The management of surgeries in patients with hemophilia is complex and requires adequate clotting factor adjustment to avoid bleeding complications and excessive factor consumption. The aim of this systematic review is to analyze the pharmacokinetic studies published on surgery in hemophilic patients, the methodologies used, the main pharmacokinetic covariates applied, and the recommendations made by clinical guidelines.
MethodA structured search was performed in Pubmed, the Cochrane Library, and the Database of Abstracts of Reviews of Effects using the search terms hemophilia (or haemophilia), surgery and pharmacokinetics (or PK). No date or language limits were established.
ResultsThe search yielded 186 results, from which 34 articles were selected. Many of these analyzed the use of continuous infusions with the aim of achieving stable factor VIII or IX levels and reducing overall factor consumption. However, continuous infusions have fallen into disuse. For decades, clinical guidelines have recommended the performance of comprehensive pharmacokinetic studies prior to surgery (9-11 samples). The clearance rate obtained is used to adjust the presurgical factor dose (or the infusion rate in case of continuous perfusion). Another approach is the use of population pharmacokinetic models, which allow adjustments to be made based on a more limited number of samples. However, the validity of these presurgical pharmacokinetic estimates ceases as soon as the surgical procedure is initiated, making it necessary to adjust the dose based on periodic peak and trough levels. In addition, depending on the type of surgery, clinical guidelines recommend maintaining factor VIII and IX levels above specific thresholds for certain periods of time, which makes it essential to use pharmacokinetics during the pre- and post-surgical process. In recent years, specific factor VIII and factor IX pharmacokinetic population models have been developed for surgery. The main covariates of these population pharmacokinetic models are age, blood type, and type of surgery for factor VIII; and age and body weight for factor IX.
ConclusionsPharmacokinetic estimation could allow individual and standardized intraoperative dose adjustments to be conducted in patients with hemophilia. The development of specific population pharmacokinetic models for surgery, including those based on extended half-life factors, will allow an optimization of current treatments, potentially reducing factor consumption and hospital stays.z
El manejo de las cirugías en pacientes hemofílicos es complejo y requiere de un ajuste adecuado de los factores de coagulación para evitar complicaciones hemorrágicas y un consumo elevado. El objetivo de esta revisión sistemática es analizar los estudios farmacocinéticos publicados en cirugía en pacientes con hemofilia, las metodologías empleadas, las principales covariables farmacocinéticas y las recomendaciones de las guías clínicas.
MétodoSe ha realizado una búsqueda estructurada sin restricciones de fecha ni idioma en Pubmed, Cochrane y Database of Abstracts of Reviews of Effects empleado los mismos términos de búsqueda: (hemophilia or haemophilia), surgery y (pharmacokinetics or PK).
ResultadosLa búsqueda sistemática obtuvo 186 resultados, de los que seleccionamos 34 artículos. Muchos estudios analizaban el uso de perfusiones continuas con el objetivo de lograr niveles estables de factor VIII o IX y reducir el consumo global, aunque su empleo ha caído en desuso. Durante décadas las guías clínicas recomendaban realizar estudios farmacocinéticos completos previos a la cirugía (9-11 muestras), según los cuales se ajusta la dosis prequirúrgica, así como la velocidad de infusión en caso de perfusión continua basándose en el aclaramiento calculado. Otra aproximación es el empleo de modelos poblacionales farmacocinéticos, ajustando con un número más limitado de muestras. Estas estimaciones farmacocinéticas prequirúrgicas pierden validez tan pronto como se inicia un procedimiento quirúrgico, y tienen que ajustarse con niveles pico y valle periódicos. Además, las guías clínicas recomiendan, en función del tipo de cirugía, mantener los niveles de factores VIII y IX por encima de los umbrales específicos durante periodos, por lo que resulta fundamental emplear la farmacocinética durante el proceso pre y postquirúrgico. En los últimos años se han desarrollado modelos poblacionales farmacocinéticos de factores VIII y IX específicos para cirugía. Las principales covariables de estos modelos son la edad, el grupo sanguíneo y el tipo de cirugía para el factor VIII, y la edad y el peso corporal para el factor IX.
