3 single?done) or (inmunologic adj2 factor?) or | 9. #8 AND (‘conference | (pharmaco* NEAR/3 management)) |
| ((tumo?r adj3 necros*) or tnf?alpha or “tnf”)).ti,ab. | abstract’/it OR ‘conference | 4. ((#3 AND #2 AND #1)) |
| 13. (((advers* or drug) adj2 effect?) or immunolog* | paper’/it OR ‘conference | 5. (#4) AND Language=(English |
| or contra?indicat*).ti,ab. | review’/it OR ‘editorial’/it OR | OR Spanish) AND Document |
| 14. (10 and 11) or (12 and 13) | ‘letter’/it OR ‘note’/it OR ‘short | Types=(Article OR Review) |
| 15. (infliximab or adalimumab or golimumab).mp. | survey’/it) | 6. TI=(clinical trial OR controlled |
| 16. 9 or 14 or 15 | 10. #8 NOT #9 | clinical trial OR randomized |
| 17. 4 and 16 | 11. #10 AND ([english]/lim OR | controlled trial OR randomization |
| 18. (letter or “case report*” or “historical article*” | [spanish]/lim) | OR single blind procedure OR |
| or (comment or editorial or in vitro or news)).pt. | 12. #11 AND ‘human’/de | double blind procedure OR |
| 19. (“reference list” or bibliography* or “hand | AND (2005:py OR 2006:py | crossover procedure OR placebo |
| search*” or “relevant journal*” or (manual | OR 2007:py OR 2008:py | OR random* OR placebo OR |
| adj1 search*) or “selection criteria” or “study | OR 2009:py OR 2010:py | blind* OR trial) OR TS=(clinical |
| selection*”).mp. | OR 2011:py OR 2012:py OR | trial OR controlled clinical trial |
| 20. 18 or 19 | 2013:py) AND (‘clinical trial’/ | OR randomized controlled trial |
| 21. humans/ or (animals/ and humans/) | de OR ‘clinical trial (topic) ’/ | OR randomization OR single |
| 22. 17 and 21 | de OR ‘controlled clinical trial’/ | blind procedure OR double blind |
| 23. limit 22 to (clinical trial, phase iii or clinical trial, | de OR ‘controlled clinical | procedure OR crossover procedure |
| phase iv or clinical trial or randomized controlled | trial (topic)’/de OR ‘phase 3 | OR placebo OR random* OR |
| trial) | clinical trial (topic)’/de OR | placebo OR blind* OR trial) |
| 24. 23 not 20 | ‘randomized controlled trial’/de | 7. #6 AND #5 |
| 25. limit 24 to (english or spanish) | OR ‘randomized controlled trial | 8. #7 Databases=SCI-EXPANDED |
| 26. limit 25 to yr=“2005 -Current” | (topic) ’/de) | Timespan=2011-2013 |
Grey literature was obtained by searching the web sites of the EMA and HTA agencies. Unpublished data were not included in this review.
Studies were chosen for inclusion in the review based on the criteria outlined below:
- •
Population: adult patients naïve to biological drugs with moderate to severe ulcerative colitis.
- •
Intervention: infliximab, adalimumab or golimumab.
- •
Comparator: other anti-TNF-drug (direct comparison between the aforementioned interventions), or placebo.
- •
Outcomes: clinical remission, clinical response and mucosal healing.
- •
Study design: randomized controlled trials (RCTs).
Selection, critical appraisal, data extraction, qualitative and quantitative synthesis of the evaluated studies were independently undertaken by two researchers. Any discrepancies between the reviewers were resolved by a third independent reviewer.
The quality of the evidence of the included studies was assessed by the section A of the Critical Appraisal Skills Programme (CASP)10.
Heterogeneity of included studies was assessed with respect to the trial design and patient populations. For drugs with more than one study, a traditional meta-analysis of the efficacy data was performed, using a fixed-effect model, in the absence of heterogeneity, and the inverse variance method. Analyses were conducted using Epidat version 4.011.
Both clinical similarity and methodological similarity should be considered in adjusted indirect comparison. If the trial similarity assumption is not fulfilled, estimates from adjusted indirect comparisons will be invalid and misleading or should be interpreted cautiously.
Finally, adjusted ITCs were conducted based on the relative effects of each biological drug against a common comparator (placebo), following the method proposed by Butcher et al12. For the calculation of the risk ratio (RR) (95% CI), the software CIT, developed by the Canadian Agency for Drugs and Technologies in Health (CADTH), was used13.
