We assessed pain, acceptability, patient preference, and tolerability of patients with psoriasis and psoriatic arthritis after switching guselkumab from a prefilled syringe to One-Press autoinjector pen.
MethodsPatients with psoriasis and psoriatic arthritis treated for at least 6 months with guselkumab syringe were recruited from Jan 2019 to Dec 2022. Gender, age, diagnosis, self-administration, and pain perception of guselkumab prefilled syringe were recorded. At the first visit, patients completed a post-auto-injection syringe questionnaire before starting auto-injection pen administration. After 2 and 6 months of guselkumab self-injection using the One-Press autoinjector pen, patient experience, adherence, preference, pain, and safety of each administration were assessed using post-guselkumab by One-Press autoinjector pen questionnaire.
Results40 patients [psoriasis n=34, psoriatic arthritis n=6] were included. All patients self-administered guselkumab by One-Press autoinjector pen. Pain at the injection site was significantly reduced with the use of the One-Press autoinjector pen. All patients considered that using One-Press autoinjector pen was easier than the syringe, 98% chose the pen as their preferred delivery system.
ConclusionThe One-Press autoinjector pen for guselkumab administration is presented as a preferred option, with a high satisfaction and less painful compared to the administration of guselkumab in a prefilled syringe.
Evaluamos el dolor, satisfacción, preferencia y tolerabilidad de los pacientes con psoriasis y artritis psoriásica tras cambiar guselkumab de jeringa precargada a pluma autoinyectora One-Press.
MétodosSe incluyeron pacientes con psoriasis y artritis psoriásica tratados durante al menos 6 meses con la jeringa de guselkumab entre enero-2019 y diciembre-2022. Se registraron sexo, edad, diagnóstico, autoadministración y percepción del dolor de la jeringa precargada previo al uso de la pluma autoinyectora. Tras 2 y 6 meses de utilizando la pluma autoinyectora One-Press, se evaluó la experiencia, la adherencia, preferencia, dolor y seguridad de la pluma mediante cuestionario.
ResultadosSe incluyeron 40 pacientes [psoriasis n = 34, artritis psoriásica n = 6]. Todos los pacientes se autoadministraron guselkumab usando la pluma autoinyectora One-Press. El dolor en el lugar de la inyección se redujo significativamente con el uso de la pluma autoinyectora One-Press. Todos los pacientes consideraron que el uso de la pluma autoinyectora One-Press era más fácil que la jeringa, el 98% eligió la pluma como su sistema de administración preferido.
ConclusiónEl empleo de la pluma autoinyectora One-Pres se presenta como opción preferida, con alto índice de satisfacción y menos dolorosa que la jeringa precargada.
Guselkumab [GUS] is a human IgG1λ monoclonal antibody that binds selectively to the interleukin 23. It has been approved in adults to treat certain types of autoimmune disorders such as moderate to severe plaque psoriasis (Ps) and active psoriatic arthritis (PsA) in patients who have had an inadequate response or who have been intolerant to a prior disease-modifying antirheumatic drug.1
GUS is administered subcutaneously and is available as a single-use prefilled glass syringe with a 27-gauge, half-inch fixed needle containing 1 mL of a sterile solution of guselkumab [100 mg/mL], for subcutaneous self-injection. To increase the convenience and comfortability of self-administration of GUS as well as to overcome dexterity difficulties, an One-Press autoinjector pen integrated, single-use, disposable autoinjector pen has been developed as a subcutaneous one-touch activation autoinjector pen.2 The One-Press autoinjector pen is easier to administer, incorporates additional usability, and safety features that hide the needle from view to offer improved safety protection than the prefilled syringe and has an ergonomic design to facilitate operation. The self-injection method of administration offers patients control and independence over the injection setting and injection schedule.3
The patients' experience with One-Press autoinjector pen was assessed in 78 psoriasis patients through a validated Self-Injection Assessment Questionnaire [SIAQ] in a phase 3, multicenter, and randomized ORION study. This study is further described in the discussion.4
In July 2022, we included in our Hospital Formulary the GUS One-Press autoinjector pen. We switched all patients with GUS prefilled syringe to GUS One-Press autoinjector pen. In order to facilitate the implementation of this new pharmaceutical form and to reassure patients when faced with the pen option, a cross-sectional study was designed. The primary objectives of this study were to evaluate the patient experience, acceptability, and preferences concerning self-injection with the One-Press autoinjector pen. Additionally, we assessed pain and tolerability associated with switching from the GUS prefilled syringe to the One-Press autoinjector pen in Ps and PsA patients.
