To determine the degree of knowledge about biological therapy and biosimilars in patients with immune-mediated inflammatory diseases treated in Outpatient Pharmaceutical Care Units.
MethodsObservational, prospective, and multicenter study during the period May 2020–March 2021. A survey (9 questions) was conducted before starting treatment in which the patients' level of knowledge about biological therapy and biosimilars was assessed.
ResultsA total of 169 patients were included in the study. The average value for the different questions was 3.3±0.6 out of 5, while the average final result was 29.4 points out of 45. 64.5% of the patients had an acceptable level before starting the medication (>27 points). The multivariate analysis showed a statistically significant correlation (p<.05) with a better score at the beginning of treatment in those patients whose prescribing service was Rheumatology.
ConclusionsIn general, the level of knowledge prior to biological therapy in patients is acceptable, being higher in dosage and administration technique related-factors and what is related to the dosage and administration technique and where to find information related to the medication; the worst rated were those on biosimilars-related. The factor of being followed by rheumatology, was associated with better knowledge.
Determinar el grado de conocimiento sobre la terapia biológica y los biosimilares en pacientes con enfermedades inmunomediadas atendidos en las Unidades de Atención Farmacéutica a Pacientes Externos.
MétodoEstudio observacional, prospectivo y multicéntrico durante el período mayo 2020-marzo 2021. Se realizó una encuesta (9 preguntas) antes de iniciar el tratamiento en la que se valoraba el nivel de conocimiento de los pacientes sobre la terapia biológica y los biosimilares.
ResultadosUn total de 169 pacientes fueron incluidos en el estudio. El valor medio para las distintas preguntas fue de 3,3 ± 0,6 sobre 5, mientras que el resultado final medio fue de 29,4/45 puntos. El 64,5% de los pacientes tenían un nivel de conocimiento aceptable antes de iniciar el tratamiento. El análisis multivariante mostró una correlación estadísticamente significativa (p < 0,05) con una mejor puntuación al inicio del tratamiento, en aquellos pacientes cuyo servicio prescriptor era Reumatología.
ConclusionesEn general, el nivel de conocimiento previo a la terapia biológica es aceptable, siendo más elevado lo relacionado con la posología y la técnica de administración y dónde encontrar información relacionada con el medicamento. Las preguntas peor valoradas fueron aquellas sobre los biosimilares. El único factor que relacionado con un mejor conocimiento, fue ser paciente seguido por Reumatología.
A patient's perception of the effectiveness and safety of a therapy may be influenced by a lack of knowledge. Salar et al. found a negative correlation between patients' perceptions of effectiveness and safety and being unaware of certain characteristics of the treatment.1
The “Seven Steps to Patient Safety” guide outlines measures to ensure safer healthcare and encourages healthcare professionals to adopt a more interactive approach with patients.2 The role of various European patient associations is noteworthy, demonstrating that effective communication, a culture of safety, and active patient involvement can improve healthcare quality and promote the safe use of medicines.3
According to data from 2012, 80% of Spaniards trust generic medicines, and 92% have heard of them at least once. In addition, 70% believe that the quality, safety, and efficacy of generic medicines are similar to those of innovative medicines.4 Just as the introduction of generic medicines faced challenges, the inclusion of biosimilar medicines (BMs) can also be a significant barrier for many patients due to their lack of knowledge. However, we know that their economic impact is very substantial, being described as “the most powerful tool for efficiency” in the 2020 report published by the Spanish Independent Authority for Fiscal Responsibility.5
For all these reasons, it is crucial to assess patients' level of knowledge about their medication before starting biological therapy (BT), as this could influence the effectiveness and safety of treatment. It would also enable individualized patient education based on their initial level of knowledge. The aim of this study was to assess patients' level of knowledge about BT and BM at the start of treatment for immune-mediated inflammatory diseases (IMIDs).