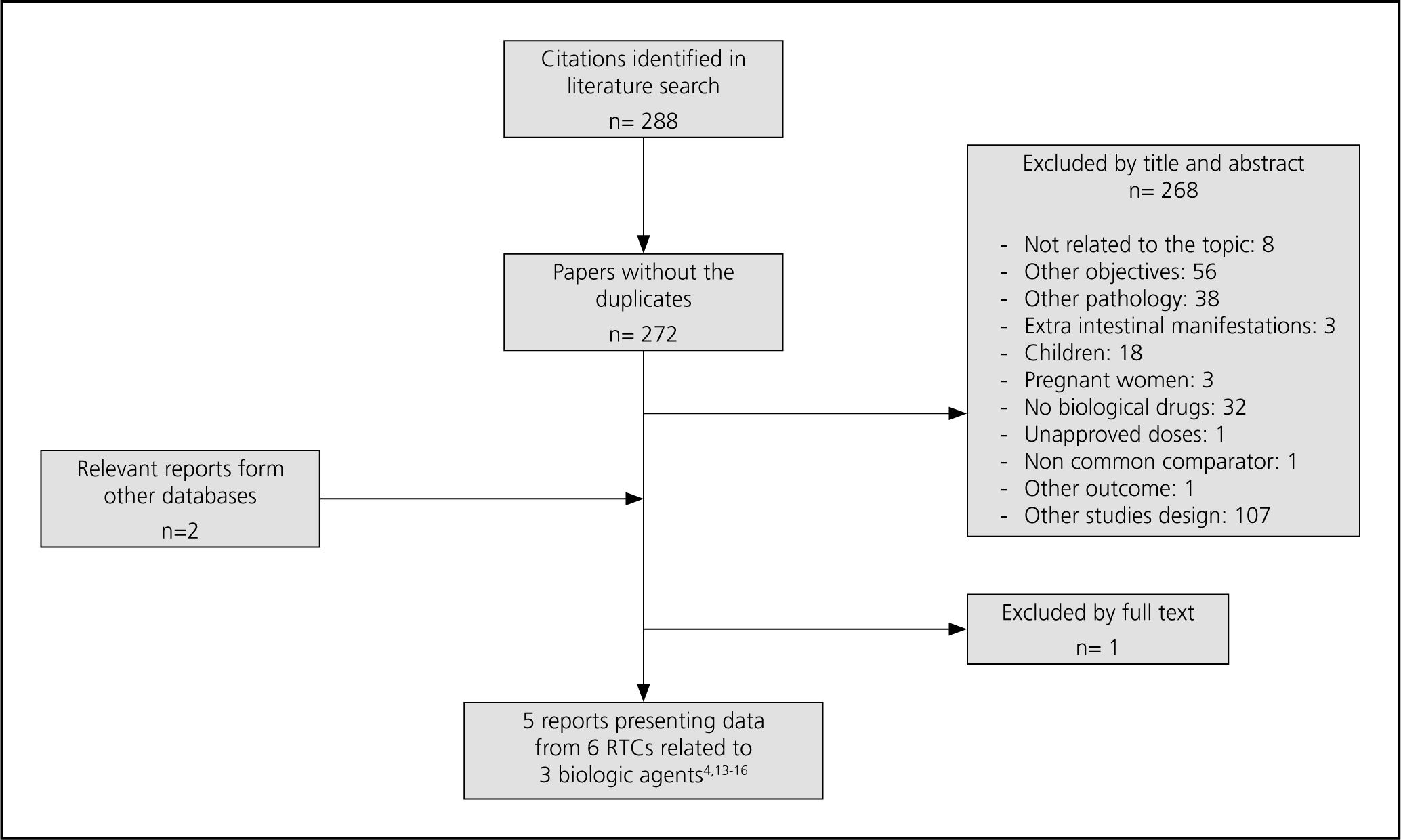
ResultsA total of 288 citations were found. 6 RCTs published in 5 papers were included: 2 for infliximab (ACT 1 and ACT 2)4, 2 for adalimumab (ULTRA 1 y ULTRA 2)14,15 and 2 for golimumab (PURSUIT-SC y PURSUIT-M)16,17. The flow diagram illustrates the way in which the trials were selected (Fig. 1).
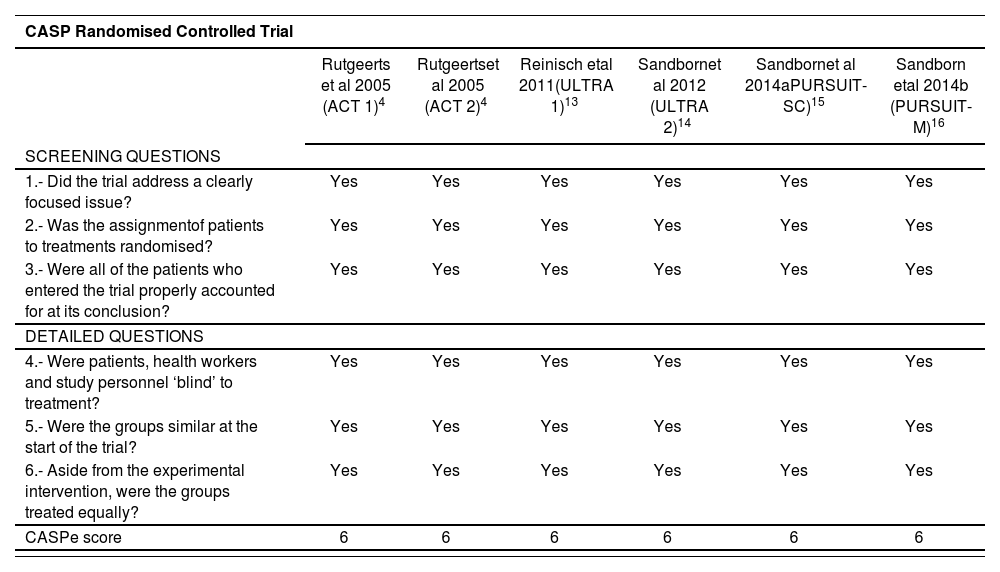
The quality of the included studies, based on CASP checklist, is detailed in table 2. All of them were of high quality (score 6 out of 6).
