To compare lipid profile changes and cardiovascular events among HIV naïve and experienced patients from a real-world cohort treated with elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide fumarate or dolutegravir/abacavir/lamivudine.
MethodA retrospective cohort study in HIV naïve and experienced people at a reference hospital in Spain was done. During the follow-up (March 2015–June 2019), patients were treated with elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide fumarate or dolutegravir/abacavir/lamivudine. Epidemiological, clinical, and immunovirological variables were recorded. A statistical analysis of the lipid profile at baseline, 48, and 120 weeks after initiating the study therapy, cardiovascular events (myocardial infarction, heart failure, cerebrovascular accident, deep venous thrombosis, myocardiopathy, non-ST-segment elevation acute coronary syndrome, and ST-segment elevation myocardial infarction), and cardiovascular risks factors was performed. Data were analysed in naïve and experienced patients from each of the study treatments. The data were obtained from the medical history. The statistical analysis was performed with SPSS v. 24 software.
ResultsA total of 266 and 191 patients receiving treatment with elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide fumarate and dolutegravir/abacavir/lamivudine were included in the study, respectively. After 120 weeks of treatment, a worsening of the lipid profile was found in the elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide fumarate group, both in naïve and experienced patients, whereas not so conspicuously observed in the dolutegravir/abacavir/lamivudine group. Statistically significant differences between both groups were found in experienced patients favouring dolutegravir/abacavir/lamivudine; in total cholesterol (204.1±38.2 vs. 187.3±29.4, P < .001) and LDL-C (126.1±31.9 vs. 113.5±28.5, P = .001) at week 48, and in total cholesterol (201.1±33.4 vs. 188.7±33.9, P = .013) and HDL-C (54.2±15.6 vs. 48.3±14.3, P = .01) at week 120. No significant differences in cardiovascular events were found, neither in naïve nor in experienced patients.
ConclusionsThe lipid profile among elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide fumarate group worsened throughout the follow-up, both in naïve and experienced patients, not so remarkable in the dolutegravir/abacavir/lamivudine group. Both regimens were well tolerated, with similar rates of cardiovascular events.
Comparar los cambios en el perfil lipídico y los eventos cardiovasculares en vida real en una cohorte de pacientes VIH naive y pretratados que han recibido elvitegravir/cobicistat/emtricitabina/tenofovir alafenamida fumarato o dolutegravir/abacavir/lamivudina.
MétodoSe realizó un estudio de cohortes retrospectivo en personas VIH naive y pretratadas que durante el periodo de seguimiento (marzo 2015 - junio 2019) recibieron elvitegravir/cobicistat/emtricitabina/tenofovir alafenamida fumarato o dolutegravir/abacavir/lamivudina en un hospital de referencia en España. Se registraron variables epidemiológicas, clínicas e inmunovirológicas. Se consideraron datos del perfil lipídico al inicio del estudio, a las 48 y 120 semanas después de iniciar la terapia del estudio, de los eventos cardiovasculares (infarto de miocardio, insuficiencia cardíaca, accidente cerebrovascular, trombosis venosa profunda, miocardiopatía, síndrome coronario agudo sin elevación del segmento ST e infarto de miocardio con elevación del segmento ST) y factores de riesgo cardiovascular. Los datos se obtuvieron de la historia clínica. Se realizó un análisis estadístico utilizando el software SPSS v.24.
ResultadosSe incluyeron en el estudio un total de 266 pacientes en tratamiento con elvitegravir/cobicistat/emtricitabina/tenofovir alafenamida fumarato y 191 con dolutegravir/abacavir/lamivudina. Después de 120 semanas de tratamiento, se observó un empeoramiento del perfil lipídico basal en el grupo elvitegravir/cobicistat/emtricitabina/tenofovir alafenamida fumarato, tanto en pacientes naive como pretratados, no siendo tan pronunciado en el grupo de pacientes que recibieron dolutegravir/abacavir/lamivudina. Cuando se comparó el perfil lipídico de ambos grupos de tratamiento, se encontraron diferencias estadísticamente significativas en pacientes pretratados a favour de dolutegravir/abacavir/lamivudina, en colesterol total (204,1±38,2 vs. 187,3±29,4, p < 0,001) y LDL-C (126,1±31,9 vs. 113,5±28,5, p = 0,001) en la semana 48, y en colesterol total (201,1±33,4 vs. 188,7±33,9, p = 0,013) y HDL-C (54,2±15,6 vs. 48,3±14,3, p = 0,01) en la semana 120. No se encontraron diferencias significativas en los eventos cardiovasculares durante el periodo de seguimiento, ni en los pacientes naive ni en los pretratados.