MethodsDesignAn observational, prospective, multicenter study in 10 hospitals conducted between May 2020 and March 2021. Inclusion criteria were patients with IMIDs naïve to BT, of legal age, and attending the pharmacy service to start treatment. The protocol was approved by the Research Ethics Committee of the Hospital Universitario de la Princesa (Spain), and informed consent was obtained from all patients. This study complied with both the Organic Law on the Protection of Personal Data and the principles of the Declaration of Helsinki. Prior to treatment, the patients completed a questionnaire (Appendix 1) to assess their level of knowledge of BT and BM.
VariablesWe included both demographic variables (sex, age, educational level, employment status, family situation) and clinical variables (diagnosis, treatment, comorbidities), which were collected through a questionnaire completed by the patients. Their level of knowledge was assessed using a specific questionnaire designed by the research team. The questionnaire comprised 9 questions scored on a Likert-type scale ranging from 1 (the lowest) to 5 (the highest), with a maximum score of 45 points (the highest level of knowledge possible). A score of at least 27 points, representing a minimum of 60% of the maximum possible score, was considered to indicate an acceptable level of knowledge.
Statistical analysisQualitative variables are expressed as frequency and percentages based on the sample size (n) and quantitative variables are expressed as mean±standard deviation (SD).
To identify factors associated with a lack of knowledge, a multivariate analysis was conducted using multiple logistic regression to calculate odds ratios (ORs) and their 95% confidence intervals (95% CIs). A p-value of <.05 was used as a cut-off for statistical significance. All statistical analyses were performed using R Studio v. 1.1.456 software.
A sample size of 170 patients was calculated with a 95% CI and a 5% sampling error, assuming a prevalence of IMIDs of 6.39%6 and that only patients starting BT with their first pharmacy visit were assessed.
ResultsThe study included a total of 169 patients. Table 1 shows the demographic and clinical characteristics of the patients. Rheumatoid arthritis was the most prevalent IMID (27.2%), adalimumab was the most commonly prescribed drug (61.5%), and rheumatology was the main prescribing service (69.5%).
Baseline patient characteristics.
| Characteristic | n=169 |
|---|---|
| Age, y | n (%) |
| Median (IQR) | 53 (41–59) |
| Sex | |
| Female | 98 (58.0) |
| Level of education | |
| Basic education | 59 (35.3) |
| Non-university higher education | 53 (31.7) |
| Higher university education | 49 (29.3) |
| No studies | 6 (3.7) |
| Lives with a partner | |
| Yes | 151 (90.4) |
| No | 16 (9.6) |
| Employment status | |
| Active | 92 (55.1) |
| Unemployed | 19 (11.4) |
| Retired | 33 (19.8) |
| Sick leave | 18 (10.8) |
| Student | 5 (2.9) |
| Service prescribing the treatment | |
| Rheumatology | 114 (69.5) |
| Dermatology | 26 (15.9) |
| Digestive | 22 (13.4) |
| Other | 2 (1.2) |
| Comorbidities | |
| None | 87 (51.5) |
| Hypertension | 31 (18.3) |
| Dyslipidemia | 24 (14.2) |
| Fatigue | 20 (11.8) |
| Depression (and other CNS disorders) | 11 (8.3) |
| Diabetes mellitus | 7 (4.1) |
| Hypothyroidism | 7 (4.1) |
| Other | 5 (3) |
| Diagnosis | |
| Rheumatoid arthritis | 46 (28.6) |
| Psoriatic arthritis | 23 (14.3) |
| Psoriasis | 22 (13.6) |
| Ankylosing spondylitis | 21 (13.0) |
| Crohn's disease | 17 (10.5) |
| Spondyloarthritis | 10 (6.2) |
| Hidradenitis suppurativa | 8 (5.0) |
| Ulcerative colitis | 5 (3.1) |
| Arteritis | 3 (1.9) |
| Lupus | 3 (1.9) |
| Other | 3 (1.9) |
IQR, interquartile range.