Quality of the included studies assessed by Critical Appraisal Skills Programme (CASP)
| CASP Randomised Controlled Trial | ||||||
|---|---|---|---|---|---|---|
| Rutgeerts et al 2005 (ACT 1)4 | Rutgeertset al 2005 (ACT 2)4 | Reinisch etal 2011(ULTRA 1)13 | Sandbornet al 2012 (ULTRA 2)14 | Sandbornet al 2014aPURSUIT-SC)15 | Sandborn etal 2014b (PURSUIT-M)16 | |
| SCREENING QUESTIONS | ||||||
| 1.- Did the trial address a clearly focused issue? | Yes | Yes | Yes | Yes | Yes | Yes |
| 2.- Was the assignmentof patients to treatments randomised? | Yes | Yes | Yes | Yes | Yes | Yes |
| 3.- Were all of the patients who entered the trial properly accounted for at its conclusion? | Yes | Yes | Yes | Yes | Yes | Yes |
| DETAILED QUESTIONS | ||||||
| 4.- Were patients, health workers and study personnel ‘blind’ to treatment? | Yes | Yes | Yes | Yes | Yes | Yes |
| 5.- Were the groups similar at the start of the trial? | Yes | Yes | Yes | Yes | Yes | Yes |
| 6.- Aside from the experimental intervention, were the groups treated equally? | Yes | Yes | Yes | Yes | Yes | Yes |
| CASPe score | 6 | 6 | 6 | 6 | 6 | 6 |
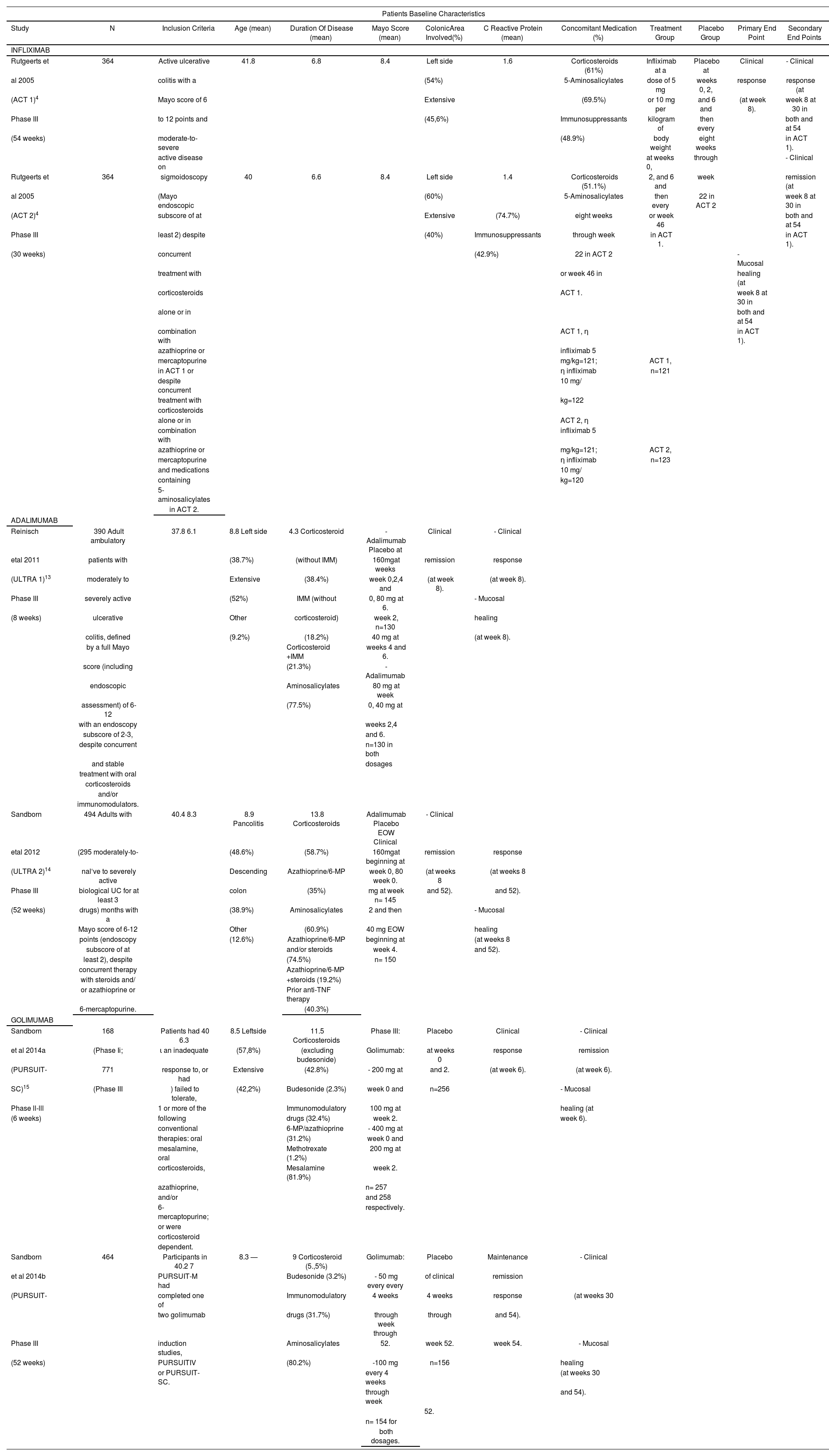
Rutgeerts et al reported the results of 2 randomized, double-blind, placebo-controlled trials (ACT 1 and ACT 2) which evaluated the efficacy of infliximab for induction and maintenance therapy in adults with ulcerative colitis5. 364 patients were included in each trial. Patients were followed for 54 weeks and 30 weeks in ACT 1 and ACT 2 studies, respectively. The primary endpoint was clinical response at week 8 in both cases.