MethodsWe conducted a cross-sectional and prospective study to assess the acceptability, patient preference, tolerability, and pain of switching GUS from a prefilled syringe to One-Press autoinjector pen in Ps and PsA patients. Eligible patients aged 18 years or older were selected for study enrollment based on hospital pharmacy dispensation records. We recruited adult patients diagnosed with Ps and PsA, who had been undergoing treatment with the GUS syringe for at least 6 months. Patients must have been self-injecting GUS using prefilled syringe with a GUS dose of 100 mg every 8 weeks. Gender, age, diagnosis, self-administration, and pain perception of GUS syringe were recorded.
During the first visit, patients completed a questionnaire regarding their observations on GUS administration using the prefilled syringe, based on the SIAQ. This is a tool used in the field of healthcare to assess patients' ability comfort, competence, and safety to self-administer injections, particularly in the case of administering injectable medications at home.5 This questionnaire was used for our group in previous studies.6 Patients were instructed from specialists in the Hospital Pharmacy regarding the proper administration of GUS using the One-Press autoinjector pen. Additionally, they were informed about the various features of the pen, such as the drug solution window for preinjection viewing, the sequential opening of safety caps to prevent accidental misfiring, and the correct positioning during injection. Each patient was provided with an educational leaflet, and they were encouraged to read it carefully at home. Moreover, a telephone number was also available to address any concerns or queries that the patients might have.
After 2 and 6 months of self-injection with the One-Press autoinjector pen, we assessed patient experience, pain perception, preference, and safety of each administration using a post-GUS autoinjection pen questionnaire based on the SIAQ.5 The questionnaire utilized a 10-point Likert-type scale to gather ratings after 6 months (3 doses) of GUS administration with the autoinjector pen. Furthermore, patients were asked to indicate their preference between the autoinjector pen and the prefilled syringe. This questionnaire was used for our group in previous studies.6
Pain related to GUS administration by prefilled syringe and autoinjector pen was recorded. A visual analog scale (VAS) was used to assess overall injection site pain within 15 min of completing the self-injections with the prefilled syringe, and the autoinjector pen. Patients were required to indicate their injection pain by placing a mark on a 10 cm line from 0 (absence of pain) to 10 (worst possible pain). Patients completed the VAS prior to the post-injection SIAQ.
Treatment adherence was obtained from the dispensation records of the Hospital Pharmacy Department. Individualized GUS dispensations and correlated dates during the study period were collected using Outpatient Clinic Hospital Pharmacy software DISPENSA [Oncopharm Health Information Technology, Valencia, Spain], which allows dispensing and follow-up of outpatient. The medication possession ratio was calculated based on information extracted from the pharmacy dispensing records.7
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and after approval of the protocol and its amendments by the local Ethics Committee. All patients gave their written informed consent for the auto-self injector pen of GUS and the local ethics committee approved the procedure.
Statistical analysisCategorical data are expressed as absolute frequency and percentage, and continuous data as mean and standard deviation (SD). Differences in the VAS score of the patient's perceived pain at the injection site between the prefilled syringe and the One-Press autoinjector pen were analyzed with the Student's t-test for paired data. Statistical significance was set at p<.05. Statistical analysis was conducted using the SPPS 19.0 working package (SPSS Inc., Chicago, IL).
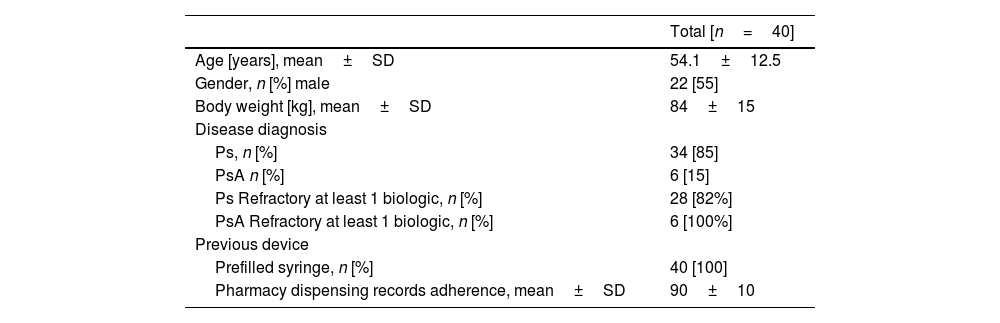
ResultsA total of 40 patients, who were currently receiving treatment with GUS subcutaneous injection via a prefilled syringe, were included in the study. Diagnoses included Ps in 34 patients and PsA in 6 patients, with a medium previous time of GUS use before the switch of at least 6 months. There were 22 men and 18 women, with a mean age of 54±12.5 years. All patients switched to self-administering GUS using the One-Press autoinjector pen, ensuring the full dose of GUS was effectively injected. Mean adherence for previous treatment or GUS measured by dispensing pharmacy records at all patients was 90±10%. In 6 Ps patients, GUS was their first biologic, and 28 Ps patients are refractory at least 1 biologic drug. All PsA patients were treated previously with 1 biological drug (Table 1).