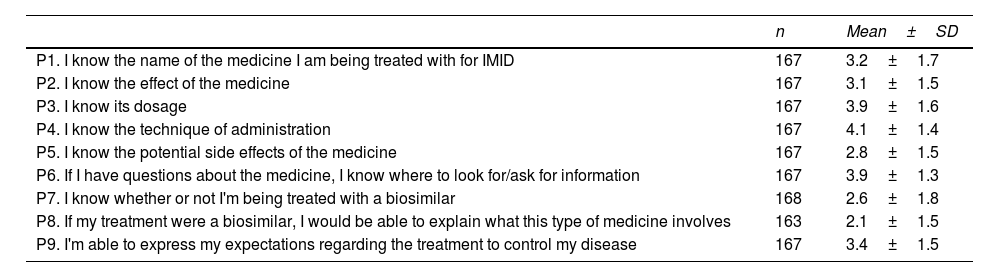
Table 2 shows the results for each question. The highest level of knowledge was related to dosage, administration techniques, and the sources for finding information about medicines. In contrast, the lowest level of knowledge was related to BM.
Results of the questionnaire on knowledge of biologic therapy and biosimilar medication.
| n | Mean±SD | |
|---|---|---|
| P1. I know the name of the medicine I am being treated with for IMID | 167 | 3.2±1.7 |
| P2. I know the effect of the medicine | 167 | 3.1±1.5 |
| P3. I know its dosage | 167 | 3.9±1.6 |
| P4. I know the technique of administration | 167 | 4.1±1.4 |
| P5. I know the potential side effects of the medicine | 167 | 2.8±1.5 |
| P6. If I have questions about the medicine, I know where to look for/ask for information | 167 | 3.9±1.3 |
| P7. I know whether or not I'm being treated with a biosimilar | 168 | 2.6±1.8 |
| P8. If my treatment were a biosimilar, I would be able to explain what this type of medicine involves | 163 | 2.1±1.5 |