ConclusionesEl perfil lipídico en el grupo elvitegravir/cobicistat/emtricitabina/tenofovir alafenamida fumarato empeoró durante el seguimiento, tanto en pacientes naïve como pretratados, no siendo tan notable en el grupo dolutegravir/abacavir/lamivudina. Ambos regímenes fueron bien tolerados, con tasas similares de eventos cardiovasculares.
Antiretroviral therapy (ART) has revolutionised the management of HIV, from becoming a chronic disease, and increasing life expectancy.1,2 This ageing of the population has brought an increase in the incidence of cardiovascular comorbidities in people living with HIV (PLWHIV), with a higher prevalence of cardiovascular risk factors (hypertension, diabetes, and dyslipidaemia).3,4 Although new drugs with better safety profiles are emerging, they are not exempt from adverse effects, such as changes in the lipid profile.5,6 Further, various studies have demonstrated that PLWHIV have an increased risk of developing heart failure, stroke, or myocardial infarction.7,8
Among the different available drugs, the most appropriate treatment for the patient, considers their individual characteristics, resistance tests, polypharmacy, and risk of interactions. Always urging individualisation, the national and international guidelines are a reference manual and guide for the management of HIV, recommending an early start after diagnosis9,10
The 2 single-tablet treatment regimens most widely used between 2015 and 2019 in our country were dolutegravir/abacavir/lamivudine (DTG/ABC/3TC) and elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide fumarate (EVG/c/FTC/TAF).
Lipid parameters variations in the randomised controlled studies of EVG/c/FTC/TAF and DTG/ABC/3TC were minimal, without clinical or statistical significance.11–16 Furthermore, there are no studies, to the best of our knowledge, directly comparing these regimens.
The primary objective of this study is to compare the lipid profile changes and cardiovascular events in a real-world cohort of naïve and treatment-experienced HIV-infected patients who had been treated with EVG/c/FTC/TAF or DTG/ABC/3TC.
MethodsA retrospective observational single-centre study (university hospital in north-western Spain) in adult PLWHIV who received EVG/c/FTC/TAF or DTG/ABC/3TC from March 2015 to June 2019 was performed. The recommendations of the national clinical practice guidelines for each year and characteristics of each patient were decisive in the selected treatment.17 The follow-up time was defined since the beginning of the study ART until its suspension, voluntary abandonment, or loss of follow-up of the patient, death, or the end of the study.
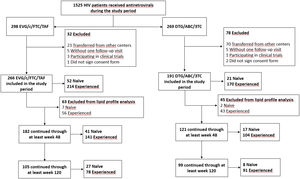
All HIV-infected adults treated with EVG/c/FTC/TAF or DTG/ABC/3TC during the study follow-up time who signed the informed consent and with at least 1 medical visit were included in the study. Those patients transferred from other centres (hospitals or penitentiaries) (N = 93), voluntary withdrawal from treatment without 1 follow-up visit (N = 10), participating in clinical trials (N = 4), or those who did not sign the informed consent (N = 3) were excluded from the study.
This study was approved by the ethics committee of our institution. The study was carried out based on the Declaration of Helsinki of Good Clinical Practices.
Epidemiological, clinical, and immunovirological variables were recorded. Data in naïve and experienced patients of each of the study treatments were analysed.
Cardiovascular risk factors such as arterial hypertension, diabetes mellitus, smoking history, and dyslipidaemia (defined as TC ≥ 200 mg/dL, TG ≥ 150 mg/dL, or LDL-C ≥ 130 mg/dL) were recorded. The data were obtained from the laboratory analysis, the medical record registrations, or the pharmacology therapy (antihypertensive drugs, insulin or oral glucose-lowering agents, or lipid-lowering treatment).
The lipid profile (including total cholesterol (TC), triglycerides (TG), LDL cholesterol (LDL-C), HDL cholesterol (HDL-C), TC:HDL-C, and LDL-C/HDL-C ratios) at 48 and 120 weeks after initiating EVG/c/FTC/TAF or DTG/ABC/3TC was compared with the baseline. For both treatment groups, those patients with lipid-lowering drugs prescription were excluded from the lipid profile analysis. Only those patients who have reached weeks 48 and 120 at the cut-off of the study and with laboratory data in those study weeks were included in the lipid profile statistical analysis, remaining in the rest of analysis.
