Neuropsychiatrists often resort to drugs with broad interindividual pharmacokinetic variability metabolized by highly polymorphic enzymes such as CYP2D6 and CYP2C19. Pharmacokinetics and pharmacogenetics offer considerable promise as techniques capable to allow individualized adjustments in treatments with psychoactive drugs. The purpose of this study was to review the existing evidence for the application of pharmacokinetics and pharmacodynamics to the dosing of drugs used in neuropsychiatry.
MethodA literature search was conducted in PubMed and Embase to find prospective studies published between January 2000 and April 2021 that used determination of psychotropic drug plasma levels or genotyping to improve response to treatment or minimize adverse events in adult patients with psychiatric conditions. MeSH terms and free search terms were used. Each article was reviewed by two independent reviewers to ensure that they met the inclusion criteria. A quantitative method was established to assess the quality of the articles selected.
ResultsA total of 27 articles met the inclusion criteria of which 16 used pharmacokinetic and 11 pharmacogenetic techniques. Fifty percent of pharmacokinetic studies met the five predefined quality criteria. Eight of the 16 papers were on antidepressants; the remainder were on antipsychotics. Two of the latter did not find an association with efficacy or safety. None of the pharmacogenetic studies met the five quality criteria. Only one of the two studies on antipsychotics found fewer adverse events with genetics-guided dosing in patients on CYP2D6 substrate antipsychotics. Six of the nine studies on antidepressants found that pharmacogenetics-based dosing improved efficacy.
ConclusionsThe evidence available on pharmacokinetics and pharmacodynamics-based personalization of treatment with psychoactive drugs is scarce. Many existing studies analyze associations between genotypes and response or toxicity but provide few data on the efficacy of treatment individualization. The results obtained suggest the existence of significant differences in pharmacokinetic parameters between responding and non-responding patients, particularly in the treatment of depression. Given that the availability of pharmacogenetic information may be useful at the beginning of treatment, combining both techniques could help optimize pharmacotherapy. However, clinical trials are needed to establish their benefits with greater accuracy.
Dentro de la neuropsiquiatría es habitual el empleo de fármacos con amplia variabilidad farmacocinética interindividual y metabolizados por enzimas altamente polimórficas como CYP2D6 y CYP2C19. La farmacocinética y la farmacogenética se vislumbran como herramientas de ayuda para conseguir un ajuste personalizado en el tratamiento con psicofármacos. El objetivo de este trabajo es revisar la evidencia existente sobre la aplicación de farmacocinética y farmacogenética en la selección de dosis de los medicamentos empleados en neuropsicofarmacología.
MétodoSe realizó una búsqueda en PubMed y Embase para localizar estudios prospectivos, publicados entre enero de 2000 y abril de 2021, que utilizasen la determinación de niveles plasmáticos de psicofármacos o genotipado para mejorar la respuesta o minimizar efectos adversos en pacientes adultos con trastornos psiquiátricos. Se emplearon términos MeSH y texto libre. Cada artículo fue revisado por dos revisores independientes para asegurar que cumplían los criterios de inclusión. Se estableció un método cuantitativo para valorar la calidad de los artículos incluidos.
ResultadosSe incluyeron 27 artículos, 16 utilizaban farmacocinética y 11 farmacogenética. El 50% de los estudios de farmacocinética cumplieron los cinco criterios de calidad predefinidos. Ocho de los 16 trabajos analizaron antidepresivos y los estudios restantes antipsicóticos. Dos de estos 8, no encontraron asociación con eficacia o seguridad. Ninguno de los estudios de farmacogenética cumplía los cinco criterios de calidad. Sólo 1 de los 2 estudios de antipsicóticos encuentra reducción de efectos adversos con dosis guiadas por genética en pacientes con antipsicóticos sustratos del CYP2D6. Seis de los 9 estudios con antidepresivos encuentran mayor eficacia al dosificar utilizando farmacogenética.
ConclusionesLa evidencia disponible sobre farmacocinética y farmacogénetica en individualización del tratamiento con psicofármacos es escasa. Gran parte de los estudios analizan asociaciones entre genotipos y respuesta o toxicidad, proporcionando pocos datos sobre la eficacia en la individualización del tratamiento. Los resultados obtenidos apuntan a la existencia de diferencias significativas en parámetros farmacocinéticos entre pacientes respondedores y no respondedores, especialmente en el tratamiento de la depresión. Disponer de información farmacogenética puede ser de utilidad al inicio del tratamiento, por lo que combinar ambas técnicas podría ayudar a optimizar la farmacoterapia, pero hacen falta ensayos clínicos para establecer claramente su beneficio.
Although all areas of pharmacotherapy are equally important, managing some of them can be slightly more complicated when they involve administration of drugs with broad inter-individual pharmacokinetic variability, whose metabolic pathways are at times controlled by highly polymorphic enzymes such as CYP2D6 or CYP2C19. One of such areas where the management of drug therapy is often more complex is neuropsychiatry. The fact that neuropsychiatric drugs are characterized by a broad pharmacodynamic variability has hampered implementation of individualized management of such medicines.
It is not unusual for terms such as precision medicine and personalized dosing to be confused. On the one hand, precision medicine, defined in the standards of national health systems as the kind of medicine that “uses information on the genes, proteins and other characteristics of the condition affecting a person to establish the diagnosis or the treatment of the said disease”, allows selecting medication in such a way that a higher likelihood of obtaining a therapeutic response is theoretically possible. On the other hand, personalized medicine also ensures that the right medication is used at the right dose in any given patient. These two concepts are confused all too frequently.