| P9. I'm able to express my expectations regarding the treatment to control my disease | 167 | 3.4±1.5 |
IMID, immune-mediated inflammatory disease.
The mean score for the questions was 3.3±0.6 out of 5, while the mean final score was 29.4±10.2 points out of 45. Overall, 64.5% of the patients had an acceptable level of knowledge before starting BT.
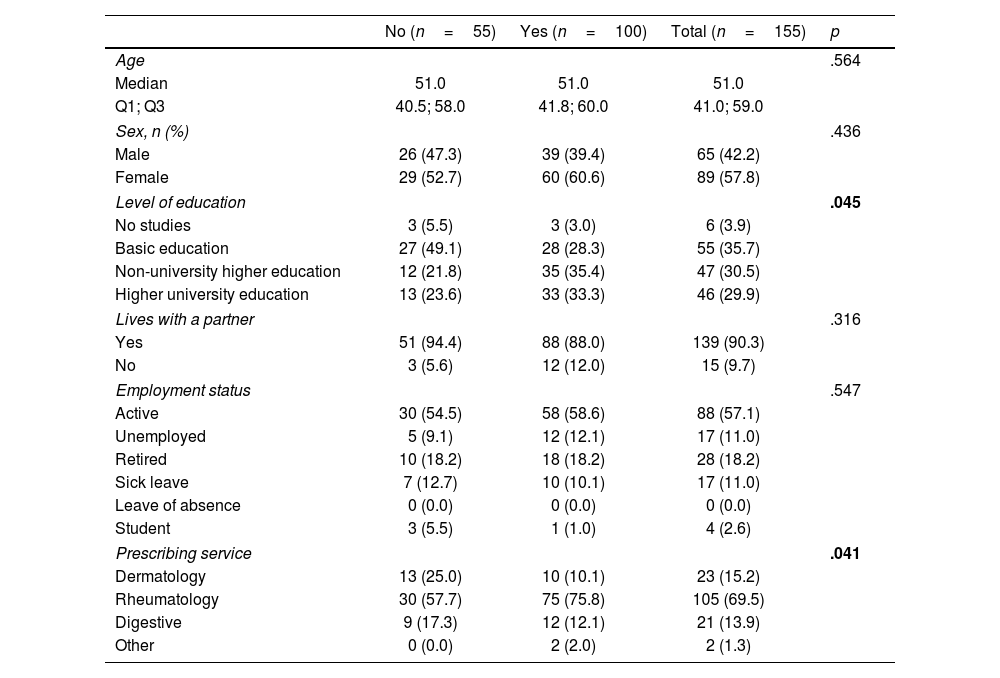
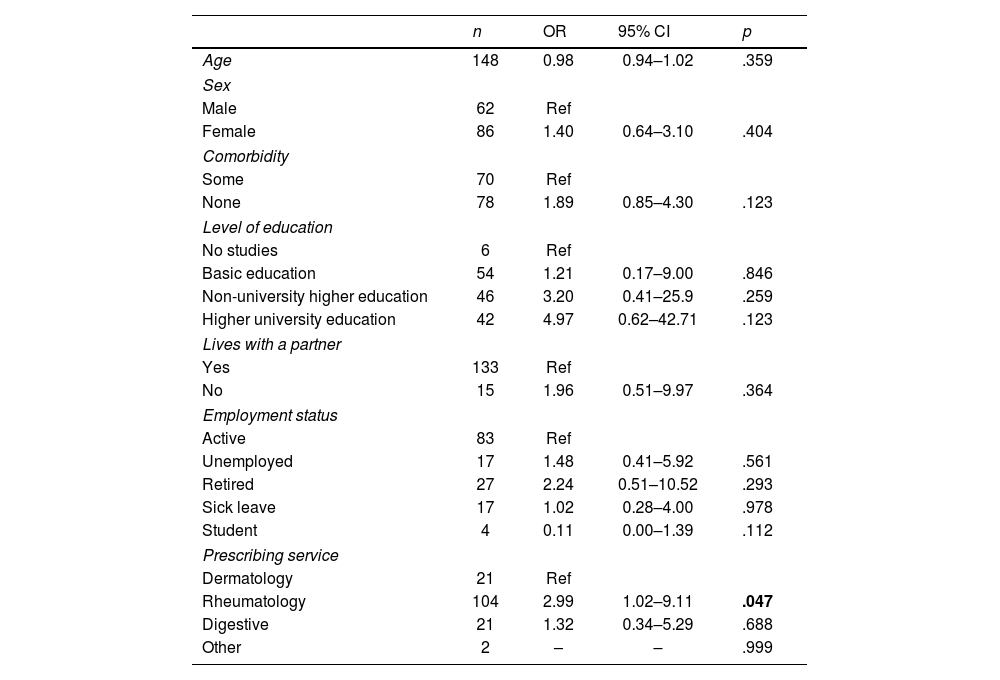
Univariate analysis was conducted to determine the factors associated with an adequate level of knowledge at the start of treatment (Table 3). A statistically significant correlation was found between educational levels (non-university vs university studies) (p=.045), and between the prescribing service and rheumatology (p=.041). After multivariate analysis (Table 4), the only characteristic associated with a better score was being a patient with rheumatology as the prescribing service (OR: 2.99; 95% CI: 1.02–9.11; p=.045).
Patient characteristics by acceptable knowledge status.