Cardiovascular events history (including myocardial infarction, heart failure, cerebrovascular accident, deep venous thrombosis, myocardiopathy, non-ST-segment elevation acute coronary syndrome, and ST-segment elevation myocardial infarction) before and during the follow-up were recorded.
While for the lipid profile analysis, those patients with lipid-lowering drugs prescription were excluded (Fig. 1), for the cardiovascular events analysis all patients were included.
The statistical analysis was performed with SPSS v. 24 software. The quantitative variables were expressed as mean±standard deviation and the qualitative variables as percentage and frequency. Group differences were compared using the Pearson χ2 or Fisher's exact test and Student's t-test or the Mann–Whitney U-test, respectively, for categorical and continuous variables. Repeated measurements were compared using paired Student's t-test or Wilcoxon signed-rank test. Univariate analysis was performed with all the covariates. To identify the risk of discontinuation because of AEs by treatment a Cox regression analysis was performed. P-values of .05 or less were considered statistically significant.
ResultsStudy design and baseline characteristics of the study cohortThe study design and all the characteristics describing the study population are collected in Fig. 1 and Table 1. A total of 457 patients were included in the study, 266 patients were treated with EVG/c/FTC/TAF and 191 with DTG/ABC/3TC. Both treatment groups were comparable, finding statistically significant differences in terms of age, number of naïve and experienced patients, acquisition risk factor for HIV infection, and among experienced patients in renal function, TG levels, hypertension, and smoking history.
Baseline characteristics of the study population.
| Variables | EVG/c/FTC/TAF (N = 266) | DTG/ABC/3TC (N = 191) | P-value |
|---|---|---|---|
| Male (%) | 76.3 | 74.9 | 0.722 |
| Age (mean ± SD) | 48.0 ± 10.5 | 50.9 ± 10.6 | 0.004 |
| 23.7 | 13.1 | 0.018 |
| 65.8 | 74.3 | |
| 10.5 | 12.6 | |
| Acquisition risk factor for HIV infection (%): | 0.030 | ||
| 36.5 | 26.7 | |
| 32.3 | 33.5 | |
| 23.3 | 35.1 | |
| 1.5 | 0.5 | |
| 6.4 | 4.2 | |
| Naive (%) | 19.5 | 11 | 0.014 |
| Inmunovirogical data | |||
| 463.1 ± 362.8 | 344.1 ± 199.7 | 0.314 |
| 4.7 ± 0.9 | 5.1 ± 0.8 | 0.025 |
| Cardiovascular risk factors (%) | |||
| 7.7 | 0 | 0.318 |
| 51.9 | 28.6 | 0.07 |
| 1.9 | 0 | 1.0 |
| 1.9 | 0 | 1.0 |
| 44.2 | 33.3 | 0.392 |
| 20.8 | 14.3 | 1.0 |
| 79.2 | 85.7 | 1.0 |
| Lipid profile data | |||
| Lipid Levels (mean ± SD) | |||
| 123.4 ± 56.2 | 95.0 ± 39.0 | 0.051 |
| 170.8 ± 41.1 | 156.2 ± 43.8 | 0.166 |
| 106.8 ± 36.4 | 116.7 ± 61.2 | 0.926 |
| 42.9 ± 9.0 | 38.3 ± 9.3 | 0.126 |
| 4.1 ± 0.9 | 4.3 ± 0.9 | 0.502 |
| 2.5 ± 0.8 | 2.8 ± 0.8 | 0.295 |
| Lipid values out of range (%) | |||
| 28.9 | 10.5 | 0.195 |
| 22.2 | 15 | - |
| 22.5 | 27.8 | - |
| Renal function | |||
| CrCl: | |||
| Normal (>60 mL/min) (%) | 98.1 | 100 | 1 |
| Mild (59–30 mL/min) (%) | 1.9 | 0 | |
| Moderate (29–15 mL/min) (%) | 0 | 0 | |
| SCr mg/dL (mean ± SD) | 0.91 ± 0.19 | 1.0 ± 0.18 | 0.08 |
| Experienced (%) | 80.5 | 89.0 | 0.014 |
| Inmunovirogical data | |||
| 633.7 ± 314.0 | 669.6 ± 332.2 | 0.274 |
| 84.0 | 85.3 | 0.727 |
| Cardiovascular risk factors (%) | |||
| 18.6 | 25.9 | 0.03 |
| 58.4 | 62.4 | 0.433 |