ConclusionesLa farmacocinética puede permitir ajustar de forma individual y protocolizada las cirugías en pacientes hemofílicos. El desarrollo de modelos farmacocinéticos poblacionales específicos para cirugía, incluyendo los factores de vida media extendida, permitirá optimizar estos tratamientos, con potencial reducción del consumo y las estancias hospitalarias.
Hemophilia is an unusual hereditary hemorrhagic disorder caused by a deficiency in coagulation factor VIII (FVIII) in the case of hemophilia A or factor IX (FIX) in the case of hemophilia B. It is associated with the appearance of often recurrent joint bleeds which, in the long term, tend to result in a disabling degenerative arthropathy1. The standard of care for severe or moderate hemophilia with a hemorrhagic phenotype is regular and continuous prophylactic administration of the deficient factor to prevent the appearance of joint bleeds and preserve quality of life2,3.
Use of any of the techniques available to measure post-infusion FVIII/FIX activity in plasma can be considered a basic pharmacokinetic (PK) application in the treatment of hemophilia. Such techniques are typically applied in three different scenarios: (i) to measure peak and trough levels during prophylaxis; (ii) to measure peak and trough levels intraoperatively; or (iii) to measure in vivo recovery (IVR) and the plasma half-life (t1/2) of FVIII/FIX to assess immune tolerance in patients with inhibitors4.
Despite the widespread use of prophylaxis, especially those patients with advanced age who did not receive primary prophylaxis at the appropriate time and therefore developed hemophilic arthropathy, often require orthopedic surgery3. Management of these surgeries is often complex and requires titrating the replacement therapy to prevent hemorrhagic complications. Titration of the dose is advisable both from a therapeutic and an economic standpoint given the high intraoperative consumption of the replacement clotting factors and their high cost4.
Historically, different approaches have been used for PK adjustments during surgery. The purpose of this systematic review is to analyze the PK-based adjustment studies published on operated patients with hemophilia A and B, as well as the methodologies applied, the main covariates used, and the recommendations made by clinical guidelines.
MethodsSearch for and selection of studiesThe search was conducted by two independent reviewers (JEMV and SBB) based on the criteria used by the systematic reviews and meta-nalyses of the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-analyses)5. A structured search with no date or language restrictions was carried out in Pubmed, the Cochrane Library and the Database of Abstracts of Reviews of Effects (DARE). Moreover, a manual search was performed of the tables of contents of the leading scientific journals in the field of coagulopathies. A secondary search was also conducted, based on the references listed in the articles selected during the first literature search. It was not necessary to contact experts to identify articles not retrieved during the review. The last literature search was carried out on 13 April 2021. Additionally, a review was conducted of the latest clinical guidelines of the International Society on Thrombosis and Haemostasis (ISTH) and the World Federation of Hemophilia (WFH) to establish the criteria currently used in PK studies on hemophilic patients undergoing surgery.
The reviewers carried out the selection of studies independently. In case of disagreement, a third reviewer (MRM) was consulted. The same search terms were used across all databases: (hemophilia or haemophilia), (surgery) and (pharmacokinetics or PK).
Inclusion criteria were as follows:
- –
Studies analyzing population PK modeling (PopPK) software or medical devices specific to surgical procedures where replacement FVIII or FIX were used.
- –
Studies analyzing a certain type of FVIII or FIX replacement therapy during surgery where dosages were titrated based on previous PK measurements.
- –
Studies analyzing the effect of the covariates on the PK of FVIII or FIX replacement therapies during surgery.
Exclusion criteria were as follows:
- –
Studies analyzing the use of a certain type of FVIII or FIX during surgery, but without stating whether PK information was used for dose titration.
- –
Studies analyzing patients in special situations (patients with inhibitors).
- –
Studies analyzing other clotting factors (Von Willlebrand factor, activated factor VII, factor XI, factor XIII, fibrinogen, etc.).
