The objective of the study was to analyse possible changes in antibiotic policy with ceftazidime-avibactam during the SARS-CoV-2 pandemic in an Intensive Care Unit (ICU) to determine patient mortality 28 days after initiation of antimicrobial therapy and to describe the microorganisms that most frequently colonise critically ill patients.
Material and methodObservational, single-centre, cohort study that included patients on treatment with ceftazidime-avibactam in ICU between March 2020 and September 2021. Demographic (age, sex), microbiological (colonisation, microorganisms isolated in blood cultures), pharmacotherapeutic (duration of treatment with ceftazidime-avibactam, antimicrobials used in synergy with ceftazidime-avibactam) and clinical (mortality, length of hospital stay and comorbidities) variables were collected. As associated comorbidities, we identified how many of the patients included in the study had diabetes mellitus (DM), chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD) or obesity.
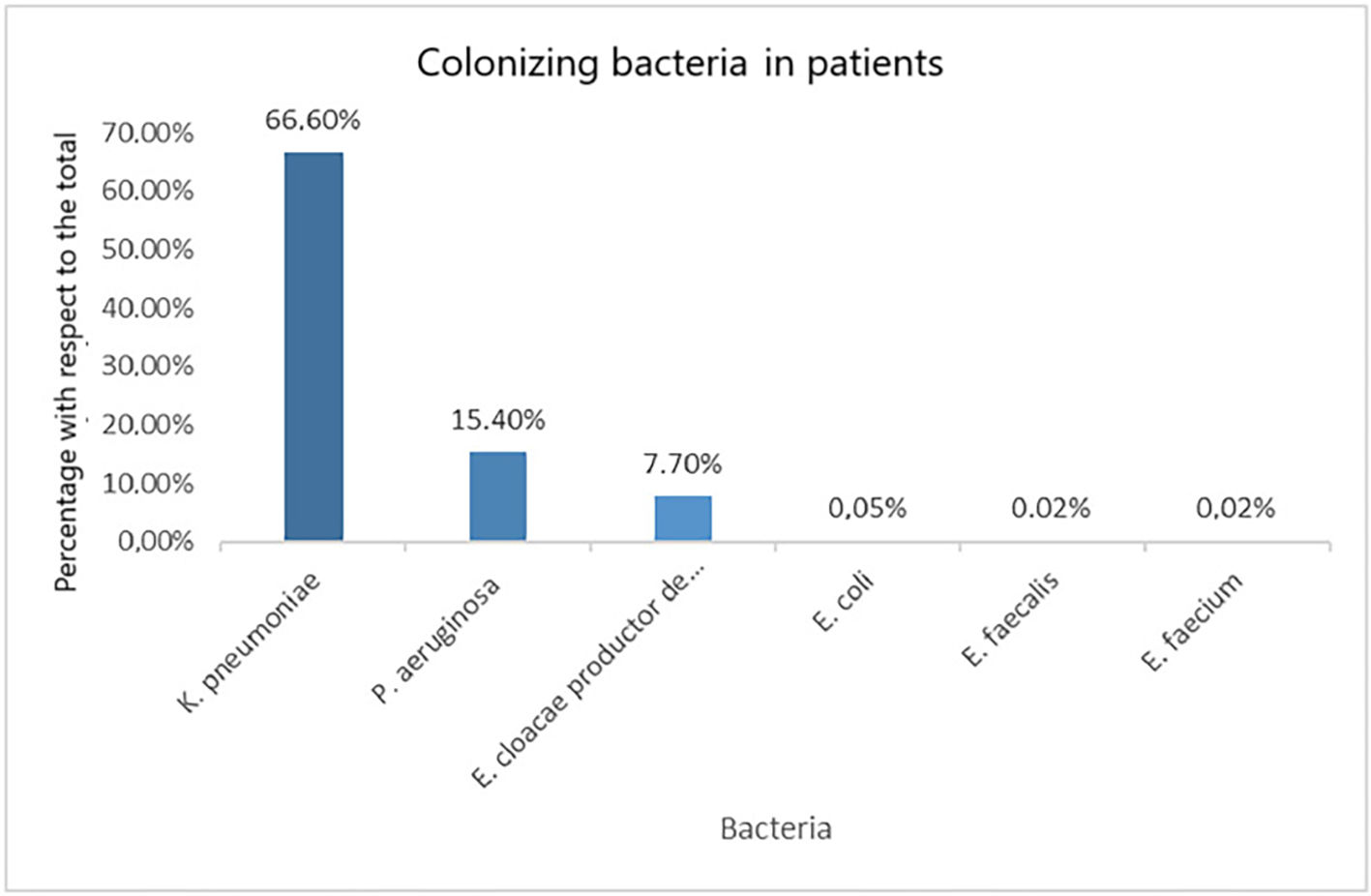
ResultsEighty-nine patients were included, 85.39% of whom were male. Forty-nine patients were infected with Sars-CoV-2. Median ICU stay was 46 days (RIQ = 58–27) in SARS-CoV-2 infected and 34 days (RIQ = 51–24) in non-infected patients. Patients were on ceftazidime-avibactam treatment for a median of 8 days (RIQ = 13–4), being 7 days (RIQ = 11–2) in COVID-19 positive patients and 11 days (RIQ = 14–6) in COVID-19 negative patients (p > 0.05). Empirical treatment with ceftazidime-avibactam was started empirically in 41.57% (n = 37) of the patients. The percentage of empiric initiations in SARS-CoV-2 infected patients was 43% and in non-infected patients 40%, with no statistically significant difference between empiric initiation according to SARS-CoV-2 diagnostic status (p > 0.05). A total of 43.8% (n = 39) of the patients were colonised by a multidrug-resistant (MDR) bacterium. Regarding on the microorganisms that colonised patients had, the most frequent was Klebsiella pneumoniae, present in 66.6% of patients (n = 26 patients). Overall mortality was 41.6%, with no statistically significant differences between SARS-CoV-2 infected and non-infected patients (42.9% and 40%, respectively; p > 0.05).
ConclusionThe SARS-CoV-2 pandemic did not lead to a change in the criteria for the use of ceftazidime-avibactam in the critically ill patient.