| 7.5 | 8.8 | 0.630 |
| 5.1 | 4.7 | 0.845 |
| 58.9 | 42.9 | 0.002 |
| 29.1 | 25.0 | 0.531 |
| 70.9 | 75.0 | |
| Lipid profile data | |||
| Lipid Levels (mean ± SD) | |||
| 121.9 ± 68.6 | 142.8 ± 91.8 | 0.035 |
| 181.9 ± 36.8 | 187.8 ± 47.1 | 0.191 |
| 111.1 ± 31.8 | 114.4 ± 49.1 | 0.913 |
| 47.8 ± 18.6 | 49.5 ± 15.6 | 0.229 |
| 4.1 ± 1.0 | 4.1 ± 1.2 | 0.796 |
| 2.5 ± 0.8 | 2.4 ± 0.9 | 0.266 |
| -Lipid values out of range (%) | |||
| 24.4 | 29.5 | - |
| 27.5 | 37.3 | - |
| 27 | 25.8 | - |
| Renal function | |||
| CrCl: | |||
| Normal (>60 mL/min) (%) | 88.7 | 81.1 | 0.035 |
| Mild (59–30 mL/min) (%) | 11.3 | 18.9 | |
| Moderate (29–15 mL/min) (%) | 0 | 0 | |
| SCr mg/dL (mean ± SD) | 1.01± 0.24 | 1.08 ± 0.24 | 0.004 |
Statistically significant differences are shown in bold.
MSM: men who have sex with men; IDU: intravenous drug use; VL: viral load; TG: triglycerides; TC: total cholesterol; LDL-C: LDL cholesterol; HDL-C: HDL cholesterol; CrCl: creatinine clearance; SCr: serum creatinine.
Most of the study patients belonged to the male gender (75.7%). The mean age was 49.2±10.7 years, with a greater number of young patients in the EVG/c/FTC/TAF group. Most patients (84%) had already received some prior ART, and the main reasons that motivated the change to any of the treatments under study were simplification (38.0%), AEs prevention (31.3%), and AEs (18.2%). In the DTG/ABC/3TC group, a higher rate of treatment experienced patients was found.
In the EVG/c/FTC/TAF group, 8 experienced patients had suffered a cardiovascular event before starting the study ART vs. 5 experienced patients in the DTG/ABC/3TC group (P = .214). Naïve patients in both groups have not suffered a previous cardiovascular event.
Lipid profile dataWhen we excluded patients with prior or during follow-up lipid-lowering treatment (N = 63) (Fig. 1), patients in the EVG/c/FTC/TAF treatment group showed significant variations in their lipid profile.
Statistically significant differences were observed in naïve patients for TC, LDL-C, and HDL-C at 48 weeks after starting EVG/c/FTC/TAF, and these differences remained at 120 weeks. Among experienced patients, statistically significant differences were found for all the lipid profile at 48 weeks, but only TC, LDL-C, and HDL-C remained statistically significant at 120 weeks (Fig. 2).
Lipid profile evolution in the EVG/c/FTC/TAF group after 48 and 120 weeks of study treatment.
Patients with lipid-lowering treatment prescription were excluded.
Statistically significant differences are shown in bold.
TG: triglycerides; TC: total cholesterol; LDL-C: LDL cholesterol; HDL-C: HDL cholesterol; w-48: week 48; w-120: week 120.
P-values: baseline lipid profile vs. lipid profile at week 48 and at week 120.
The rate of naïve patients with TC values above the normal range at 48 (53.6%) and 120 (48.1%) weeks after prescription of EVG/c/FTC/TAF was higher than those of baseline values (22.2%). The LDL-C values in naïve patients were also higher than those of baseline values (47.5% and 48.1% at 48, and 120 weeks, respectively, vs. 22.5% at baseline). In experienced patients, these values were also above the normal range: TC at 48 weeks (57.9%) and 120 weeks (56.4%) vs. baseline value (27.5%); LDL-C at 48 (47.7%) and 120 weeks (38.5%) vs. baseline value (27.0%); TG at 48 weeks (29.7%) and 120 weeks (25.7%) vs. baseline levels (24.4%).