- –
Studies analyzing non-factor replacement therapies (emicizumab).
The results of the primary search were used to select articles that met the inclusion criteria based on their title and abstract. Subsequently, a secondary selection was made based on the full articles.
ResultsThe systematic search performed produced a total of 181 hits from the above mentioned databases; five hits were also obtained from the additional sources (Figure 1). As a result of the primary selection, a total of 41 citations were selected for full reading, with 34 of those eventually meeting the selection criteria. Concordance between the reviewers was excellent (kappa = 0.98). The most significant data from the different studies is summarized in Table 1.
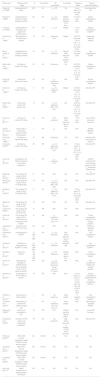
Pharmacokinetic studies on hemophilic patients undergoing surgery included in the systematic review
| Study, year | Purpose of the study | N | Hemophilia | PK parameters | Covariates | Sampling times | Type of pharmacokinetics |
|---|---|---|---|---|---|---|---|
| Kasper et al. (1985)7 | Retrospective PK study | 350 | HA | t1/2, IVR | NA | 2: pre-dose & 10 min post-dose | Semilogarithmic role |
| Ruffo et al. (1986)8 | Development of PopPK & limited sampling method | NA | HA | t1/2, Vd, clearance | Weight, age and baseline FVIII level | 2: 3 & 9 h post-dose | Single-compartment PopPK |
| Longo et al. (1985)9 | Development of nomogram for 3 target FVIII concentrations | 20 | HA | t1/2, Vd | Weight | 1: 10 h post-dose | Single-compartment PopPK8 |
| Durisová et al. (1998)11 | Development of PK estimation method | 18 | HA | Clearance, AUC | Weight | 9 (ISTH): pre-dose, 15, 30 min, 1, 3, 6, 9, 12, 24 h post-dose | Frequency-response method |
| Bolon-Larger et al. (2007)11 | Development of PopPK & limited sampling method | 33 | HA | t1/2, Vd, clearance | Weight, body area and FVIII baseline level | 2: 0,5 & 6-8 h post-dose | Two-compartment PopPK |
| Martinowitz et al. (1992)13 | Continuous infusion PK study | 24 | HA | Clearance | NA | 9 (ISTH): pre-dose, 15, 30 min, 1, 3, 6, 9, 12, 24 h post-dose | Single-compartment PopPK9 |
| Hay et al. (1996)14 | Continuous infusion PK study | 24 | HA | t1/2, clearance, clearance rate | Nav | NAv | Single-compartment PopPK |
| Rochat et al. (1999)15 | Continuous infusion PK study | 5 | HA | t1/2, Vd, clearance, MRT | Weight | 9 (ISTH): pre-dose, 15, 30 min, 1, 3, 6, 9, 12, 24 h post-dose | Individual PK |
| Martinowitz et al. (2009)16 | Continuous infusion PK phase II trial | 14 | HA | t1/2, Vd, clearance, MRT, IVR, AUC | Nav | 9 (ISTH): pre-dose, 15, 30 min, 1, 3, 6, 9, 12, 24 h post-dose | Individual PK |
| Suzuki et al. (2017)17 | Continuous infusion PK study | 34 | HA | IVR, clearance | Body mass index and type of FVIII | 1: 70 h post-dose | Use of IVR & clearance formulas |
| Kremer Hovinga et al. (2018)18 | Case study on continuous infusion of EHL FVIII products | 1 | HA | t1/2 | NA | NAv | PopPK (WAPPS-Hemo) |
| Schulman et al. (1999)19 | Continuous infusion PK study | 10 | HB | Clearance | NA | NAv | Individual PK and clearance formula |
| Hoots et al. (2003)20 | Continuous infusion PK study | 28 | HB | t1/2, Vd, clearance, MRT, IVR, AUC | NA | 10: pre-dose, 15 min, 1, 4, 8, 24, 48, 52, 72, 76 h post-dose | Individual PK |
| Suzuki et al. (2015)22 | Comparison of 5 different methods to calculate clearance | 7 | HB | t1/2, clearance, IVR | NA | NAv | PopPK, Individual PK and formulas based on AUC, t1/2 distribution and terminal t1/2 |
| Mahlangu et al. (2016)23 | Pre-surgical PK study on EHL FVIII (rFVIIIFc) | 21 | HA | NAv | NA | NAv | Individual PK |