analizar posibles cambios en la política antibiótica de ceftazidima-avibactam durante la pandemia por SARS-CoV-2 en una unidad de medicina intensiva, determinar la mortalidad de los pacientes a los 28 días del inicio del antimicrobiano y describir los microorganismos que más frecuentemente colonizan a los pacientes críticos.
Material y métodosestudio observacional, unicéntrico y de cohortes que incluyó a pacientes en tratamiento con ceftazidima-avibactam en medicina intensiva entre marzo de 2020 y septiembre de 2021. Se recogieron variables demográficas (edad, sexo), microbiológicas (colonización, microorganismos aislados en hemocultivos), farmacoterapéuticas (duración de tratamiento con ceftazidima-avibactam, antimicrobianos empleados en sinergia con ceftazidima-avibactam) y clínicas (mortalidad, tiempo de estancia hospitalaria y comorbilidades). Como comorbilidades asociadas, se identificaron cuántos de los pacientes incluidos en el estudio presentaron diabetes mellitus (DM), enfermedad renal crónica (ERC), enfermedad pulmonar obstructiva crónica (EPOC) u obesidad.
Resultadosse incluyeron 89 pacientes, siendo el 85,39% hombres. Presentaron infección por SARS-CoV-2 49 pacientes. La mediana de estancia en la UCI fue de 46 días (RIQ = 58–27) en infectados por SARS-CoV-2 y de 34 días (RIQ = 51–24) en pacientes no infectados. Los pacientes estuvieron en tratamiento con ceftazidima-avibactam durante una mediana de 8 días (RIQ = 13–4), siendo de 7 días (RIQ = 11–2) en pacientes COVID-19 positivos y de 11 días (RIQ = 14–6) en los negativos (p > 0,05). El 41,57% (n = 37) de los pacientes había comenzado el tratamiento con ceftazidima-avibactam de forma empírica. El porcentaje de inicios empíricos en pacientes infectados por SARS-CoV-2 fue del 43% y en los pacientes no infectados del 40%, no habiendo diferencias estadísticamente significativas entre el inicio de forma empírica según el estado de diagnóstico de SARS-CoV-2 (p > 0,05). El 43,8% (n = 39) de los pacientes estaba colonizado por alguna bacteria multirresistente (BMR). Con respecto a los microorganismos que presentaban los pacientes colonizados, el más frecuente fue Klebsiella pneumoniae, presente en el 66,6% de los pacientes (n = 26 pacientes). La mortalidad global fue del 41,6%, no observándose diferencias estadísticamente significativas entre los infectados y los no infectados por SARS-CoV-2 (42,9 y 40%, respectivamente; p > 0,05).