For adalimumab, 2 randomized, double-blind and placebo controlled trials were included, ULTRA 1 and ULTRA 214,15. The ULTRA 1 study evaluated the efficacy of two dosing regimens of adalimumab in the induction period. A total of 390 patients were included and the primary endpoint was clinical remission at week 8. Moreover, in the ULTRA 2 study, the efficacy of adalimumab in the maintenance period was evaluated. A total of 494 patients were included. However, the subset of patients naïve to biological drugs (the study population in this review) was 295, as patients previously treated with biological agents could be included in the study. The primary end point was clinical remission at weeks 8 and 52.
PURSUIT-SC and PURSUIT-M trials assessed the efficacy of golimumab in the induction and maintenance period, respectively16,17. PURSUIT-SC integrated data from phase II and III trials. For phase III, a total of 771 patients, followed for 6 weeks, were included. The primary endpoint was clinical response at week 6. PURSUIT-M included 464 patients and the primary endpoint was clinical sustained response through week 54.
Patients baseline characteristics are showed in table 3. In order to asses the relative efficacy of the biologic drugs in the induction and maintenance periods, relevant and common clinical endpoint in the studies for the three drugs were selected: clinical remission, clinical response and mucosal healing, measured in the 6-8 (induction) and 52-54 (maintenance) weeks.
Summary of kev features of clinical trials of bioloaical aaents selected for indirect comparisons
| Patients Baseline Characteristics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | N | Inclusion Criteria | Age (mean) | Duration Of Disease (mean) | Mayo Score (mean) | ColonicArea Involved(%) | C Reactive Protein (mean) | Concomitant Medication (%) | Treatment Group | Placebo Group | Primary End Point | Secondary End Points |
| INFLIXIMAB | ||||||||||||
| Rutgeerts et | 364 | Active ulcerative | 41.8 | 6.8 | 8.4 | Left side | 1.6 | Corticosteroids (61%) | Infliximab at a | Placebo at | Clinical | - Clinical |
| al 2005 | colitis with a | (54%) | 5-Aminosalicylates | dose of 5 mg | weeks 0, 2, | response | response (at | |||||
| (ACT 1)4 | Mayo score of 6 | Extensive | (69.5%) | or 10 mg per | and 6 and | (at week 8). | week 8 at 30 in | |||||
| Phase III | to 12 points and | (45,6%) | Immunosuppressants | kilogram of | then every | both and at 54 | ||||||
| (54 weeks) | moderate-to-severe | (48.9%) | body weight | eight weeks | in ACT 1). | |||||||
| active disease on | at weeks 0, | through | - Clinical | |||||||||
| Rutgeerts et | 364 | sigmoidoscopy | 40 | 6.6 | 8.4 | Left side | 1.4 | Corticosteroids (51.1%) | 2, and 6 and | week | remission (at | |
| al 2005 | (Mayo endoscopic | (60%) | 5-Aminosalicylates | then every | 22 in ACT 2 | week 8 at 30 in | ||||||
| (ACT 2)4 | subscore of at | Extensive | (74.7%) | eight weeks | or week 46 | both and at 54 | ||||||
| Phase III | least 2) despite | (40%) | Immunosuppressants | through week | in ACT 1. | in ACT 1). | ||||||
| (30 weeks) | concurrent | (42.9%) | 22 in ACT 2 | - Mucosal | ||||||||
| treatment with | or week 46 in | healing (at | ||||||||||
| corticosteroids | ACT 1. | week 8 at 30 in | ||||||||||
| alone or in | both and at 54 | |||||||||||
| combination with | ACT 1, η | in ACT 1). | ||||||||||
| azathioprine or | infliximab 5 | |||||||||||
| mercaptopurine | mg/kg=121; | ACT 1, | ||||||||||
| in ACT 1 or | η infliximab | n=121 | ||||||||||
| despite concurrent | 10 mg/ | |||||||||||
| treatment with | kg=122 | |||||||||||
| corticosteroids | ||||||||||||
| alone or in | ACT 2, η | |||||||||||
| combination with | infliximab 5 | |||||||||||
| azathioprine or | mg/kg=121; | ACT 2, | ||||||||||
| mercaptopurine | η infliximab | n=123 | ||||||||||
| and medications | 10 mg/ | |||||||||||
| containing | kg=120 | |||||||||||
| 5-aminosalicylates | ||||||||||||
| in ACT 2. | ||||||||||||
| ADALIMUMAB | ||||||||||||
| Reinisch | 390 Adult ambulatory | 37.8 6.1 | 8.8 Left side | 4.3 Corticosteroid | -Adalimumab Placebo at | Clinical | - Clinical | |||||
| etal 2011 | patients with | (38.7%) | (without IMM) | 160mgat weeks | remission | response | ||||||