| Brand et al. (2016)24 | Pre-surgical PK study on EHL FVIII (BAX 855) | 15 | HA | t1/2, Vd, clearance, MRT, IVR, AUC | NA | NAv | Individual PK |
| Gruppo et al. (2019)25 | Pre-surgical PK study on EHL FVIII (BAX 855) | 21 | HA | t1/2, Vd, clearance, MRT, IVR, AUC | NA | 10: pre-dose, 15 min, 3, 9, 32, 56, 96 h post-dose | Individual PK |
| Négrier et al. (2016)26 | Pre-surgical PK study on EHL FVIII (rIX-FP) | 19 | HB | NAv | NA | Nav | Individual PK |
| Curtin et al. (2020)27 | Pre-surgical PK study on EHL FVIII (rIX-FP) | 21 | HB | NAv | NA | Nav | Individual PK |
| Powell et al. (2015)28 | Pre-surgical PK study on EHL FVIII (rFIXFc) & comparison with PopPK | 12 | HB | t1/2, clearance, IVR, time up to 1% | NAv | NAv | Three-compartment PopPK29 (not surgery-specific) |
| Hazendonk et al. (2015)30 | Protocol of the OPTI-CLOT trial | NA | HA | NAv | NA | NA | PopPK vs standard dosing |
| Hazendonk et al. (2016)31 | Development of PopPK | 75 ADU 44 PED | HA | t1/2, Vd, clearance | Age, blood type and type of surgery | NAv | Two-compartment PopPK |
| Preijers et al. (2021)32 | Validation & readjustment of PopPK31 in PED | 87 PED (206 total) | HA | t1/2, Vd, clearance | Weight & age | NAV | Two-compartment PopPK31 |
| Preijers et al. (2018)33 | Desarrollo PopPK | 82 AD 32 PED | HB | t1/2, Vd, clearance | Weight, age & type of FIX | NAV | Three-compartment PopPK |
| Collins et al. (2012)34 | Development, validation of PopPK N9-GP and comparison of PK of rFIX, pdFIX & N9-GP | NA | HB | t1/2, Vd, clearance, peak level, trough level at 3 & 7 days | NAv | NAV | Two-compartment PopPK (not surgery-specific) |
| Simpson et al. (2019)36 | Comparison of PK of rFIXFc & N9-GP | 15 | HB | NAv | NAv | 14: pre-dose, 10, 30 min, 1, 3, 6, 8, 24, 48, 96, 144, 168, 192 & 240 h post-dose | Single-compartment PopPK (N9-GP) & three-compartment (rFIXFc) |
| Preijers et al. (2019)38 | Case report on obese patient | 1 | HA | Vd, clearance | Ideal weight | 8 samples (NAv) | Two-compartment PopPK31 |
| Van Moort et al. (2019)39 | Case report on patient with extreme weight loss | 1 | HA | t1/2, clearance, IVR, time up to 1% | Ideal weight | NAv | Ideal weight-adjusted PopPK |
| White et al. (1995)40 | Influence of covariates on IVR | 72 | HB | IVR | Weight, age and type of pdFIX | NAv | Use of IVR formulas |
| Hazendonk et al. (2016)41 | Analysis of FVIII under- and overdosing predictors | 119 | HA | Vd, clearance | Age, blood type, type of surgery, of FVIII and of perfusion | NAv | Individual PK |
| WFH et al. (2005)35 | WFH 2005 Clinical Guidelines: target FVIII/FIX levels during surgery | NA | HA-HB | NA | NA | NA | NA |
| Srivastava et al. (2013)37 | WFH 2013 Clinical Guidelines: target FVIII/FIX levels during surgery | NA | HA-HB | NA | NA | NA | NA |
| Srivastava et al. (2020)3 | WFH 2020 Clinical Guidelines: target FVIII/FIX levels during surgery | NA | HA-HB | NA | NA | NA | NA |
| Iorio et al. (2017)43 | Delphi Consensus for defining target FVIII levels | NA | HA | NA | NA | NA | NA |
ADU: adult patients; AUC: area under the curve; BAX 855: ruríoctocog alfa pegol; EHL: extended half-life; FVIII: factor VIII; FIX: factor FIX; HA: hemophilia A; IVR: in vivo 'ecovery index; N9-GP: nonacog beta pegol; NA: not applicable; NAv: not available; pdFIX: plasma-.derived FIX; PED: pediatric patients; PK: pharmacokinetics; PopPK: population PK models; rFIX: recombinant FIX; rFIXFc: eftrenonacog alfa; rFVIIIFc: efmoroctocog alfa; rIX-FP: albutrepenonacog alfa; t1/2: half-life; MRT: mean residence time; Vd: distribution volume; WFH: World Federation of Haemophilia.