| No (n=55) | Yes (n=100) | Total (n=155) | p | |
|---|---|---|---|---|
| Age | .564 | |||
| Median | 51.0 | 51.0 | 51.0 | |
| Q1; Q3 | 40.5; 58.0 | 41.8; 60.0 | 41.0; 59.0 | |
| Sex, n (%) | .436 | |||
| Male | 26 (47.3) | 39 (39.4) | 65 (42.2) | |
| Female | 29 (52.7) | 60 (60.6) | 89 (57.8) | |
| Level of education | .045 | |||
| No studies | 3 (5.5) | 3 (3.0) | 6 (3.9) | |
| Basic education | 27 (49.1) | 28 (28.3) | 55 (35.7) | |
| Non-university higher education | 12 (21.8) | 35 (35.4) | 47 (30.5) | |
| Higher university education | 13 (23.6) | 33 (33.3) | 46 (29.9) | |
| Lives with a partner | .316 | |||
| Yes | 51 (94.4) | 88 (88.0) | 139 (90.3) | |
| No | 3 (5.6) | 12 (12.0) | 15 (9.7) | |
| Employment status | .547 | |||
| Active | 30 (54.5) | 58 (58.6) | 88 (57.1) | |
| Unemployed | 5 (9.1) | 12 (12.1) | 17 (11.0) | |
| Retired | 10 (18.2) | 18 (18.2) | 28 (18.2) | |
| Sick leave | 7 (12.7) | 10 (10.1) | 17 (11.0) | |
| Leave of absence | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Student | 3 (5.5) | 1 (1.0) | 4 (2.6) | |
| Prescribing service | .041 | |||
| Dermatology | 13 (25.0) | 10 (10.1) | 23 (15.2) | |
| Rheumatology | 30 (57.7) | 75 (75.8) | 105 (69.5) | |
| Digestive | 9 (17.3) | 12 (12.1) | 21 (13.9) | |
| Other | 0 (0.0) | 2 (2.0) | 2 (1.3) | |
Factors associated with higher levels of knowledge.
| n | OR | 95% CI | p | |
|---|---|---|---|---|
| Age | 148 | 0.98 | 0.94–1.02 | .359 |
| Sex | ||||
| Male | 62 | Ref | ||
| Female | 86 | 1.40 | 0.64–3.10 | .404 |
| Comorbidity | ||||
| Some | 70 | Ref | ||
| None | 78 | 1.89 | 0.85–4.30 | .123 |
| Level of education | ||||
| No studies | 6 | Ref | ||
| Basic education | 54 | 1.21 | 0.17–9.00 | .846 |
| Non-university higher education | 46 | 3.20 | 0.41–25.9 | .259 |
| Higher university education | 42 | 4.97 | 0.62–42.71 | .123 |
| Lives with a partner | ||||
| Yes | 133 | Ref | ||
| No | 15 | 1.96 | 0.51–9.97 | .364 |
| Employment status | ||||
| Active | 83 | Ref | ||
| Unemployed | 17 | 1.48 | 0.41–5.92 | .561 |
| Retired | 27 | 2.24 | 0.51–10.52 | .293 |
| Sick leave | 17 | 1.02 | 0.28–4.00 | .978 |
| Student | 4 | 0.11 | 0.00–1.39 | .112 |
| Prescribing service | ||||
| Dermatology | 21 | Ref | ||
| Rheumatology | 104 | 2.99 | 1.02–9.11 | .047 |
| Digestive | 21 | 1.32 | 0.34–5.29 | .688 |
| Other | 2 | – | – | .999 |
95% CI, 95% confidence interval; OR, odds ratio; Ref, reference.
The results show that, overall, patients have an acceptable level of knowledge about BT and BMs. The questions associated with the highest scores were related to dosage, administration technique, obtaining information, and expectations regarding disease control, whereas those associated with the lowest scores were related to adverse effects and knowledge of BMs. The only factor associated with a higher level of knowledge was receiving a prescription for BT from the rheumatology service. This association could be due to several factors, including greater experience of the service in using BMs or the introduction of training resources.
Recent studies have assessed patients' knowledge of BM and generally found that, despite limited knowledge, they have a high level of satisfaction with their medications.7 In the setting of IMID, a noteworthy study by Peyrin-Biroulet et al. assessed the perspective of patients with inflammatory bowel disease (IBD) receiving BM in Europe. The results showed that although 36% of the patients had heard of BM, 32% would be completely confident and trusting if the healthcare professional explained the BM concept to them.8 Our study found that the level of knowledge about BMs was higher, probably due to the prescribing and dispensing system in Spain, where the provision of pharmaceutical care by hospital pharmacists is a key factor in increasing the patients' level of knowledge about their medication.