Conclusiónla pandemia por SARS-CoV-2 no conllevó un cambio en los criterios de utilización de ceftazidima-avibactam en el paciente crítico.
Antimicrobial resistance is associated with high morbidity and mortality rates, representing a major public health concern.1 This complication is partly due to the inappropriate and excessive use of antibiotic agents2 In a recent publication, around 4,950,000 deaths in 2019 were estimated to be associated with antimicrobial resistance.3
Beta-lactams and carbapenems are effective in combating gram-negative bacteria infections. However, their effectiveness is decreasing owing to the mechanisms of resistance developed by microorganisms, including efflux pumps, beta-lactamases or target mutations.4 Efforts are being made to develop and approve new antibiotic agents that escape these mechanisms and effectively combat these microorganisms.
Ceftazidime-avibactam is a combination of two active substances that evade one of the cephalosporin resistance mechanisms of microorganisms.5 Ceftazidime is a third-generation, broad-spectrum cephalosporin with high activity against aerobial gram-negative bacilli. However, this agent can be inactivated by cephamycinases (AmpC), carbapenemases, and extended-spectrum beta-lactamases (ESBLs).6 Avibactam has a diazabicyclooctane structure and is a covalent reversible beta-lactamase inhibitor.5 The antibiotic ceftazidime-avibactam was approved in Spain in 2016. Coverage of this combination by the public health system is restricted to severe infections by carbapenem-resistant bacterial strains, when no other therapeutic option is available. When used as an empirical treatment, coverage is limited to KPC/OXA-48-producing Klebsiella.7
Empirical use of ceftazidime-avibactam is justified in certain settings, such as in patients colonized by multidrug-resistant microorganisms, or in critically ill patients with a high risk of developing multidrug-resistant infection. A retrospective study conducted in India revealed that ceftazidime-avibactam was frequently administered as an empirical treatment to critical patients with suspicion of nosocomial infection.8
Hospital overload during the first waves of the SARS-CoV-2 pandemic, added to the severity of disease, led to a global increase in the use of some antimicrobials.9 Reportedly, the most frequently prescribed antibiotics during the first wave of the pandemic were fluoroquinolones and third-generation cephalosporins.10 The years after the pandemic have witnessed an increase in the incidence of antimicrobial resistance.11
Initiation of antimicrobial treatment in intensive care units (ICUs) is generally empirical, considering the patient's comorbidities, site of infection, and severity of patient's condition.12
The purpose of this study was to compare the pattern of ceftazidime-avibactam use in an ICU during the pandemic.
Materials and methodsA retrospective, observational, single-center, cohort study was conducted in a class-5 hospital according to the Spanish hospital Cluster classification. The study included all ICU patients who received ceftazidime-avibactam treatment between March 2020 and September 2021. One of the cohorts included patients with a diagnosis of SARS-CoV-2 (confirmed by SARS-CoV-2 RNA detection by real-time reverse transcription polymerase chain reaction [RT-PCR] or by IgM and IgG antibody detection). SARS-CoV-2-negative patients were included in the control cohort. Data were extracted from electronic medical records integrated with the computerized physician order entry (CPOE).
The primary objective of this study was to compare the pattern of ceftazidime-avibactam use in ICU patients with and without SARS-CoV-2 infection during the pandemic. Secondary objectives included determining mortality 28 days after the initiation of antimicrobial treatment and describing the most frequent isolates in critically ill patients.