Since the 1960's and 1970's, pharmacokinetic monitoring came to be gradually —if somewhat slowly— incorporated to clinical practice. Although drugs such as digoxin, theophylline and some antibiotics have been subjected to routine monitoring in some hospitals, most centers have failed to implement any surveillance measures. Recently, with the introduction of immunopharmacotherapy to specialties such as gastroenterology, dermatology, oncology and rheumatology, there seems to have been an increase in the clinical use of pharmacokinetics, although practitioners tend to confuse monitoring with quantification and application of a widely used algorithm.
Although the AGNP (Arbeitsgemeinschaft für Neuropsycopharmacologye und PharmaKopsychiatrie) group, made up of chemists, biochemists, pharmacists and psychiatrists, published its first consensus with evidence-based recommendations for pharmacokinetic monitoring in the realm of psychopharmacology back in 2004, application of such guidelines has been very limited1. Some hospitals do monitor clozapine, probably due to the restrictions on its use, but very few centers have extended their monitoring protocols to all antipsychotics, antidepressants, hypnotics and mood stabilizers.
At the same time, different platforms have been developed informing psychiatrists about the genotype of different enzyme isoforms involved in metabolic, transport and pharmacodynamic processes. Although such platforms are presented as precision medicine tools, the information they provide usually includes dosing recommendations, which gives rise to considerable ambiguity and even confusion as they are not in themselves useful tools to determine a patient's dosing schedule. For example, although a heterozygous genotype is associated with wide phenotypic variability, with drug clearance coefficients of variation as high as 65%, it has been abundantly demonstrated that one same dose cannot lead to the same outcome in all patients2. These platforms allow selection of the initial dosing regimen, albeit with a certain error margin, but they can under no circumstances be used as a basis for dosing individualization. Their dosing recommendations are usually inspired in the CPIC (Clinical Pharmacogenetics Implementation Consortium) guidelines3–5. Apart from genetic factors, there are other elements, i.e, anthropometry, comorbidity conditions, age, etc., that play a role in the determination of medicines’ serum or plasma concentrations. As shown by several studies, their potential benefit in terms of preventing toxicity and limiting adverse events has been reported in several studies on poor metabolizers. Use of these platforms should be made in due consideration of phenoconversion, defined as a mismatch between a given genotype and its functional expression (phenotype) as a result of an interaction with a drug, a food item or a natural product. In their study on venlafaxine, Preskorn et al.6 dwell extensively on this topic. It is therefore essential to carefully review the patient's entire medication to prevent phenoconversion phenomena that could alter dosing recommendations, i.e., inclusion of bupropion in a patient on venlafaxine7.
The debate that has arisen on the role of pharmacokinetics and pharmacodynamics in the management of narrow therapeutic index drugs prompts us to ask the key question in this systematic review: What evidence is there on the application of pharmacokinetic or pharmacodynamic criteria to dosing in the realm of neuropsychopharmacology?
MethodsData sources and search strategyA systematic literature review was carried out in the PubMed and Embase electronic databases with a view to identifying all articles published between January 2000 and April 2021 that used pharmacogenetic and pharmacokinetic monitoring techniques to improve health outcomes in patients with psychiatric disorders treated with antidepressants, anti-psychotics and mood stabilizers. The analysis was carried out following the PRISMA guidelines, designed to improve the quality of this kind of systematic review8.
An initial search was conducted in PubMed and Embase in May 2021 to identify all articles published during the above-mentioned period on the subject of interest. The search strategy is duly described in Annex I. The initial search was followed by a cross-reference search for other articles on the subject that met the inclusion criteria.
Inclusion and exclusion criteriaTo be included, articles had to comprise adult patients over the age of 19 years diagnosed with a psychiatric disorder and treated with psychotropic drugs. Furthermore, they had to resort to determination of plasma levels of the drugs employed and/or to genotyping of certain polymorphisms to improve response to treatment or minimize adverse events. Studies that only analyzed the association between one or several polymorphisms and the patients’ response to treatment or the appearance of an adverse event without providing data on the efficacy of the intervention were excluded. Inclusion and exclusion criteria are spelled out in Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
DSM: Diagnostic and Statistical Manual of Mental Disorders; ICD: International Classification of Diseases.
After excluding all duplicate articles, the Mendeley computer application was used to perform a first title and abstract screen of all the articles identified, classifying them as either valid or not valid in accordance with the inclusion criteria established. The review was carried out by two independent reviewers. Discrepancies between them were resolved by consensus among all the reviewers. The articles included following this first review were read in full ensuring that the above-mentioned inclusion criteria were in fact met.
All relevant data from every study was collected to evaluate their quality and perform the subsequent analysis (condition, age, psychotropic drug, type of study, sample size, clinical scales used, methodology, length of follow-up, and concomitant treatments).
Quality of the studies selectedA series of criteria were defined (Table 2) with the aim of objectively appraising the quality of each of the studies included. These criteria are explained below.