Traditional PK estimates individual parameters on the basis of the concentrations of the drug obtained at different sequential sample collections following administration of one dose of the replacement factor. No population-based models are used. According to the ISTH, measuring the individual pharmacokinetic profile of FVIII and FIX requires 9-11 adult patient samples (4 collected during the distribution phase and 5-7 during the excretion phase) and at least five samples in children6. A washout period of five t1/2 is also needed. The procedure also requires a firm commitment from the patient and their family given the large number of samples and the amount of time necessary, which makes its application difficult in clinical practice. These kinds of analyses are normally restricted to clinical trials that include small and homogeneous groups of hemophilic patients, where the reference technique selected must be subject to a low intrinsic error rate.
Population PK (PopPK) analyses. Bayesian estimationBayesian analysis is a statistical procedure used to adjust patient data to a previously proposed general model. It uses the experimental information obtained from the individual (individual information) plus the information known ex ante about a drug's performance in a given population (population-based information) whose physiopathological characteristics are similar to those of the patient under analysis. If experimental information is limited, the influence of population-based values will be high. However, the influence of population-based values decreases as more experimental data become available. PopPK is capable of estimating individual parameters without the need of the exhaustive sample collection required by traditional PK4.
Initial studies analyzing the methodology used to estimate the FVIII/FIX PK profile during surgeryThe first few PK studies on hemophilic patients undergoing surgery were published in the 1980's7–9. At that time, a retrospective study analyzed the PK values obtained in 350 surgical procedures in patients with hemophilia A7. A pre-infusion sample was collected at baseline, followed by a post-infusion one al 10 minutes to calculate the t1/2 and IVR of the replacement factor. In 1986, Ruffo et al. published the first software ever based on a PopPK model applied to surgery8, modifying previous models used for prophylaxis10. The new PopPK, based on FVIII, used a non-linear single-compartment strategy that assumed that the t1/2 of FVIII peaked immediately after surgery and gradually decreased over the next few days. The model suggested obtaining two samples, one at 3 hours and the other at 9 hours post-infusion. Sometime later, a nomogram was put together based on the PK data from 20 patients with hemophilia A undergoing surgery, which allowed determination of the required maintenance dose on the basis of the FVIII concentration observed 10 hours after application of the loading dose for three target FVIII steady-state concentrations (30, 60 or 90 IU/dL)9.
Durisová et al. estimated the PK of FVIII intraoperatively by using the “frequency-response” method, based not only on post-surgical FVIII levels as previous models, but also considering pre-surgical FVIII concentrations11. The sheer complexity of the model combined with the lack of a software that facilitated its application and the failure to titer the dose in some patients meant that the model was soon neglected.