Regarding DTG/ABC/3TC group, naïve patients showed significant variations when comparing the baseline data of TC, LDL-C, and HDL-C with those at 48 weeks, and TC and HDL-C at 120 weeks after the start of the study treatments, when patients with lipid-lowering treatment were excluded (N = 2, Fig. 1). Among experienced patients, no statistically significant differences were observed in the lipid levels (Fig. 3).
Lipid profile evolution in the DTG/ABC/3TC group after 48 and 120 weeks of study treatment.
Patients with lipid-lowering treatment prescription were excluded.
Statistically significant differences are shown in bold.
TG: triglycerides; TC: total cholesterol; LDL-C: LDL cholesterol; HDL-C: HDL cholesterol; w-48: week 48; w-120: week 120.
P-values: baseline lipid profile vs. lipid profile at week 48 and at week 120.
The rate of naïve patients with TC values above the normal range at 48 (17.6%) and 120 (25%) weeks after DTG/ABC/3TC prescription was similar to the TC baseline values (15%). TG values in naïve patients were above the normal range at 48 (23.5%) and 120 (25%) weeks vs. baseline values (10.5%). Nevertheless, the rate of naïve patients with LDL-C values out of range decreased from the baseline (27.8%) compared to those at 48 (25.1%) and 120 (12.5%) weeks. In experienced patients, the rate with values above the normal range was higher at 48 (TG 33.6%, LDL-C 29.8%) and 120 weeks (TG 33%, LDL-C 33.3%) than that of the baseline (TG 29.5%, LDL-C 25.8%). Patients with TC levels above normal range at 120 weeks were similar to those at baseline (37.4% vs. 37.3%, respectively).
Comparing the 2 study groups, similar baseline lipid levels were seen in the 2 study groups in both naïve and experienced patients (Table 1). When we compared the 2 antiretroviral treatments excluding those patients with lipid-lowering drug prescription, statistically significant differences in TC (204.1±38.2 vs. 187.3±29.4, P < .001) and LDL-C (126.1±31.9 vs. 113.5±28.5, P = .001) levels were seen in experienced patients at week 48 and in TC (201.1±33.4 vs. 188.7±33.9, P = .013) and HDL (54.2±15.6 vs. 48.3±14.3, P = .010) at week 120 for EVG/c/FTC/TAF and DTG/ABC/3TC, respectively. No statistically significant differences were found when naïve patients were compared at 48 or 120 weeks (Table 2).
Lipid profile changes in the EVG/c/FTC/TAF and DTG/ABC/3TC group after 48 and 120 weeks of study treatment.
| W48 | W120 | |||||
|---|---|---|---|---|---|---|
| EVG/c/FTC/TAF | DTG/ABC/3TC | P-value1 | EVG/c/FTC/TAF | DTG/ABC/3TC | P-value2 | |
| Naive patients | (N = 41) | (N = 17) | (N = 27) | (N = 8) | ||
| Lipid Levels (mean ± SD) | ||||||
| TG (mg/dL) | 126.8 ± 60.7 | 117.2 ± 51.6 | 0.626 | 127.1 ± 53.7 | 161.3 ± 180.6 | 0.444 |
| TC (mg/dL) | 202.1 ± 40.5 | 184.8 ± 50.4 | 0.078 | 195.0 ± 34.0 | 173.0 ± 28.9 | 0.080 |
| LDL-C (mg/dL) | 127.6 ± 37.7 | 116.3 ± 43.1 | 0.261 | 121.1 ± 32.2 | 101.8 ± 22.0 | 0.080 |
| HDL-C (mg/dL) | 50.1 ± 12.1 | 46.9 ± 7.8 | 0.531 | 48.4 ± 10.9 | 45.8 ± 10.8 | 0.665 |
| TC:HDL-C ratio | 4.2 ± 1.2 | 4.0 ± 1.0 | 0.690 | 4.2 ± 1.0 | 3.9 ± 1.1 | 0.610 |
| LDL-C/HDL-C ratio | 2.7 ± 1.0 | 2.5 ± 0.8 | 0.650 | 2.6 ± 0.8 | 2.3 ± 0.6 | 0.492 |
| Experienced patients | (N = 141) | (N = 104) | (N = 78) | (N=91) | ||
| Lipid Levels (mean ± SD) | ||||||
| TG (mg/dL) | 145.6 ± 114.2 | 135 ± 66.1 | 0.811 | 123.7 ± 66.6 | 156.6 ± 123.2 | 0.058 |
| TC (mg/dL) | 204.1 ± 38.2 | 187.3 ± 29.4 | <0.001 | 201.1 ± 33.4 | 188.7 ± 33.9 | 0.013 |
| LDL-C (mg/dL) | 126.1 ± 32.0 | 113.5 ± 28.5 | <0.001 | 122.2 ± 26.2 | 113.0 ± 30.0 | 0.058 |
| HDL-C (mg/dL) | 50.7 ± 13.3 | 48.0 ± 13.2 | 0.068 | 54.2 ± 15.6 | 48.3 ± 14.3 | 0.010 |
| TC:HDL-C ratio | 4.2 ± 1.0 | 4.1 ± 1.0 | 0.705 | 3.9 ± 0.9 | 4.2 ± 1.2 | 0.146 |
| LDL-C/HDL-C ratio | 2.6 ± 0.8 | 2.5 ± 0.8 | 0.631 | 2.4 ± 0.6 | 2.5 ± 0.9 | 0.336 |
Patients with lipid-lowering treatment prescription were excluded.