| (ULTRA 1)13 | moderately to | Extensive | (38.4%) | week 0,2,4 and | (at week 8). | (at week 8). | ||||||
| Phase III | severely active | (52%) | IMM (without | 0, 80 mg at 6. | - Mucosal | |||||||
| (8 weeks) | ulcerative | Other | corticosteroid) | week 2, n=130 | healing | |||||||
| colitis, defined | (9.2%) | (18.2%) | 40 mg at | (at week 8). | ||||||||
| by a full Mayo | Corticosteroid +IMM | weeks 4 and 6. | ||||||||||
| score (including | (21.3%) | - Adalimumab | ||||||||||
| endoscopic | Aminosalicylates | 80 mg at week | ||||||||||
| assessment) of 6-12 | (77.5%) | 0, 40 mg at | ||||||||||
| with an endoscopy | weeks 2,4 | |||||||||||
| subscore of 2-3, | and 6. | |||||||||||
| despite concurrent | n=130 in both | |||||||||||
| and stable | dosages | |||||||||||
| treatment with oral | ||||||||||||
| corticosteroids | ||||||||||||
| and/or | ||||||||||||
| immunomodulators. | ||||||||||||
| Sandborn | 494 Adults with | 40.4 8.3 | 8.9 Pancolitis | 13.8 Corticosteroids | Adalimumab Placebo EOW Clinical | - Clinical | ||||||
| etal 2012 | (295 moderately-to- | (48.6%) | (58.7%) | 160mgat beginning at | remission | response | ||||||
| (ULTRA 2)14 | nal‘ve to severely active | Descending | Azathioprine/6-MP | week 0, 80 week 0. | (at weeks 8 | (at weeks 8 | ||||||
| Phase III | biological UC for at least 3 | colon | (35%) | mg at week n= 145 | and 52). | and 52). | ||||||
| (52 weeks) | drugs) months with a | (38.9%) | Aminosalicylates | 2 and then | - Mucosal | |||||||
| Mayo score of 6-12 | Other | (60.9%) | 40 mg EOW | healing | ||||||||
| points (endoscopy | (12.6%) | Azathioprine/6-MP | beginning at | (at weeks 8 | ||||||||
| subscore of at | and/or steroids | week 4. | and 52). | |||||||||
| least 2), despite | (74.5%) | n= 150 | ||||||||||
| concurrent therapy | Azathioprine/6-MP | |||||||||||
| with steroids and/ | +steroids (19.2%) | |||||||||||
| or azathioprine or | Prior anti-TNF therapy | |||||||||||
| 6-mercaptopurine. | (40.3%) | |||||||||||
| GOLIMUMAB | ||||||||||||
| Sandborn | 168 | Patients had 40 6.3 | 8.5 Leftside | 11.5 Corticosteroids | Phase III: | Placebo | Clinical | - Clinical | ||||
| et al 2014a | (Phase Ii; | ι an inadequate | (57,8%) | (excluding budesonide) | Golimumab: | at weeks 0 | response | remission | ||||
| (PURSUIT- | 771 | response to, or had | Extensive | (42.8%) | - 200 mg at | and 2. | (at week 6). | (at week 6). | ||||
| SC)15 | (Phase III | ) failed to tolerate, | (42,2%) | Budesonide (2.3%) | week 0 and | n=256 | - Mucosal | |||||
| Phase ll-lll | 1 or more of the | Immunomodulatory | 100 mg at | healing (at | ||||||||
| (6 weeks) | following | drugs (32.4%) | week 2. | week 6). | ||||||||
| conventional | 6-MP/azathioprine | - 400 mg at | ||||||||||
| therapies: oral | (31.2%) | week 0 and | ||||||||||
| mesalamine, oral | Methotrexate (1.2%) | 200 mg at | ||||||||||
| corticosteroids, | Mesalamine (81.9%) | week 2. | ||||||||||
| azathioprine, | n= 257 | |||||||||||
| and/or | and 258 | |||||||||||
| 6-mercaptopurine; | respectively. | |||||||||||
| or were | ||||||||||||
| corticosteroid | ||||||||||||
| dependent. | ||||||||||||
| Sandborn | 464 | Participants in 40.2 7 | 8.3 — | 9 Corticosteroid (5.,5%) | Golimumab: | Placebo | Maintenance | - Clinical | ||||
| et al 2014b | PURSUIT-M had | Budesonide (3.2%) | - 50 mg every every | of clinical | remission | |||||||
| (PURSUIT- | completed one of | Immunomodulatory | 4 weeks | 4 weeks | response | (at weeks 30 | ||||||
| two golimumab | drugs (31.7%) | through week through | through | and 54). | ||||||||
| Phase III | induction studies, | Aminosalicylates | 52. | week 52. | week 54. | - Mucosal | ||||||
| (52 weeks) | PURSUITIV | (80.2%) | -100 mg | n=156 | healing | |||||||
| or PURSUIT-SC. | every 4 weeks | (at weeks 30 | ||||||||||
| through week | and 54). | |||||||||||
| 52. | ||||||||||||
| n= 154 for | ||||||||||||
| both dosages. | ||||||||||||
Clinical response was defined as a decrease from baseline in the total Mayo score of at least 3 points and at least 30 percent, with an accompanying decrease in the subscore for rectal bleeding of at least 1 point or an absolute subscore for rectal bleeding of 0 or 1.