Bolon-Larger et al. developed a two-compartment PopPK model using a non-linear mixed effects modeling (NONMEM) strategy based on data from 33 patients with hemophilia A12. Of the different samples analyzed, the ones exhibiting the greatest accuracy and the fewest biases were those obtained at 0.5 and at 6-8 hours post infusion. Body weight, body surface area, and the baseline FVIII concentration were the covariates with the greatest influence on the distribution volume (Vd).
PK studies in patients on continuous infusion undergoing surgeryIntraoperative use of continuous infusion (CI) of coagulation products was for some time promoted over intermittent injections with a view to achieving more stable levels of FVIII/FIX and reducing overall factor consumption. However, continuous infusion has lately fallen into disuse. The literature search performed as part of this study detected 8 studies on the use of CI during surgery, 6 of which with FVIII13–18 and 2 with FIX19,20. These studies usually included a preoperative individual PK analysis with nine sample collections and using non-compartmental models to titer the dosage of the clotting factors, following the recommendations of the ISTH6. These studies confirmed that clearance decreases over the first five days post-op, making it possible to adjust the dosing schedule and reduce factor consumption. Use of CI with extended t1/2 factor (EHL) products during surgery was only reported in the case of one patient, who was being treated with efmoroctocog alfa18. In that case, WAPPS-Hemo® was used to analyze previous PK values21.
A study by Suzuki et al. compared five different methods for calculating the clearance of nonacog alfa in patients on CI22. The method, based on IVR and t1/2 distribution, obtained similar clearance rates as direct CI calculations, while clearance calculations using terminal t1/2 and AUC values underestimated the clearance rate. The simulated single-compartment model also obtained good correlations.
PK analyses of the new FVIII/FIX products used intraoperativelyThe advent of the new EHL factor products has resulted in the performance of new research into the intraoperative behavior of PK, both for EHL FVIII23–25 and FIX26–28. Most of these studies carry out a preoperative individual non-compartmental PK analysis to estimate the PK parameters. The exception is a study on eftrenonacog alfa (rFIXFc) that used the PopPK model in patients on prophylaxis29 and compared the estimated levels with the real-life levels, obtaining an excellent correlation between them28.
Use of new PopPK models during surgeryThe OPTICLOT trial was designed to create a FVIII PopPK model for surgical use and compare it to the results of standard dosing30. A bicompartmental surgical model was developed using the NONMEM technique with data from 140 procedures on 75 adult patients and 58 procedures on 44 children with hemophilia A31. Covariates in this PopPK analysis included age, blood type and type of surgery. The model was validated through a cohort of 87 pediatric patients, and a new model comprising a total of 206 patients was generated32. This new model significantly improved the available predictions, with the estimation accuracy improving from a median underestimation of 17 IU/dL to a median overestimation of 2 IU/dL. Similarly, a three-compartment surgical PopPK analysis was developed using the NONMEM technique, with data from 255 procedures on 118 patients with hemophilia B33. Body weight, age and type of FIX were the main covariates.
Few studies have compared the PK values of the new EHL factors with those of standard t1/2 (SHL) factors or other EHL factors during surgery. Noteworthy among them is a study on nonacog beta pegol (N9-GP). The authors developed a specific bicompartmental PopPK and analyzed its performance in different scenarios against recombinant FIX (rFIX) and plasma-derived FIX (pdFIX)34. Intraoperative simulations were carried out to compare the dosing regimens of N9-GP, rFIX and pdFIX needed to achieve the target FIX levels established by the WFH (100-120 IU/dL pre-op, 40 IU/dL at days 1-3; 30 IU/dL at days 4-6; and 20 IU/dL at 7-14 post-op)35. Use of N9-GP, as measured in IUs/kg, was 80% lower than that of rFIX and pdFIX; the number of infusions required was also lower (2 vs.16).
With the help of the results of a cross-sectional clinical trial comparing the PK of EHL FIX products with that of N9-GP and rFIXFc, specific PopPK (single- and three-compartment, respectively) models were designed for surgical and on-demand applications36. The model was used to make estimations on the basis of the recommendations of the WFH for factor administration during surgery37, with N9-GP requiring the lowest number of infusions (67% and 55% in major and minor surgery, respectively) and the lowest product consumption (67% and 58% in major and minor surgery, respectively).