Definition of the quality criteria of the selected articles
| Code | Quality criterion | Requirements |
|---|---|---|
| 1 | Patient selection | Homogeneous characterization |
| 2 | Number of patients | ≥ 10 in each experimental group |
| 3 | Efficacy and/or safety scales | Defined and validated |
| 4 |
| Validated analytical method, biological matrix, Css, C≥10-12 h psot-administration Validated genotyping method, biological matrix |
| 5 | Concomitant treatment | Indication of permitted medicines during the analysis |
Css: steady-state plasma concentration; C≥10-12 h psot-administration: plasma concentration at least 10-12h post-administration.
- 1.
Patient selection.
The patient sample must be homogeneous and representative of the study undertaken. The psychiatric condition analyzed and the diagnostic score used must be explicitly stated.
- 2.
Number of patients.
At least 10 patients must be included in each experimental group, as suggested by Kloosterboer et al.9.
- 3.
Effectiveness and/or safety scales.
The scales employed and the respondent patient concept must be properly defined. The baseline clinical situation of each patient must be clearly defined at the outset of the study according to the scale employed. Effectiveness and safety scales must be validated and the length of follow-up must be clearly stated.
- 4.
Methodology:
- 4.1.
Pharmacokinetic studies.
The analytical technique employed had to be specific and sensitive, preferably based on high-resolution liquid chromatography or mass spectrometry coupled to liquid chromatography. The analytical method must be validated for reliability and reproducibility. The study must indicate the type of biological matrices used: serum, plasma or whole blood. The sampling time must be long enough to allow for a steady state to be reached and for plasma levels to be equal to the concentrations attained 10-12 hours post-administration.
- 4.2.
Pharmacogenetic study.
The analytical technique employed ought to have been well described and validated in previous publications. Techniques based on DNA extraction from peripheral blood were preferred, accompanied by appropriate integrity, purity (260 nm/280 nm absor-bance) and quantification analyses. All studied populations had to be in Hardy-Weinberg equilibrium. In studies of genetic variants in different cytochromes P450, to determine whether there was a genotype/phenotype correlation, an analysis had to be carried out of the genetic variants described in the official nomenclature of the Pharmacogene Variation Consortium3,10.
- 4.1.
- 5.
Concomitant treatment.
Studies had to indicate the type of drugs allowed as co-medication, especially as regards any enzyme inducing or inhibiting medication that could alter the studied drug's kinetic behavior or contribute to its phenoconversion.
On completion of the article selection process, after applying the inclusion, exclusion and quality criteria, the effect of the drugs analyzed on health outcomes was evaluated. The evaluation was based on the identification of whether any statistically significant differences were found between respondents and non-respondents, and between the presence or absence of toxicity.
ResultsApplication of the inclusion and exclusion criteria yielded a total of 41 articles with a proper pharmacokinetics/pharmacogenetics balance. The reviewers carried out the selection in pairs, there being four reviewers the process was divided between two groups of two reviewers each.
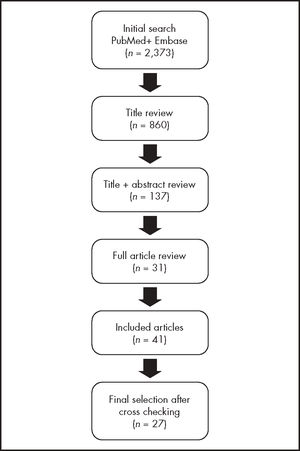
Article selectionFigure 1 shows the process followed to review and select the articles included in this study.
Of the 899 articles initially selected, 39 were discarded after reading their title as they were duplicates of studies already included. The title and abstract screen resulted in 137 articles being exhaustively reviewed by the two pairs of reviewers. The majority of them were eventually excluded because they were association studies and did not provide any information on the efficacy of the dosing individualization techniques used. The screening process yielded a total of 31 studies, which were supplemented by another 10 articles, all of them of a pharmacogenetic nature, drawn from the literature analysis included in some of the papers selected. The final sample comprised 11 pharmacogenetic and 16 pharmacokinetic studies.
Quality of the selected articlesIt must be mentioned that 50% of the pharmacokinetic studies fulfilled the five pre-defined quality criteria. Four studies (25%) comprised a very small patient sample with less than 10 subjects in the responding and the non-responding patient groups. Two studies (12.5%) provided no information on the length of the sampling period or on the kind of biological matrix used for the pharmacokinetic study. The description of the concomitant treatment administered during the study was not indicated in 12.5% of the papers reviewed.
The systematic search of pharmacogenetic studies eventually identified 11 papers that fulfilled the five inclusion criteria. Although the excluded studies did analyze the correlation of various genotypes with the response to treatment or the toxicity to antidepressants or antipsychotics, they did not report on any therapeutic decisions being based on genotyping or, if they did, the results of such decisions were not evaluated in terms of the efficacy or toxicity associated to the treatment. Seventy-five percent of the 11 pharmacogenetic studies selected, presented with poor genotyping quality (quality criterion 4.2) as they did not include all the genetic variants defining a given phenotype according to international guidelines3,10, because the Hardy-Weinberg principle was not complied with for the studied populations, or because no quality data was provided about the studied sample. None of the studies specified what concomitant therapy or combination of drugs was administered to each patient, making it impossible to rule out the occurrence of phenoconversion (quality criterion 5). Four studies provided no details on the scale used to measure safety (quality criterion 3) and two studies failed to define the diagnostic scale employed (quality criterion 1).