The data collected included demographic (age, sex); microbiological (colonization, isolated microorganisms in blood cultures); pharmacotherapeutic (duration of ceftazidime-avibactam treatment, antimicrobials used concurrently to ceftazidime-avibactam); and clinical (mortality, length of hospital stay and comorbidities) variables. Associated comorbidities considered in the study included diabetes mellitus (DM), chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), and obesity.
Bacteremia was defined as the presence of bacteria in one or more blood cultures. The definition of sepsis was established in 2016 as “life-threatening organ failure caused by a dysregulated host response to infection, with organ dysfunction identified on the basis of an increase of at least 2 points in the sequential organ failure assessment (SOFA) score”.13
Descriptive statistics and inferential analysis were used for statistical analysis. Descriptive results were expressed as mean, standard deviation, median and interquartile range (ICR). For inferential analysis, a comparison of quantitative variables was performed using Student's t-test. Differences between variables were considered significant when p < 0.05.
The study was approved by the local Ethics Committee (local code 24/010).
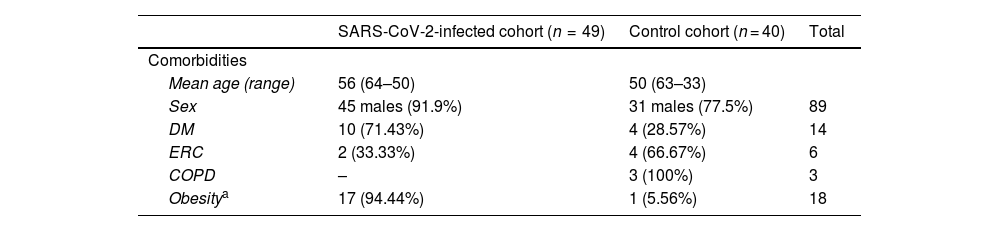
ResultsA total of 89 patients were included, of whom 85.40% were male. As many as 55% (n = 49) had SARS-COV-2 infection. Table 1 summarizes the characteristics of patients.
Characteristics of patients.
| SARS-CoV-2-infected cohort (n = 49) | Control cohort (n = 40) | Total | |
|---|---|---|---|
| Comorbidities | |||
| Mean age (range) | 56 (64–50) | 50 (63–33) | |
| Sex | 45 males (91.9%) | 31 males (77.5%) | 89 |
| DM | 10 (71.43%) | 4 (28.57%) | 14 |
| ERC | 2 (33.33%) | 4 (66.67%) | 6 |
| COPD | – | 3 (100%) | 3 |
| Obesitya | 17 (94.44%) | 1 (5.56%) | 18 |
DM: diabetes mellitus; CKD: chronic kidney disease COPD: chronic obstructive disease.
The median length of hospital stay was 45 days (RIQ = 66–33), with a median length of ICU stay of 40 days (RIQ = 54–25), with the latter being 46 days (RIQ = 58–27) in SARS-CoV-2-infected patients versus 34 days (RIQ = 51–24) in non-infected patients. The median duration of ceftazidime-avibactam treatment was 8 days (RIQ = 13–4), being 7 days (RIQ = 11–2) for COVID-19-positive patients and 11 days (RIQ = 14–6) for negative patients (p > 0.05).
Empirical ceftazidime-avibactam was initiated in 41.57% (n = 37) of patients. In total, 43% and 40% of SARS-CoV-2-positive and -negative patients received empirical treatment, respectively. No statistically significant differences were observed between positive and negative patients in the rate of initiation of empirical treatment (p > 0.05).
As many as 43.8% (n = 39) of patients were colonized by multi-drug resistant bacteria (MDRB). Of them, 51.3% (n = 20) had SARS-CoV-2 infection, without statistically significant differences between groups (COVID-19-positive and -negative) (p > 0.05). The most commonly isolated microorganism was Klebsiella Pneumoniae (K. pneumoniae), which was found in 26 patients (66.6%). Of them, 19 were VIM-producing strains.
Fig. 1 describes all colonizing microorganisms isolated in patients.