Garcia et al. found similar results in Brazilian IBD patients, with 31% indicating they would be convinced to use BMs if they were explained to them beforehand, and 37% expressing concern but accepting BM therapy.9 Macaluso et al. studied BMs in IBD patients in Italy and found that 45% had received no information about BMs, while 58% considered their knowledge of this treatment to be poor.10 These results show that there is significant room for improvement in educating patients about BMs, with the goal of increasing their confidence in the treatment.
Moreover, despite the increasing use of BMs in clinical practice—which contributes to the sustainability of the healthcare system—the lack of training among healthcare professionals may limit their acceptance.11 The study by Marín-Jiménez et al. offers insights into the barriers and facilitators affecting the use of BMs among specialist physicians and hospital pharmacists in Spain.12 Looking ahead, detecting these barriers, and encouraging collaboration among professionals in educating patients about BMs will help reduce patient mistrust.13,14
The main strengths of this study are its multicenter, prospective design and that, to our knowledge, it is the first study to assess the level of knowledge about BT and BMs in patients with IMID. Another relevant aspect is the threshold used to assess an acceptable level of knowledge (more than 60%), which guarantees a minimum level of knowledge.
A possible limitation is the use of a questionnaire specifically designed for the study. However, it should be noted that there is currently no validated questionnaire. Another potential limitation in identifying predictors is the large number of rheumatology patients compared to other patients.
In conclusion, the overall level of knowledge about BT in patients with IMIDs is generally high. However, knowledge about BMs could be improved. It is therefore crucial to promote education about BMs in order to increase patients' confidence in their treatment and thus improve its effectiveness and safety.
Contribution to the scientific literatureThe use of BT in patients with IMID is becoming increasingly widespread. However, we know that some of these patients may not have all the information they need to achieve the best therapeutic outcomes. The results show that the level of knowledge about BT is appropriate for patients with IMID. Nevertheless, there is a lack of knowledge about BMs, which may hinder their use in routine clinical practice.
Understanding the key areas where there is a lack of information about BMs in patients with IMIDs will enable hospital pharmacies to focus their training and patient education efforts on these therapies.
FundingNone declared.
Statement of authorshipAll authors have contributed to the study in accordance with the international consensus on authorship (study conception and design, data collection, data analysis and interpretation, article writing, and approval of the final draft) and have read and approved the final version submitted.
CRediT authorship contribution statementCarlos Seguí-Solanes: Writing – original draft, Visualization, Validation, Methodology, Investigation. Lidia Estrada: Writing – original draft, Validation, Supervision, Resources. Esther Ramírez Herráiz: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. Silvia Ruiz-García: Writing – original draft, Visualization, Validation, Supervision. Tomás Palanques-Pastor: Writing – review & editing, Writing – original draft, Visualization, Validation. Vicente Merino Bohórquez: Writing – review & editing, Writing – original draft, Visualization, Validation, Software. Cristina Capilla Montes: Writing – review & editing, Visualization, Validation, Supervision. Joaquín Borras-Blasco: Writing – review & editing, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
The authors would like to thank all of the hospitals that enrolled patients in the study for their willingness to participate: Rosa M. Romero Jiménez, Hospital Gregorio Marañón (Madrid), Amaya Arrondo Velasco, Complejo Hospitalario de Navarra (Navarra), Nuria Rudi Sola, Hospital General de Granollers (Barcelona), Nuria Carballo Martínez, Hospital del Mar (Barcelona), Nadia Méndez Cabaleiro, Hospital San Rafael (Barcelona), Oihana Mora Atorrasagasti, Hospitalario U. Galdakao (Bizkaia), Jose Vicente Aparici Bolufer, Hospital Comarcal de Monforte (Lugo).