The characteristics, results and quality standards of the selected pharmacokinetic studies are shown in tables 3a and 3b. Two of the studies on antipsychotics (Table 3a)11,12 did not obtain any association between efficacy or safety and plasma concentrations. Riedel et al.13 is of particular interest for presenting paradoxical results; patients not responding to treatment with risperidone exhibited higher plasma concentrations than patients who did respond despite receiving similar oral doses. The authors suggest that failure to respond may be associated with an alteration of phase II metabolism, which means that dose escalation would not be a judicious option in these cases as pharmacokinetic monitoring is extremely useful in these patients. Dettling et al.14 also observed greater plasma clearance in patients who responded to treatment with clozapine, although statistically significant differences were only observed on parametric tests. Fellows et al.15 defined a minimum effective concentration of olanzapine of 23-25 ng/mL with low specificity and sensitivity levels as the leftward deviation of the identity line of the ROC curve was very small. Kondo et al.16 defined a curvilinear relationship between plasma concentrations of nemonapride and improvement in the BPRS score. Lin et al.17 for their part found a greater exposure to dehydroaripiprazole in responding patients, and Volonteri et al.18 identified risperidone's metabolic quotient as a predictor of a therapeutic response and observed a relationship between the extrapyramidal effects and plasma concentrations of risperidone + 9OH-risperidone.
Pharmacokinetic articles selected on antipsychotics used in patients with schizophrenia
| Ref. | Drug | Follow-up design | Efficacy | Safety | Results | Quality criteria |
|---|---|---|---|---|---|---|
| [14] | Clozapine | POn (n = 34) 10 weeks | BPRS | C/D = 0.6 ± 0.3 ng/mL per mg (R) vs 1.0 ± 0.6 ng/mL (nR); t-test p = 0.05. MWU test p = 0.09 | 1, 2, 3, 4, 5 | |
| [15] | Olanzapine | POn (n = 53) 6 weeks | PANSS | AIMS, SAS, BAS | Cut-off 23-25 ng/ml AUC ROC 55% | 1, 2, 3, 4 |
| [16] | Nemonapride | PO. DF (n = 31) 3 weeks | BPRS | % improvement BPRS = 47.9 + 73.9Cp-44.2Cp2 r2 = 0.427, p < 0.001 | 1, 3, 4, 5 | |
| [11] | Risperidone | PO. DF (n = 30) 42 days | PANSS | ESRS, UKU | n.s. | 1, 3, 4, 5 |
| AUC curve ROC (A + DHA) = 0.68 ng/mL (0.52-0.84), p = 0.047 | ||||||
| [17] | Aripiprazole | POn (n = 45) 6 weeks | PANSS | AIMS, SAS, BAS |
| 1, 2, 3, 5 |
| [12] | Fluphenazine | PO (n = 31) 52 weeks | BPRS, SANS | SAS, BAS | n.s. | 1, 2, 3, 5 |
| [13] | Risperidone | POn (n = 82) 6 weeks | PANSS, CGI | SAS, BAS | Cp = 49.9 ± 30.7 ng/mL (nR) vs Cp = 38.2 ± 17 ng/mL (R), p = 0.045 | 1, 2, 3, 4, 5 |
| [18] | Risperidone depot | POn (n = 30) 6 months | BPRS, PANSS, CGI | SAS |
| 1, 2, 3, 4, 5 |
9OH-R: 9hydroxy-risperidone; A: aripiprazole; AIMS: Abnormal Involuntary Movement Scale; AUC: area under the curve; BARS: Barnes Akathisia Rating Scales; BPRS: Brief Psychiatric Rating Scale; CGI: clinical global impresión; C/D: relationship between plasma concentration and daily doce; Cp: trough plasma concentration; DHA: dehydroaripiprazole; ESRS: Extrapyramidal Symptom Rating Scale; FD: fixed doce; MWU: Mann-Whitney U Test; nR: non-responding patient; n.s.: no significant data; OPn: prospective naturalistic observational study; OR: odds ratio; PANSS: Positive and Negative Syndrome Scale for Schizophrenia; R: responding patient; RISP: risperidone; SANS: Scale for the Assessment of Negative Symptoms; SAS: Simpson-Agnus Scale; UKU: Udvalg for Kliniske Unders⊘gelser Scale.
As regards antidepressants (Table 3b), the eight studies analyzed19–26 found a correlation between plasma concentrations and therapeutic response. Moreover, Grasmäder et al.20 observed an inversely proportional relationship between duration of sleep and mirtazapine plasma concentrations.