Clinical remission was defined as a total Mayo score of 2 points or lower, with no individual subscore exceeding 1 point. Mucosal healing was defined as an absolute subscore for endoscopy of 0 or 1.
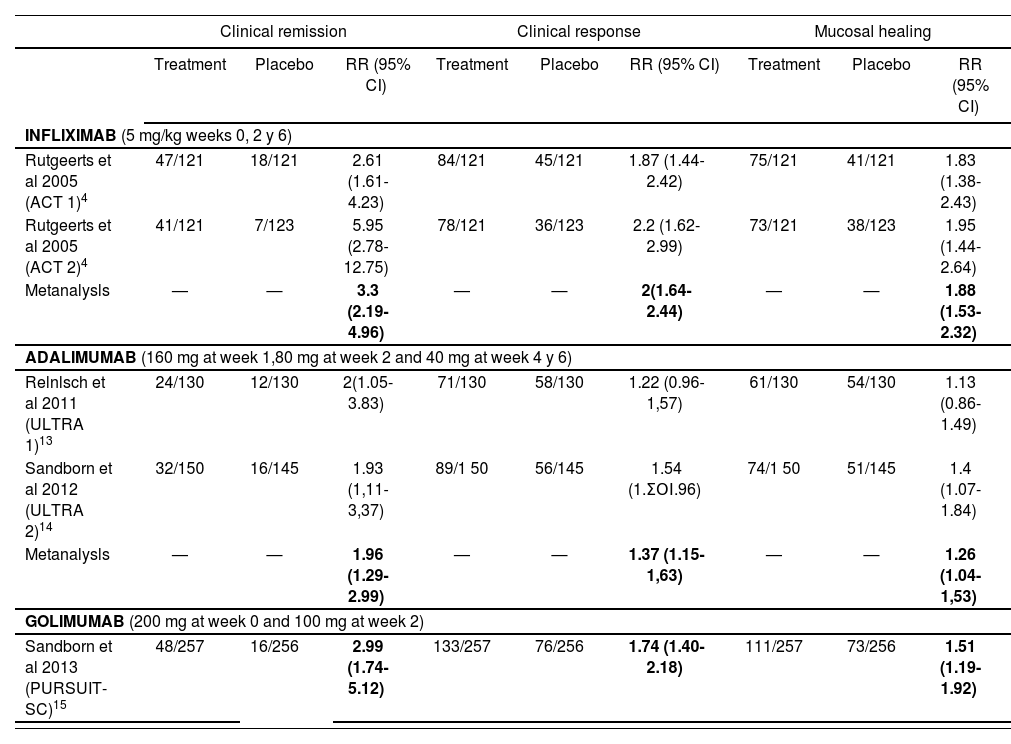
The efficacy results for the endpoints clinical remission, clinical response and mucosal healing of each clinical trial included are listed in tables 4 and 5, for the induction and maintenance periods, respectively.
Efficacy results in the induction period (week 8 for infliximab y adalimumab; week 6 for golimumab).
| Clinical remission | Clinical response | Mucosal healing | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Placebo | RR (95% CI) | Treatment | Placebo | RR (95% CI) | Treatment | Placebo | RR (95% CI) | |
| INFLIXIMAB (5 mg/kg weeks 0, 2 y 6) | |||||||||
| Rutgeerts et al 2005 (ACT 1)4 | 47/121 | 18/121 | 2.61 (1.61-4.23) | 84/121 | 45/121 | 1.87 (1.44-2.42) | 75/121 | 41/121 | 1.83 (1.38-2.43) |
| Rutgeerts et al 2005 (ACT 2)4 | 41/121 | 7/123 | 5.95 (2.78-12.75) | 78/121 | 36/123 | 2.2 (1.62-2.99) | 73/121 | 38/123 | 1.95 (1.44-2.64) |
| Metanalysls | — | — | 3.3 (2.19-4.96) | — | — | 2(1.64-2.44) | — | — | 1.88 (1.53-2.32) |
| ADALIMUMAB (160 mg at week 1,80 mg at week 2 and 40 mg at week 4 y 6) | |||||||||
| Relnlsch et al 2011 (ULTRA 1)13 | 24/130 | 12/130 | 2(1.05-3.83) | 71/130 | 58/130 | 1.22 (0.96-1,57) | 61/130 | 54/130 | 1.13 (0.86-1.49) |
| Sandborn et al 2012 (ULTRA 2)14 | 32/150 | 16/145 | 1.93 (1,11-3,37) | 89/1 50 | 56/145 | 1.54 (1.ΣΟΙ.96) | 74/1 50 | 51/145 | 1.4 (1.07-1.84) |
| Metanalysls | — | — | 1.96 (1.29-2.99) | — | — | 1.37 (1.15-1,63) | — | — | 1.26 (1.04-1,53) |
| GOLIMUMAB (200 mg at week 0 and 100 mg at week 2) | |||||||||
| Sandborn et al 2013 (PURSUIT-SC)15 | 48/257 | 16/256 | 2.99 (1.74-5.12) | 133/257 | 76/256 | 1.74 (1.40-2.18) | 111/257 | 73/256 | 1.51 (1.19-1.92) |
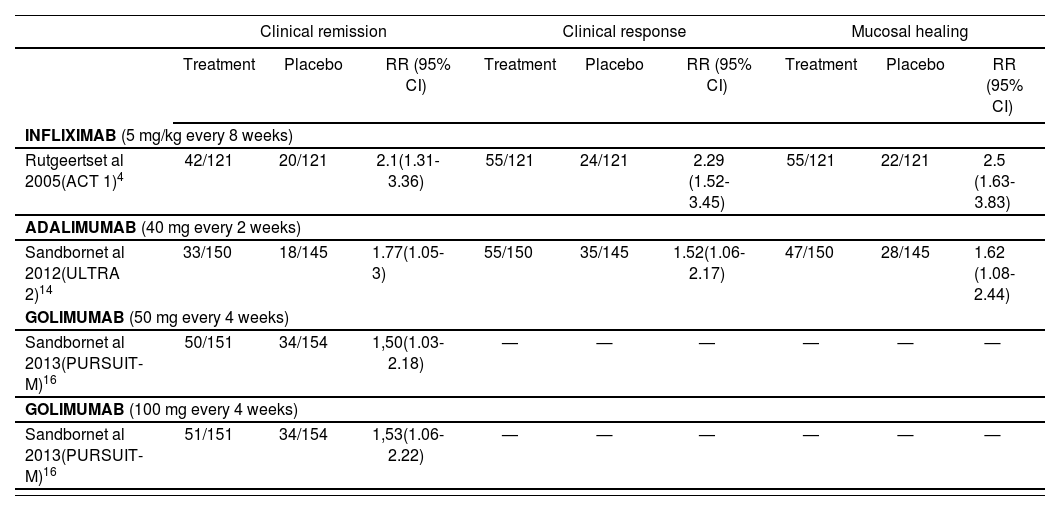
Efficacy results in the maintenance period (week 54 for infliximab and golimumab; week 52 for adalimumab).
| Clinical remission | Clinical response | Mucosal healing | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Placebo | RR (95% CI) | Treatment | Placebo | RR (95% CI) | Treatment | Placebo | RR (95% CI) | |