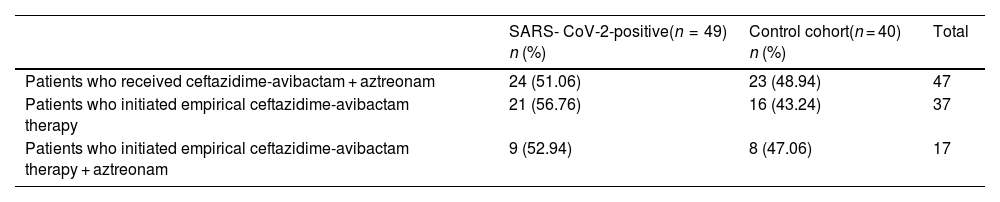
Concomitant aztreonam was administered for its synergic effect in 52.8% (n = 47) of patients. Of them, 36.17% (n = 17) had initiated empirical treatment. The percentages of patients in each cohort who received ceftazidime-avibactam plus aztreonam are shown in Table 2.
shows the patients in each cohort who received ceftazidime-avibactam and aztreonam.
| SARS- CoV-2-positive(n = 49) n (%) | Control cohort(n = 40) n (%) | Total | |
|---|---|---|---|
| Patients who received ceftazidime-avibactam + aztreonam | 24 (51.06) | 23 (48.94) | 47 |
| Patients who initiated empirical ceftazidime-avibactam therapy | 21 (56.76) | 16 (43.24) | 37 |
| Patients who initiated empirical ceftazidime-avibactam therapy + aztreonam | 9 (52.94) | 8 (47.06) | 17 |
Of all patients, the presence of bacteremia was confirmed in 10.1% (n = 9) of patients. Of them, 88.88% (n = 8) had a diagnosis of SARS-CoV-2. Of them, bacteremia was caused by K. pneumoniae in 4 patients and by Pseudomonas aeruginosa in 3 patients. A patient had Enterococcus faecium bacteremia. The isolate identified in the COVID-19-negative patient was Enterobacter asburiae.
Overall mortality was 41.6%, with no statistically significant differences observed between SARS-CoV-2-positive and -negative patients (42.9 and 40%, respectively; p > 0.05).
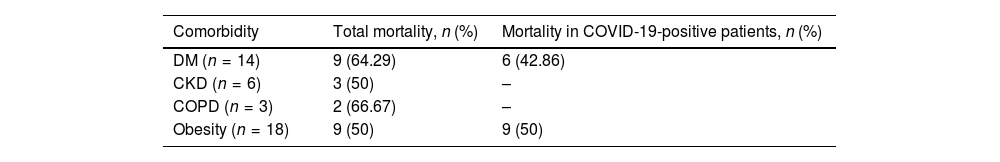
As many as 81% of deaths occurred within 28 days from initiation of the ceftazidime-avibactam therapy. Table 3 shows mortality by patient comorbidity.
Mortality by patient's comorbidity.
| Comorbidity | Total mortality, n (%) | Mortality in COVID-19-positive patients, n (%) |
|---|---|---|
| DM (n = 14) | 9 (64.29) | 6 (42.86) |
| CKD (n = 6) | 3 (50) | – |
| COPD (n = 3) | 2 (66.67) | – |
| Obesity (n = 18) | 9 (50) | 9 (50) |
DM: diabetes mellitus; CKD: chronic kidney disease COPD: chronic obstructive disease.
This retrospective, observational study conducted to assess the use of ceftazidime-avibactam in an ICU during the first waves of the pandemic revealed no differences in the pattern of use of this treatment between COVID-19-positive and -negative patients.
In a previous Spanish study involving COVID-19 patients treated in a third-level hospital, 80% of ICU patients had received empirical broad-spectrum treatment.14 However, results cannot be compared, since the empirical treatment most commonly administered in the Nebreda-Mayoral T. study was ceftriaxone, whereas our study only considered empirical ceftazidime-avibactam treatment, with the latter having revealed that 43% of SARS-CoV-2 patients had initiated this treatment empirically.