Effect of co-variables on PopPK values during surgeryThe FVIII PopPK model developed under the OPTICLOT trial showed that clearance decreased with age and was 26% higher in patients with blood type 031. Moreover, a 7% decrease in clearance was observed in major surgeries as compared with minor ones. Two case reports on PK-based FVIII dose titration during surgery concluded that the recommended weight is the ideal body weight both for obese patients38 and in cases of extreme weight loss39.
FIX PopPK showed that clearance and the distribution volume in the central compartment (V1) grew gradually with age and increasing body weight until the age of 2033. Patients treated with pdFIX showed lower clearance and V1 levels than those treated with rFIX (11% and 17%, respectively). Similarly, V1 in patients with moderate hemophilia B was 10% lower than in those with severe hemophilia B.
Other studies analyzed the influence of the covariates on PK during surgery. A study on pdFIX compared IVR before and after surgery and demonstrated an age, weight and pdFIX type-dependent effect on dosing40. A retrospective study analyzed the variables that influenced IVR and clearance in patients on CI of FVIII during surgery and detected differences in the clearance rate depending on the subjects’ body mass index and the type of FVIII used17.
Another study analyzed the factors capable of predicting FVIII under- and overdosing in 198 surgeries on 119 patients41. Blood type 0 and major surgery turned out to be predictors of overdosing, while underdosing was associated with increasing age, plasma-derived FVIII and intermittent infusion. Blood type 0 was also associated with an increased bleeding rate.
Intraoperative factor concentrations recommended by clinical guidelinesTo ensure bleeding control during surgery, the clinical guidelines recommend maintaining FVIII/FIX levels above specific thresholds for specific time periods. Exact target levels will depend on the type of surgery performed. These recommendations have undergone certain changes over time3,35,37. Table 2 contains the recommendations of the latest WFH guidelines3.
Recommended peak FVIII/FIX levels and length of treatment depending on type of surgery. Adapted from Srivastava et al. 20203
| Hemophilia A | Hemophilia B | |||||||
|---|---|---|---|---|---|---|---|---|
| Type of surgery | Low-dose pattern | High-dose pattern | Low-dose pattern | High-dose pattern | ||||
| Peak level (IU/dL) | Length of treatment (days) | Peak level (IU/dL) | Length of treatment (days) | Peak level (IU/dL) | Length of treatment (days) | Peak level (IU/dL) | Length of treatment (days) | |
| Major surgery | ||||||||
| Preoperative | 60-80 | 80-100 | 50-70 | 60-80 | ||||
| 30-40 | 1-3 | 60-80 | 1-3 | 30-40 | 1-3 | 40-60 | 1-3 | |
| Postoperative | 20-30 | 4-6 | 40-60 | 4-6 | 20-30 | 4-6 | 30-50 | 4-6 |
| 10-20 | 7-14 | 30-50 | 7-14 | 10-20 | 7-14 | 20-40 | 7-14 | |
| Minor surgery | ||||||||
| Preoperative | 40-80 | 50-80 | 40-80 | 50-80 | ||||
| Postoperative | 20-50 | 1-5 | 30-80 | 1-5 | 20-50 | 1-5 | 30-80 | 1-5 |
Another approach is the one followed by the Delphi expert consensus, which redefined the target plasma levels of FVIII, replacing the traditional target level of 1 IU/dL by 8 different target levels42. Four of these target levels are related to surgery, with thresholds being established depending on the different surgical stages and the complexity of the surgery (Figure 2).
Target intraoperative FVIII plasma levels. Adapted from lorio et al. 201743.