| INFLIXIMAB (5 mg/kg every 8 weeks) | |||||||||
| Rutgeertset al 2005(ACT 1)4 | 42/121 | 20/121 | 2.1(1.31-3.36) | 55/121 | 24/121 | 2.29 (1.52-3.45) | 55/121 | 22/121 | 2.5 (1.63-3.83) |
| ADALIMUMAB (40 mg every 2 weeks) | |||||||||
| Sandbornet al 2012(ULTRA 2)14 | 33/150 | 18/145 | 1.77(1.05-3) | 55/150 | 35/145 | 1.52(1.06-2.17) | 47/150 | 28/145 | 1.62 (1.08-2.44) |
| GOLIMUMAB (50 mg every 4 weeks) | |||||||||
| Sandbornet al 2013(PURSUIT-M)16 | 50/151 | 34/154 | 1,50(1.03-2.18) | — | — | — | — | — | — |
| GOLIMUMAB (100 mg every 4 weeks) | |||||||||
| Sandbornet al 2013(PURSUIT-M)16 | 51/151 | 34/154 | 1,53(1.06-2.22) | — | — | — | — | — | — |
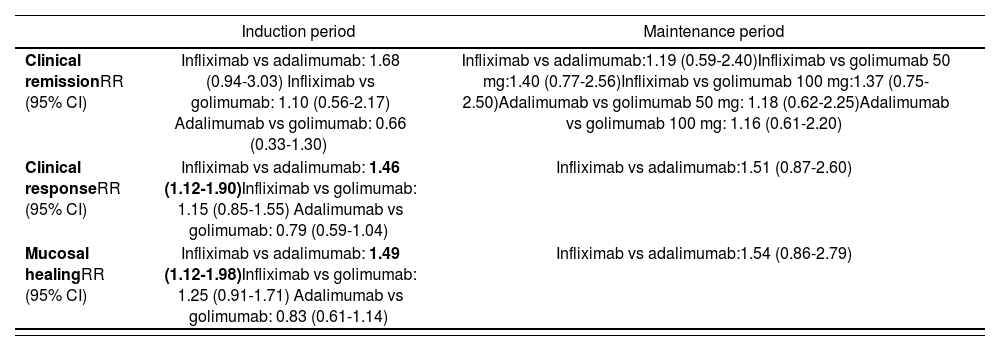
The results of the adjusted ITCs (table 6) for the selected outcomes (clinical remission, clinical response and mucosal healing) revealed that:
Results of the adjusted indirect comparisons
| Induction period | Maintenance period | |
|---|---|---|
| Clinical remissionRR (95% CI) | Infliximab vs adalimumab: 1.68 (0.94-3.03) Infliximab vs golimumab: 1.10 (0.56-2.17) Adalimumab vs golimumab: 0.66 (0.33-1.30) | Infliximab vs adalimumab:1.19 (0.59-2.40)Infliximab vs golimumab 50 mg:1.40 (0.77-2.56)Infliximab vs golimumab 100 mg:1.37 (0.75-2.50)Adalimumab vs golimumab 50 mg: 1.18 (0.62-2.25)Adalimumab vs golimumab 100 mg: 1.16 (0.61-2.20) |
| Clinical responseRR (95% CI) | Infliximab vs adalimumab: 1.46 (1.12-1.90)Infliximab vs golimumab: 1.15 (0.85-1.55) Adalimumab vs golimumab: 0.79 (0.59-1.04) | Infliximab vs adalimumab:1.51 (0.87-2.60) |
| Mucosal healingRR (95% CI) | Infliximab vs adalimumab: 1.49 (1.12-1.98)Infliximab vs golimumab: 1.25 (0.91-1.71) Adalimumab vs golimumab: 0.83 (0.61-1.14) | Infliximab vs adalimumab:1.54 (0.86-2.79) |
In the induction period, there were no statistically significant differences between the 3 drugs in terms of clinical remission. In relation to the efficacy endpoints clinical response and mucosal healing, statistically significant differences were observed between infliximab and adalimumab.