Selected pharmacokinetic articles on antidepressants
| Ref. | Disease/medication | Follow-up design | Efficacy | Safety | Results | Quality |
|---|---|---|---|---|---|---|
| [19] | MD Venlafaxine | POn (n = 22) 6 weeks | MADRS, CGI | ESRS | Relationship Cp (venlafaxine + O-desmethylvenlafaxine)-MADRS, r2 = 0.402. p = 0.0268. Responders: Cp = 123-387 μg/L | 1, 3, 4, 5 |
| [20] | MD Mirtazapine | POn (n = 45) 70 days | HDRS | UKU | Cut-off: 30 ng/mL, OR = 1.054. p = 0.031 Sleep duration-Cp ratio OR = 0.925. p = 0.034 | 1, 2, 3, 4 |
| [21] | MD Fluvoxamine | POn (n = 12) 28 days | HDRS |
| 1, 3, 4, 5 | |
| [22] | OCD Clomipramine | PO (n = 22) 12 weeks | CGI, HDRS HARS, YBOCS | Association between CGI & C/D (CMI + DCMI), p = 0.03 & con low Cp DCMI, p = 0.04 | 1, 2, 3, 4, 5 | |
| [23] | MD Sertraline | POn (n = 23) 1 year | BPRS, HRS |
| 1, 2, 3, 4, 5 | |
| [24] | MD, BD Lamotrigine | POn (n = 37) 8 weeks | MADRS |
| 1, 2, 3, 4, 5 | |
| [25] | MD Fluvoxamine | POn (n = 51) 12 weeks | HDRS | Cut-off 61.4 ng/mL, p < 0.01 for patients with initial HDRS = 17 > 20. | 1, 2, 3, 4, 5 | |
| [26] | MD Duloxetine | POn (n = 45) 12 weeks | HARS, CGI |
| 1, 2, 3, 4, 5 |
BD: bipolar disorder; BPRS: Brief Psychiatric Rating Scale; CGI: clinical global impression; C/D: relationship between plasma concentration and daily dose; CMI: clomipramine; Cp: trough plasma concentration; DCMI: N-desmethylclompramine; ESRS: Extrapyramidal Symptom Rating Scale; HRS: Hamilton Rating Scale; HARS: Hamilton Anxiety Rating Scale; HDRS: Hamilton Depression Rating Scale; MADRS: Montgomery-Asberg Depression Rating Scale; MD: major depression; nR: non-responding patient; OCD: obsessive-compulsive disorder; OR: odds ratio; POn: naturalistic prospective observational study; R: responding patient; UKU: Udvalg for Kliniske Unders⊘gelser Scale; YBOCS: Yale-Brown Obsessive Compulsive Scale.
The characteristics, results and quality standards of the selected pharmacogenetic studies are shown in Table 4. The two clinical trials on psychotropic drugs27,28 presented in Table 4 found no significant differences regarding the efficacy or the toxicity of antipsychotic treatment between CYP1A2, CYP2C19, CYP2D6 or CYP3A5 genotype dosing and dosing determined by standard clinical practice, neither at the beginning of treatment nor when a change was made to the treatment. Only Arranz et al.27 found a tendency toward a reduction in adverse events in patients on pharmacogenetics-guided dosing. The authors found this tendency in the subgroup of patients on CYP2D6 substrates such as risperidone and aripiprazole who carried the ultrarapid or slow metabolizing genotype.
Description of prospective clinical trials where the clinical decision was based on pharmacogenetic analyses in patients with schizophrenia and major depression
| Ref. | Disease | Medication | E/C | Follow-up design | Efficacy | Safety | Analyzed genes | Analytical method | Results | Dosing recommendation/recommended drug indication | Quality criteria |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [27] | Schizophrenia | APs | 123/167 | RDBCT 12 ws | PANSS | UKU-SERS | CYP1A2 CYP2C19 CYP2D6 CYP3A5 | MassARRAY platform, TaqMan PCR | n.s. on PANSS; p < 0.05 on UKU-SERS subgroups | % of dose modification according to dedicated protocol | 1, 3 |
| [28] | Schizophrenia | APs | 311/217 | RDBCT 1 year | Persistence or treatment failure | UKU | CYP2D6 CYP2C19 | Real time PCR (TaqMan) | n.s. | Dosing based on CYP (CPIC) guidelines | 1, 3 |
| [29] | Major depression | ADs | 352/333 | RBDCT 12 ws | HDRS17 | ND | YP1A2 CYP2C9 CYP2C19 CYP2D6 CYP3A4 CYP3A5 SLC6A4 COMT HTR2A, MTHFR | NeuroIDgenetix® Test | p < 0.01 in genotype-guided group vs control group | NeuroIDgenetix® test (classifies indication and dosing based on genotype) | 1 |
| [35] | Major depression, bipolar disorder | ADs substrate P-GP | 38/30 | RCT 28 days | HDRS | AMDP | ABCB1 | Real-time PCR | n.s. | Maximum antidepressant dose in patients with an ABCB1 genotype | 2, 3, 4 |
| [31] | Major depression | ADs | 22/22 | POc 8 ws | QIDS-C16 HDRS17 | Patient-reported AEs | CYP2D6 CYP2C19 CYP1A2 SLC6A4 HTR2A | Luminex xTAG system and restriction enzymes | p < 0.01 in the genotype-guided group vs standard treatment | Dosing recommendation and genotype-guided drug selection | 1, 3 |
| [30] | Major depression | ADs | 114/113 | POc 8 ws | HDRS-17 QIDS-C16 PHQ-9 | ND | CYP2D6 CYP2C19 CYP1A2 SLC6A4 HTR2A | Luminex xTAG system and restriction enzymes | p < 0.01 in the genotype-guided group vs standard treatment | Pharmacogenetics-based treatment based on GeneSight assay | 1 |
| [32] | Depression, anxiety, ADHD, psychosis | ADs & APs | 178/59 | RCT 3:13 months | NPQ SDC | Interview on AEs | 13 genes | IDgenetix neuropsychiatric test panel | Reduction of AEs at month 3; p < 0.05 vs control | Medication/dosing selection according to (Dgenet/x®-based genotyping (CPIC guidelines + literature) | 2, 3, 4 |
| [33] | Major depression | ADs | 155/161 | RDBCT 12 ws | HDRS-17 CGI-S MSQ | FIBSER | CYP2D6 | Real time PCR QuantStudio™ 12 K Flex Real-Time PCR System | p < 0.05 in genotype-guided dosing, with HDRS reduction and FIBSER improvement |
| 1, 3 |
| [34] | Major depression | ADs | 74/74 | RDBCT 12 ws | HDRS | ND | CYP2D6, CYP2C19 (CNSDose® panel genético) | Sequenom® Matrix | p < 0.01 in genotype-guided dosing, with HDRS reduction vs non-guided group | Recommended dosing based on CNSDose® pharmacogenic dosing report | 1 |
| [36] | Major depression | ADs | 26/25 | RDBCT 10 ws | HDRS-17, PHQ-9, QIDS-SR, QIDS-CR | ND | CYP2D6 CYP2C19 CYP1A2 SLC6A4 HTR2A | Luminex xTAG system and restriction enzymes | n.s. | Recommended dosing based on GeneSight assay results | 1 |
| [41] | Major depression, ADHD | Ads (SSRI, SNRI, Mix, folate) | 468 total, MTHFR: 195WT/272 risk; SLC6A4 125 WT/334 risk | Open-label RCT 3 months | CGI-I, CGI-S | UKU, QUIDS-SR, Q-LES-Q-SF | SLC6A4 MTHFR | Genecept assay | n.s. | Selection of medication base don Genecept Report® genotyping | 1, 3 |
The experimental group is the one for which the treatment is modified as a function of genotype. The control group is the one where patients are treated according to standard practice.