Against this background, hemophilic patients undergoing surgery should have their peak levels measured 15-30 minutes after the replacement factor has been infused; trough levels should also be regularly measured. It has also been suggested that a full preoperative PK or a PopPK analysis be conducted. The former would require a larger number of samples (9-11) than the latter to adjust the preoperative dose and, if needed, the continuous infusion rate, based on the calculated clearance rate3. These preoperative PK estimations must be adjusted during surgery by regularly measuring peak and trough factor concentrations. Several studies have shown that the FVIII/FIX concentrations obtained with this method tend to fall outside the established target range, leading to under- or overdosing41,43. This is the reason why the OPTICLOT group is promoting the use of surgery-specific PopPK models to make these kinds of adjustments31,33.
DiscussionPK has become a new tool to adjust prophylactic treatment in hemophilic patients. Thanks to the fact that PopPK, unlike traditional direct calculation and multiple sampling methods, requires only 2-3 samples3,4, PK has now become widely used, among other things, to manage changes between SHL clotting factors44 or between SHL factors and the new EHL ones45,46. Nonetheless, while clear recommendations have been published concerning the sampling times required to estimate the PK of FVIII and FIX used prophylactically47, there is still no standard concerning the optimal number of samples required for PK analyses performed during surgery.
CI is commonly used in major surgery given the convenience it provides by preventing peaks and troughs. However, its drawbacks include the high level of expertise required to appropriately design the required dose, the need to use specifically designed pumps, and the need to determine the stability of FVIII or FIX concentrations after reconstitution within the infusion device3. Use of CI has been associated with lower clearance rates, which allow a reduction of the dose and of factor consumption as a whole3. Nevertheless, stability issues may require changes every 12 hours or additional bolus injections to ensure effective circulating clotting factor levels. The technique is nowadays only considered useful in patients with severe hemophilia A or B, as in patients with milder phenotypes dosage titration tends to be more difficult. Moreover, CI has been related with a higher risk of inhibitor development in these patients48,49.
Although the role of covariates of FVIII and FIX PK in prophylaxis is well understood, many knowledge gaps still exist regarding their influence during surgery. The uncertainty is even greater when it comes to EHL factors, for which few PopPK models are available for the surgical setting. The role of the extravascular space may be particularly important as that space is the site at which FIX accumulates and binds to collagen. The strength of this bond is believed to vary across the different types of FIX50, which leads to a higher Vd for rFIXFc given its broad extravascular distribution and to a lower Vd for N9-GP, depending on the PK model employed; Vd is three-compartmental for rFIXFc29 and single-compartmental for N9-GP51.
As is the case with all regression models, a PopPK model's predictive precision depends on it being used in the same conditions as it was developed. For that reason, studies using PopPK models developed in the context of prophylaxis are only valid for the preoperative dose and fail when it comes to making estimations during the procedure41,43. The OPTICLOT group has in the last few years developed surgery-specific PopPK models for FVIII and FIX31,33. They are furthermore conducting a randomized clinical trial in order to show that PopPK models provide more precise estimations than traditional approaches, permitting more effective dosing and minimizing hemorrhagic complications and overall factor consumption30. Prophylaxis-specific collaborative models such as WAPPS-Hemo could in the near future incorporate these surgery-based approaches and facilitate their use in clinical practice21.
In short, la PK may allow an individualized and standardized adjustment not only of the design of replacement factor prophylaxis but also of the surgical administration of clotting factors during surgery. There still remains to define the best suited PopPK model for each case as well as the most appropriate sampling times for PK analyses. In addition, the recent development of EHL clotting factors may result in the design of surgical protocols with fewer infusions, which would allow a reduction in the bleeding risk associated to peak and trough levels, in the consumption of clotting factors and thereby in the overall cost of surgical procedures and in the patients’ hospital stay. Significant as it already is, the contribution of pharmacists to multidisciplinary teams dedicated to the management of PK within the Congenital Coagulopathies Unit will become even more decisive thanks to the new developments discussed in this study. Indeed, pharmacists could become a key figure in the management of these patients.
FundingNo funding.
AcknowledgementsThe authors would like to thank SEFH's PKGen Group for inviting them to contribute to the Revista's Special Issue on personalized drug therapy in clinical practice.
Conflict of interestsNo conflicto of interests.