Statistically significant differences are shown in bold.
TG: triglycerides; TC: total cholesterol; LDL-C: LDL cholesterol; HDL-C: HDL cholesterol; W48: week 48; W120: week 120.
P-value1: lipid profile EVG/c/FTC/TAF vs. DTG/ABC/3TC at week 48.
P-value2: lipid profile EVG/c/FTC/TAF vs. DTG/ABC/3TC at week 120.
For the analysis of the variations in the lipid profile, all patients with lipid-lowering drugs were excluded. The global prescription of these antilipemic drugs in experienced patients was similar for both groups at the end of the study (EVG/c/FTC/TAF 26.2% vs. DTG/ABC/3TC 25.3%, P = .846). In naïve patients, the prescription of lipid-lowering drugs was lower compared with that of experienced patients, and it was also similar for both groups (13.5% vs. 9.5%, P = .999). During the follow-up, 9.4% (4 naïve and 22 experienced) and 8.4% (1 naïve and 15 experienced) of patients using EVG/c/FTC/TAF and DTG/ABC/3TC, respectively, required lipid-lowering drugs prescription (P = .706).
Cardiovascular risk dataAs previously described in the baseline characteristics of the population, comparing each cardiovascular risk factor between groups, we only have found significant differences in experienced patients with smoking habit and hypertension.
However, we have found that 63.5% of naive patients in the EVG/c/FTC/TAF group presented at least 1 cardiovascular risk factor, compared to 33.3% in the DTG/ABC/3TC group (P = .019). Among experienced patients, 74.8% in the EVG/c/FTC/TAF group and 63.5% in the DTG/ABC/3TC group (P = .07).
Eleven (11) experienced patients in the EVG/c/FTC/TAF group had suffered a cardiovascular event, 3 of whom developed the event after initiation of study treatment (N = 1 non-ST-segment elevation acute coronary syndrome; N = 1 ST-segment elevation myocardial infarction; N = 1 heart failure). While in the DTG/ABC/3TC group, of the 12 patients who suffered the event, 7 of them ocurred once the study treatment had started (N = 2 non-ST-segment elevation acute coronary syndrome; N = 2 cerebrovascular accident; N = 1 myocardial infarction; N = 1 deep venous thrombosis; N = 1 myocardiopathy). When comparing both groups, no significant statistical differences were found (P = .214).
In regard to naïve patients, only 1 in the EVG/c/FTC/TAF group had suffered a cardiovascular event (ST-segment elevation myocardial infarction), and it was after starting the study drug (P = 1.0).
Follow up dataA total of 39 and 33 patients in the EVG/c/FTC/TAF and the DTG/ABC/3TC group discontinued the ART during the follow-up, respectively (p = 0.449). In both groups, AEs were the main reason for the discontinuation (16 patients in the EVG/c/FTC/TAF group and 21 patients in the DTG/ABC/3TC group), with similar risk of interruption due to AEs [HR = 1.77, 95%IC=(0.92–3.39), P = .087].
Discontinuation due to hypercholesterolemia was only observed in the EVG/c/FTC/TAF group, and it was also the main reason to discontinued therapy (N = 7), being central nervous system disorders (CNSd) the main cause in the DTG/ABC/3TC group (N = 14).