In the maintenance period, there were no statistically significant differences between the 3 drugs in terms of clinical remission, clinical response and mucosal healing.
DiscussionIn the six RCTs included, patients had similar baseline characteristics and the efficacy outcomes used were the same, although the primary endpoint was not the same in all trials. In addition, the six trials evaluated the results at weeks 6-8 and 52-54 for induction and maintenance periods, respectively. Based on the homogeneity and similarity of the trials, it was possible to realize indirect comparisons between the three biological agents.
The internal validity of the analyses is contingent on three factors: 1) the appropriate identification of the studies that make up the evidence network, 2) the quality of the individual RCTs, and 3) the extent of confounding bias due to similarity violations. Appropriate search and selection methods of all relevant RCTs was conducted. The internal validity of the single RCTs included was high. Studies did not differ with respect to the characteristics of the patients, the way in which the outcomes were measured or defined, the protocol requirements including the concomitant interventions allowed, the length of follow-up as well as differential loss to follow-up.
There are several limitations to consider in these analyses. The study ULTRA 2 included patients who could have been previously treated with anti-TNF drugs. However, the patients were stratified according to prior exposure to the same or not, and the results were reported independently for each subgroup of patients. Moreover, in this study, patients not responding to adali-mumab treatment could continue with it, but they entered to an open trial, assuming these losses as treatment failure (this does not happen with trials of infliximab and golimumab). Finally, the Mayo score was calculated in ULTRA 2 as the worst score of the last three days for stool frequency and rectal bleeding, while in RCTs of in-fliximab and golimumab was calculated as the average score of the last three days for these items.
The statistical approach that we employed is widely accepted by agencies such as the National Institute for Health and Care Excellence (NICE), and the CAD-TH. However, many clinicians may be unfamiliar with this approach and few guides are available to critically appraise such studies. The ITCs rely on many of the same assumptions as a standard pair-wise meta-analysis. There is a necessary consideration that the trials of each agent are sufficiently similar to pool together in terms of populations, interventions and outcomes. A further necessary consideration is that these similarities exist across the different agents.
For both infliximab and golimumab, the results of ACT 1 and ACT 2 and PURSUIT-SC and PURSUIT- M, were consistent with each other respectively. However, in the case of adalimumab, in ULTRA 1 and ULTRA 2, the results for the primary endpoint (clinical remission at week 8) were similar, but these studies differed in the results of the secondary endpoints. In ULTRA 1, there were no statistically significant differences between adalimumab and placebo for clinical response and mucosal healing at week 8, while in ULTRA 2 there were. This discrepancy may be due to higher response rates in the placebo group in ULTRA 1.
The three biological agents showed statistically superior efficacy to placebo. The available evidence is limited, as there are no comparative head to head trials.
The results of the adjusted ITCs for the outcomes evaluated were heterogeneous and insufficient to suggest differences between the three drugs. Therefore, they can be considered therapeutic alternatives with similar efficacy.
The results of the RCTs can be extrapolated to the population of interest, because the baseline characteristics of the patients do not have substantially differences with the patients treated in the routine clinical practice. Moreover, the end points used in the studies are the recommended for this condition.
Given the lack of ITCs related to biological agents in Spain, this work could be an important contribution for the evidence available so far. Nevertheless, a network meta-analysis18, which includes vedolizumab (a biological agent recently approved by EMA), had been published after we finished our systematic review. This new meta-analysis concludes that biological agents are effective treatments for UC. However head-to-head trials are necessary to select the best treatment option.
There is no evidence to suggest the superiority of one drug over the other. In view of the results obtained, inflixi-mab, adalimumab and golimumab appear to be similarly effective therapeutic alternatives. Therefore, other considerations such as safety, tolerance and cost-effectiveness should be taken into account in order to select the most appropriate treatment for individuals with ulcerative colitis.