ADs: antidepressants; AEs: adverse events; AMDP: Arbeitsgemeinschaft für Methodik und Dokumentation in der Psychiatrie; APs: antipsychotics; CGI: clinical global impression; CGI-S: severity score at the beginning of the study; CGI-I: clinical improvement at 3 months; E/C: nr of subjects in the experimental/control group; FIBSER: frequency, intensity, burden of side effects rating; HDRS: Hamilton Depression Rating Scale; MSQ: medication satisfaction questionnaire; ND: not determined; NPQ: Baseline Neuropsychiatric Questionnaire; n.s. no significant data; PANSS: Positive and Negative Symptom Scale for Schizophrenia; P-GP: p glycoprotein; PHQ9: Patient Depression Questionnaire; POc: prospective observational cohort study; QIDS-C16: Quick Inventory of Depressive Symptomatology Clinician-Rated; QIDS-SR: Quick Inventory of Depressive Symptomatology Self Report; Q-LES-Q-SF: Quality of Life Enjoyment and Satisfaction Questionnaire, short form; RDBCT: randomized double-blind clinical trial; RCT: randomized clinical trial; SAPS: Scale for the Assessment of Positive Symptoms; SDC: symbol digit coding; SNRIs: serotonin norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; UKU-SERS: Udvalg for Kliniske Unders⊘gelser Adverse Event Scale; ws: weeks.
In the case of antidepressants, the variability was wider. Six clinical trials comparing pharmacogenetics-guided antidepressant treatment with treatment according to routine clinical practice found the former to be more effective29–34, whereas two studies found no improvement whatsoever in terms of efficacy or reduction of adverse events35,36.
DiscussionThe present systematic review seeks to answer an apparently simple question: What are the clinical benefits of pharmacokinetics and pharmacogenetics for individualizing the dosing of psychotropic drugs? Contrary to what may be expected, the number of clinical studies on the subject is relatively scarce, which means that any conclusions drawn should be taken with caution. Over the last decade there has been a mushrooming of genetic studies aimed mainly at correlating genetic variants with exposure to psychoactive drugs. An example of this is an exhaustive review published in Molecular Psychiatry in 200437 on the genotype-phenotype relations that exist between the different antidepressants and antipsychotics and on different dosing modification proposals intended to compensate for differences in plasma concentrations. A metanalysis published in JAMA-Psychiatry in 202138 found a strong association between exposure to different psychotropic drugs and different CYP2C19 and CYP2D6 genotypes. These studies have led to the publication of several clinical guidelines such as the CPIC (Clinical Pharmacogenetics Implementation Consortium) guidelines, the DPWG (Dutch Pharmacogenetics Working Group) guidelines, the CPNDS (Canadian Pharmacogenomics Network for Drug Safety) guidelines, and the RNPGx (French National Network of Pharmacogenetics) guidelines39, among others. They have also resulted in the inclusion of the relevant information in the summary of product characteristics (SmPC) of drugs like aripiprazole. These clinical guidelines made recommendations both related to indications and dose modifications based on the CYP2C19 and CYP2D6 genotypes. Nonetheless, the information selected to make such recommendations comes from studies correlating genotypes with drug exposure, which is indicative that evidence based on clinical results is too scarce to make recommendations. The information contained in these guidelines makes reference to “potential risks”, suggesting that the metabolization of these drugs is subjected to the action of other genes such as CYP1A2, CYP2C9 and CYP3A4 as well as that of other environmental, epigenetic or dietary factors, comorbidities or concomitant medication40.