The other causes of discontinuation due to AE were CNSd (N = 3), gastrointestinal disorders (GI) (N = 2), weight gain (N = 1), arthralgia (N = 1), rash (N = 1), and renal function alteration (N = 1) in the EVG/c/FTC/TAF group, and GI (N = 5) and renal function alteration (N = 2) in the DTG/ABC/3TC group.
Of all the deaths that occurred during the study, 6 in the EVG/c/FTC/TAF group and 11 in the DTG/ABC/3TC, none of them were associated with cardiac events.
DiscussionThis is the first real-world observation cohort comparing EVG/c/FTC/TAF and DTG/ABC/3TC in terms of lipid profile variations from baseline values, and cardiovascular events, to the best of our knowledge. The findings from this study showed a higher deterioration of the baseline lipid profile in the EVG/c/FTC/TAF group than that in the DTG/ABC/3TC group after 120 weeks of treatment. However, when we compared both groups, we observed significant differences only between treatment experienced patients, in TC and LDL-C values at 48 weeks, and in TC and HDL-C at 120 weeks, favouring the DTG/ABC/3TC group. While there were no differences in cardiovascular events after initiating the study. Furthermore, no significant differences were observed in the discontinuation of study treatments, with similar risk of interruption due to AEs. It must be taken into account that discontinuation due to hypercholesterolemia was only observed in the EVG/c/FTC/TAF group.
It is known that TAF has lost the lipid-lowering effect of tenofovir disoproxil fumarate (TDF), and has also been associated with the worsening in lipid parameters.18 This was confirmed in our study, where approximately half of our patients had TC and LDL levels above the normal range after 48 and 120 weeks, considering that less than a third of the patients presented those levels at baseline.
Pivotal studies of EVG/c/FTC/TAF in naïve patients found significant increases in TG, TC, HDL-C, and LDL-C compared with EVG/c/FTC/TDF (P < .001), but not in TC:HDL-C ratio (associated with cardiovascular disease risk) at weeks 48 and 144.11,12 These differences were attributed to significant reductions in plasma tenofovir concentrations with TAF. Further, Huhn et al. compared EVG/c/FTC/TAF and EVG/c/FTC/TDF treatment in naïve patients and observed an increase in median fasting lipids (TC, LDL-C, and HDL-C) at week 96 in the EVG/c/FTC/TAF group, with a similar TC:HDL ratio between the groups. Despite the significant increase in fasting lipids in the EVG/c/FTC/TAF group, they concluded that it does not crucially affect the cardiovascular risk profile.19
These results are similar to those we observed in our naïve patients treated with EVG/c/FTC/TAF when we compared the baseline data with those at weeks 48 and 120. As previously found in clinical studies, we also found no differences in the TC:HDL-C ratio between groups, cardiovascular events, or in the proportion of participants initiating lipid-modifying agents in naïve patients, although the proportion in our study (7.7%) was higher than in clinical trials (5.5%) for patients receiving EVG/c/FTC/TAF.
We found significant differences in all lipid values at week 48, including TC:HDL-C, and LDL-C/HDL ratios, but only in TC, LDL-C, and HDL-C at week 120 compared with those of the baseline data in experienced patients in EVG/c/FTC/TAF group. Kuo et al. observed similar results, with an increase in TG, TC, LDL-C, and HDL-C values, but not in the TC:HDL-C ratio at week 48.20 However, Huang et al. also found increases in the TC:HDL-C ratio at week 48 in patients who switched to EVG/c/FTC/TAF, as observed in our study.21
One study showed that patients who switched to DTG/ABC/3TC improved the lipid profile.22 On the other hand, there is a long-standing controversy surrounding the association between ABC and an increased risk of cardiovascular diseases in addition to the hypersensitivity reactions caused by ABC.23
In the DTG/ABC/3TC group, we found significant differences in TC and HDL-C levels in naïve patients at weeks 48 and 120, and in LDL-C only at week 48. However, patients with TG, TC, or LDL-C values above the normal range after 48 and 120 weeks were similar than those at baseline or better in the case of LDL-C. In pivotal studies, no clinically significant changes were observed over time (up to week 144) in the fasting lipid profile in the DTG group. These studies observed a small increase in LDL-C and TC levels and in the TC:HDL-C ratio, with a modest rise in TG, similar to what we observed in our patients.14,15
In pivotal trials of patients who switched to DTG, an increased in TC, LDL-C, HDL-C, and TG were observed at 24 and 48 weeks.16 This is totally different from what was observed in experienced patients, where no statistically significant differences were found in any lipid values or in the TC:HDL-C and LDL-C:HDL ratios during the follow-up. The results described by Bagella et al. in their study of patients who switched to DTG/ABC/3TC suggested an improvement in the lipid profile at 48 weeks, especially in the group of patients who changed from a PI-based regimen (reduction in TC and LDL-C, small increase in HDL-C, and a significant decrease in TG and TG:HDL ratio at weeks 24 and 48).22
Our study confirms the results obtained in previous works, where, as it is well known, the lipid profile worsens after starting ART, although the difference is more notable in the EVG/c/FTC/TAF group. Winston et al. compared patients switching to TAF/FTC or ABC/3TC, while continuing the same third drug. They did not find statistical differences in fasting TC, LDL-C, TG, or TC:HDL ratio at week 48; changes in HDL-C were significantly different yet not clinically relevant. Altogether, they considered both treatments alternatives, effective, and safe.24
No differences were observed in the prescription of lipid-lowering drugs, similar in both treatment groups and between naïve and experienced patients, although in the latter case, up to 26% of patients have been prescribed.