Other studies have explored the empirical use of ceftazidime-avibactam, such as that conducted by Hernández-Terciado et al.,15 which was conducted prior to ours (between July 2018 and June 2019, before the pandemic). The authors reported that 67% of patients who had received at least a dose of ceftazidime-avibactam initiated the treatment empirically. In our study, 41.57% of patients were prescribed this empirical treatment, including SARS-CoV-2-positive and -negative patients. Although Hernández-Terciado C. et al. did not disaggregate the use of this antibiotic by prescribing clinical department, and our study only included ICU patients, evidence suggests that the empirical use of this antibiotic was already high in the pre-pandemic period.
The mean age of the patients in our study was lower than that of the patients in the Docherty et al.16 study (72 years), where COVID-19-positive patients were included. In contrast, the mean age of our patients (53 years) was comparable to that (59 years) of patients in the systematic review by García-Vidal et al.17
The systematic review conducted by Rees EM et al.18 uncovered a median ICU stay of COVID-19-positive patients of 8 days in China and 7 days in other countries. This length of stay is far shorter than in our study, with a median ICU stay of 46 days (RIQ = 58–27) in patients with SARS-CoV-2 infection.
The subgroup analysis carried out by Hulme KD et al.19 demonstrated an association between obesity and higher mortality in SARS-CoV-2-positive patients, regardless of the use of invasive mechanical ventilation (IMV) or the presence of respiratory failure. These findings are consistent with those of our study, which unveiled that 100% of obese patients who died were COVID-19-positive. In the same line, 66.6% of patients with DM who died were COVID-19-positive. Lim S. et al.20 reported that hyperglycemia might favor viral replication, leading to increased morbidity and mortality in patients suffering from SARS-CoV-2 infection.
In agreement with the Aguilera-Calzadilla et al. study,21 the most frequent isolates detected in our study were gram-negative bacteria. This aligns with the findings of the Grau et al. review, which revealed that most of the isolated microorganisms in ICUs are gram-negative bacteria, accounting for 67% of isolates identified in this unit.22 In the Aguilera-Calzadilla et al. study,21 the most frequent bacteria in SARS-CoV-2 patients were Escherichia coli and K. pneumoniae, which contrasts with our finding of a higher frequency of K. pneumoniae followed by P. aeruginosa.
A limitation of this study is that the use of ceftazidime-avibactam during the pre-pandemic period was not explored. Another limitation is that secondary COVID-19-associated infections and bacterial co-infections in COVID-19-positive patients were not considered separately. A variety of studies highlight the differences between secondary COVID-19-associated infection, or overinfection, and bacterial co-infection in COVID-19-positive patients. According to these studies, co-infection is established when it occurs within the first 48 h after hospital admission, whereas secondary infection occurs 48 h after admission.17 Data on the time point where a BMR was isolated were not collected in our study, which hinders assessing the prevalence of bacterial co-infection and secondary COVID-19-associated infection. The use of ceftazidime-avibactam in ICU patients with SARS-CoV-2 infection with ventilator-associated pneumonia has been examined in several studies, such as that conducted by Burastero et al.23 However, no evidence is available on the pattern of ceftazidime-avibactam use in the setting of SARS-CoV-2 infection.
Contribution to the scientific literatureThis study reports our experience with the use of ceftazidime-avibactam during the SARS-CoV-2 pandemic. The evidence available in Spain about the use of antibiotics during the SARS-CoV-2 pandemic is limited. In addition, few studies have been conducted to determine the most common colonizing microorganisms identified in this period. This study was conducted to fill these gaps of knowledge and determine whether the empirical use of a restricted-use antibiotic increased or not during the pandemic.
CRediT authorship contribution statementFátima Mayo Olveira: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. José Manuel Caro Teller: Methodology, Visualization, Writing – original draft, Writing – review & editing. María Dolores Canales Siguero: Conceptualization, Formal analysis, Investigation. Sara Ortiz Pérez: Funding acquisition, Software, Validation. María del Carmen Jiménez León: Formal analysis, Validation. José Miguel Ferrari Piquero: Investigation, Project administration, Resources, Visualization.