This systematic review responds to the need to analyze the clinical evidence for improving the efficacy and toxicity profile of psychotropic drugs when pharmacokinetics and pharmacogenetics are used for treatment individualization. Although clinical trials that use pharmacogenetics as a tool to individualize treatment with antipsychotics do not show significant differences regarding the effectiveness of treatment or the reduction of adverse events27,28, certain patients with a rapid or slow metabolizer CYP2D6 genotype treated with drugs metabolized by this pathway could benefit from a reduction of the dose at the beginning of their treatment27.
Data is less clear in the realm of clinical trials on antidepressants. It should be underscored that the nine clinical trials that use pharmacogenetics as a tool to select the right medication and individualize the dose to be administered to the patient tend to be based on different designs. None of the studies focuses on a specific antidepressant but rather they select the antidepressant to be used based on different tests and selection algorithms for each patient, which makes it difficult to draw any conclusions for any specific drug. They also use different genotyping, dose calculation and therapeutic indication algorithms such as Genecept®41, GeneSight®36, CNSDose®34, Neuropharmagen®33, and Neuro IDgenetix®32, in addition to the above mentioned CPIC guidelines. These platforms have been approved to support physicians in their decisions on both the indication and the dosing of psychotropic drugs, establishing usage alerts for the different drugs as a function of genotype. It must be said, however, that there is wide variability across such platforms, both in terms of the number of genes analyzed and therapeutic recommendations, which makes it difficult to compare the various studies analyzed in this review. Around 40 pharmacogenetic platforms are currently being used to provide information and recommendations on the use of psychotropic drugs, which is indicative of the wide variability that exists. The review performed in this paper only found clinical trials that used five of these platforms, with negative results having been obtained with Genecept®41 and GeneSight®36. Other public platforms such as Sequecnce2Script42 (sequence2script.com) make an attempt to provide more than just genotyping information, with their dose-calculation and indication algorithms including other aspects like the potential occurrence of phenoconversion as a result of the concomitant medication used.
Nevertheless, pharmacogenetic recommendations for individualizing the dosing of psychoactive drugs can only be useful if they are implemented before initiation of treatment as pharmacogenetic information ceases to be clinically useful if the treatment is already underway. Pharmacogenetic information therefore provides a one-time snapshot, with limited information to follow up patients and make dosing adjustments according to their changing clinical situation. This means that the evolution of a psychoactive drug's plasma levels during treatment does not depend on the genotype but rather on the environment that surrounds the patient and on their clinical situation. In other words, although the genotype does not change, plasma concentrations may do so.
As regards studies on the usefulness of clinical pharmacokinetics (Tables 3a and 3b), a total of 16 of these studies were selected after ensuring that they met al.l the inclusion criteria. Half of these studies (n = 8) reported on the effects of antipsychotics in patients diagnosed with schizophrenia and the other half on the effects of antidepressants typically used in subjects with major depression. One study22 analyzed subjects with obsessive-compulsive disorder and another24 evaluated the role of lamotrigine as a mood stabilizer in patients with bipolar disorder.
Many of the excluded studies were merely descriptive, with a significant amount of these describing the concentrations and metabolic quotients of a certain drug as a function of the polymorphisms of an enzyme isoform, generally the one most closely involved in the drug's metabolism. However, this did not result in the use of distinctive doses in those genetic subgroups and therefore did not lead to distinctive clinical results.
Unlike the pharmacogenetic studies analyzed, most of which were randomized clinical trials, all the pharmacogenetic studies in the sample were prospective, observational and usually naturalistic. Another difference between both groups was the sample size. Pharmacokinetic studies included fewer patients, albeit enough to draw meaningful conclusions. This is to a certain extent indicative of the lack of economic support for pharmacokinetic studies, generally based on clinical practice. Although pharmacokinetics was introduced into hospitals in the 1960's, its implementation has faced many difficulties. It has often been used as a way of controlling toxicity rather than increasing efficacy, for example in the case of digoxin. Its use has been cast aside because of alleged increases in healthcare costs.
In the field of neuropsychiatry, although scientific evidence has supported the implementation of pharmacokinetics techniques more firmly than in the case of, say, aminoglycosides1, few hospitals use such procedures routinely, least of all those that extend their practice beyond clozapine and tricyclic antidepressants. The results obtained in this systematic review suggest the existence of significant differences in drug exposure-related pharmacokinetic parameters (concentrations, concentration-dose indices) between responding and non-responding patients treated for depression. This tool might be instrumental in optimizing drug therapy, which is a priority in this population of patients. Needless to say, more randomized clinical trials are needed to clearly establish the benefit of pharmacokinetic techniques. Pharmacokinetic studies carried out in this area by the pharmaceutical industry, which is the only actor capable of defraying the cost of a clinical trial, are limited to the items required for them to be able to register their products. The same can be said about other areas such as therapeutic monitoring of antibiotics, antifungals or cytostatics, which has been based on findings made by isolated researchers, groups or scientific societies and not on the information provided in their SmPCs.
At any rate, the most logical thing to do would be to implement optimization strategies based on the study of potential drug-drug interactions in order to avoid genetic interpretation errors. Treatment should be initiated based on the different genetic variants known to be associated with neuropsychiatric disorders and pharmacokinetic techniques should be applied to optimize dosing and identify potential unreported drug-drug interactions.
FundingNo funding.
Conflict of interestNo conflict of interests.