Due to ART and chronic HIV infection, PLWHIV have a long-life expectancy, which can lead to atherosclerotic lesions and cardiovascular disease. In the D:A:D study, they concluded that the use of ABC increased the risk of myocardial infarction.25 Otherwise, no differences were found in our study regarding the number of patients (naïve or experienced) who suffered a cardiovascular adverse event between the 2 drugs studied, despite significant differences in terms of presenting a cardiovascular risk factor.
The observed limitations of this study were the different baseline group characteristics, and the retrospective observational design that might have introduced uncontrolled bias (for instance, prescriber/selection bias is present and probably patients with renal disease were prescribed DTG/ABC/3TC preferentially to avoid tenofovir, yet renal patients probably have a greater chance of having hyperlipidaemia over time). It also could have introduced some bias the fact that have eliminated from the analysis of the lipid profile variation the patients who had been prescribed lipid-lowering treatment. Furthermore, the main limitation was the single-centre study design with a limited sample size. The conflicting results detected between trials and some cohorts, such as our data, could be partially explained by several factors, including study populations heterogeneity, patients´ habits, the follow-up time, and the research design.
In summary, when we compared both study groups, differences were found in TC in experienced patients favouring DTG/ABC/3TC during the follow-up. Accordingly, both regimens can therefore be alternatives in terms of lipid profile, tolerance, and incidence of cardiovascular events, hence future studies should monitor patients receiving TAF-based regimens more closely.
Declaration of authorshipSandra Rotea-Salvo contributed to the data collection, interpretation and writing of the article. Víctor Giménez-Arufe in data collection, interpretation and critical revision. Alejandro Martínez-Pradeda and Carla Fernández-Oliveira in data collection and critical revision. Álvaro Mena-De-Cea, Luis Margusino-Framiñán and María Isabel Martín-Herranz in the interpretation of results, critical review, and journal selection. And Purificación Cid-Silva, in the conception and design of the paper, and in the critical review of the article. All authors have approved the final version for publication.
FundingThis work was supported in part by the Professor Novoa Santos Foundation, A Coruña.
Contribution to scientific literatureElvitegravir/cobicistat/emtricitabine/tenofovir alafenamide fumarate and dolutegravir/abacavir/lamivudine are usually safe and well-tolerated drugs used in the management of HIV in clinical practice. However, it has been described that they modify the lipid profile and cardiovascular risk. Although EVG/c/FTC/TAF is no longer recommended as the first line for the management of HIV, the importance of this study lies in the direct comparison of TAF-based vs. ABC-based regimens.
A worsening of the lipid profile was observed in both treatment groups, being more pronounced among treatment experienced patients in TAF-based regimen. Despite these differences, the risk of cardiovascular events was similar in both groups, with no significant differences found. Both regimens continued to be well tolerated, with similar rates of drug-related adverse events or discontinuation. This is the first real-world observation cohort comparing both treatments in terms of lipid profile and cardiovascular events, providing more data to clinicians to choose the best regimen for each patient.
To Vanesa Balboa-Barreiro for her collaboration in this work in the statistical analysis and interpretation of results. And we also thank the Pharmacy and Infectious Internal Medicine Services.