(((((pharmacokinetic:ti, ab OR pharmacogenomic:ti, ab OR “pharmacogenomic variants”:ti, ab OR pharmacogenetic:ti, ab OR 'pharmacokinetics’/ exp OR 'pharmacogenetics/exp OR 'pharmacogenomic variants/exp) AND (“drug dosage”:ti, ab OR dose-response:ti, ab OR 'dose response relationship, drug/exp)) AND (antidepressant:ti, ab OR antipsychotic:ti, ab OR “antidepressive agents”:ti, ab OR “psychotropic drugs”:ti, ab OR “antipsychotic agents”:ti, ab OR 'psychotropic drugs/exp OR 'antidepressive agents/exp OR 'antidepressive agents/pharmacokinetics/exp OR 'antipsychotic agents/exp OR 'antipsychotic agents/pharmacokinetics/exp)) AND (“clinical outcome”:ti, ab OR “treatment outcome”:ti, ab OR “therapeutic uses”:ti, ab OR 'therapeutic uses/exp OR 'therapeutic uses/pharmacokinetics/exp OR 'treatment outcome/exp)) AND (adult:ti, ab OR “Young adult”:ti, ab OR “middle aged”:ti, ab OR aged:ti, ab OR elderly:ti, ab OR 'adult/exp OR 'aged/exp OR 'middle aged’/exp OR 'young adult’/ exp)) AND (“randomized controlled trial[Text Word]”
OR “controlled clinical trial[Text Word]” OR “cohort study[Text Word]” OR “longitudinal study[Text Word]” OR “clinical trial[Text Word]” OR 'randomized controlled trials as topic’/exp OR 'controlled clinical trials as topic’/exp OR 'cohort studies’/exp OR (controlled AND 'clinical trials as topic’/ exp) OR 'longitudinal studies’/exp OR 'clinical trials as topic’/exp
The number of articles identified using this strategy was 501.
Six (#1 a #6) independent term searches were carried out using both MeSH terms and text words. The MeSH terms included were “pharmacokinetics, pharmacogenetics, pharmacogenomic variants, dose-response relationship, drug, psychotropic drugs, antidepressive agents, antidepressive agents/pharmacokinetics, antipsychotic agents, antipsychotic agents/pharmacokinetics, therapeutic uses, therapeutic uses/pharmacokinetics, treatment outcome, adult, aged, middle aged, young adult, randomized controlled trials as topic, controlled clinical trials as topic, cohort studies, longitudinal studies, clinical trials as topic” and the free text terms (text words) used were: “pharmacokinetic, pharmacogenomic, pharmacogenomic variants, pharmacogenetic, drug dosage, dose-response, antidepressant, antipsychotic, antidepressive agents, psychotropic drugs, antipsychotic agents, clinical outcome, treatment outcome, therapeutic uses, adult, young adult, middle aged, aged, elderly, randomized controlled trial, controlled clinical trial, cohort study, prospective study, longitudinal study, clinical trial”. The terms were searched in the title & abstract of the articles to ensure that every article on the subject of interest was included.
#1: ((((((pharmacokinetic[Text Word]) OR (pharmacogenomic[Text Word])) OR (pharmacogenomic variants[Text Word])) OR (pharmacogenetic[Text Word])) OR (pharmacokinetics[MeSH Terms])) OR (pharmacogenomics[MeSH Terms])) OR (pharmacogenomic variants[MeSH Terms])
#2 = ((drug dosage[Text Word]) OR (dose-response[Text Word])) OR (dose response relationship, drug[MeSH Terms])
#3: (((((((((antidepressant[Text Word]) OR (antipsychotic[Text Word])) OR (antidepressive agent[Text Word])) OR (psychotropic drugs[Text Word])) OR (antipsychotic agents[Text Word])) OR (psychotropic drugs[MeSH Terms])) OR (antidepressive agents[MeSH Terms])) OR (antidepressive agents/pharmacokinetics[MeSH Terms])) OR (antipsychotic agents[MeSH Terms])) OR (antipsychotic agents/pharmacokinetics[MeSH Terms])
#4: (((((clinical outcome[Text Word]) OR (treatment outcome[Text Word])) OR (therapeutic uses[Text Word])) OR (therapeutic uses[MeSH Terms])) OR (therapeutic uses/pharmacokinetics[MeSH Terms])) OR (treatment outcome[MeSH Terms])
#5: ((((((((adult[Text Word]) OR (young adult[Text Word])) OR (middle aged[Text Word])) OR (aged[Text Word])) OR (elderly[Text Word])) OR (adult[MeSH Terms])) OR (aged[MeSH Terms])) OR (middle aged[MeSH Terms])) OR (young adult[MeSH Terms])
#6: (((((((((((randomized controlled trial[Text Word]) OR (controlled clinical trial[Text Word])) OR (cohort study[Text Word])) OR (incidence study[Text Word])) OR (longitudinal study[Text Word])) OR (clinical trial[Text Word])) OR (randomized controlled trials as topic[MeSH Terms])) OR (randomized controlled trials as topic[MeSH Terms])) OR (cohort studies[MeSH Terms])) OR (controlled clinical trials as topic[MeSH Terms])) OR (longitudinal studies[MeSH Terms])) OR (clinical trials as topic[MeSH Terms])
Once the six searches were completed, the items were combined giving rise to four additional searches, the process being concluded with search #11 which produced to 398 articles.
Combined search:
#1 AND #2 = #7
#7 AND #3 = #8
#8 AND #4 = #9
#9AND #5 = #10
#10 AND #6 = #11