Characteristics of the patients that used One-Press autoinjector pen.
| Total [n=40] | |
|---|---|
| Age [years], mean±SD | 54.1±12.5 |
| Gender, n [%] male | 22 [55] |
| Body weight [kg], mean±SD | 84±15 |
| Disease diagnosis | |
| Ps, n [%] | 34 [85] |
| PsA n [%] | 6 [15] |
| Ps Refractory at least 1 biologic, n [%] | 28 [82%] |
| PsA Refractory at least 1 biologic, n [%] | 6 [100%] |
| Previous device | |
| Prefilled syringe, n [%] | 40 [100] |
| Pharmacy dispensing records adherence, mean±SD | 90±10 |
Ps: psoriasis; PsA: psoriatic arthritis.
At the 2-month visit, all patients switched to the One-Press device pen and continued with GUS 100 mg/Q8W. During the next 6-month visit, 39 patients who switched from the syringe to the One-Press device pen also continued with GUS 100 mg/Q8W. Any patient returned to the prefilled syringe due to self-administration problems with the One-Press device pen and only 1 patient stopped GUS due to PsA reactivation.
Patient mean adherence to GUS was 91.2% after 6 months. This assessment was based on individualized drug dispensations and correlated dates during the study period were collected from the Outpatient Clinic Hospital Pharmacy database. Throughout the study, all patients successfully self-administered GUS using the One-Press autoinjector pen and consistently injected the full dose in each administration.
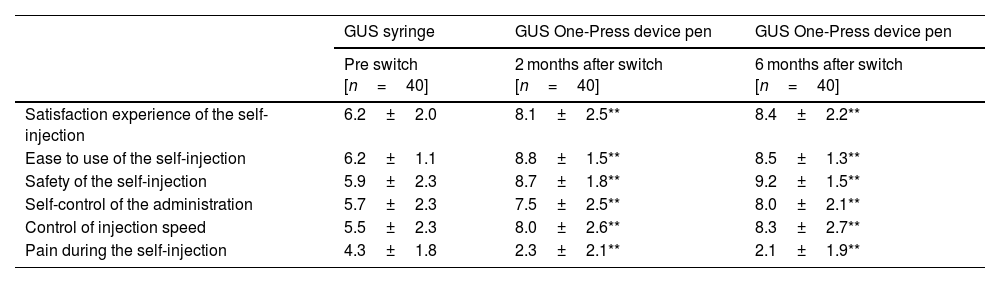
Patients reported a high level of satisfaction with the self-injection experience using the One-Press autoinjector pen compared to the syringe at both the 2- and 6-month follow-up. In terms of ease of use, patients indicated that the One-Press autoinjector pen was preferred over the syringe and perceived more safety during the self-injection process in comparison to self-injection with the prefilled syringe (Table 2).
Patient experience from the pre-injection [syringe] and post-injection [One-Press autoinjector pen] Assessment of Self-Injection questionnaire after 2 and 6 months of patient self-injection of GUS via One-Press autoinjector pen.
| GUS syringe | GUS One-Press device pen | GUS One-Press device pen | |
|---|---|---|---|
| Pre switch [n=40] | 2 months after switch [n=40] | 6 months after switch [n=40] | |
| Satisfaction experience of the self-injection | 6.2±2.0 | 8.1±2.5** | 8.4±2.2** |
| Ease to use of the self-injection | 6.2±1.1 | 8.8±1.5** | 8.5±1.3** |
| Safety of the self-injection | 5.9±2.3 | 8.7±1.8** | 9.2±1.5** |
| Self-control of the administration | 5.7±2.3 | 7.5±2.5** | 8.0±2.1** |
| Control of injection speed | 5.5±2.3 | 8.0±2.6** | 8.3±2.7** |
| Pain during the self-injection | 4.3±1.8 | 2.3±2.1** | 2.1±1.9** |
* All parameters were evaluated with a 0–10 scale. ** p<.05].
Overall, patients scored the One-Press autoinjector pen favorably across all SIAQ4 domains following each injection, when compared to both the prefilled syringe and the One-Press autoinjector pen. An analysis of the individual added value questions for the One-Press autoinjector pen revealed that patients highly scored the device attributes, such as control of injection speed and administration, with ratings ranging from 8.0 to 9.2.
Furthermore, all patients agreed that the One-Press autoinjector pen was easier to use compared to the syringe. 39 out of 40 patients [98%] expressed a clear preference for the One-Press autoinjector pen as their preferred delivery system. Only 1 patient indicated an equal preference for both the subcutaneous One-Press autoinjector pen and the prefilled syringe (Table 2).
Pain at the injection site was significantly reduced with the use of One-Press autoinjector pen in comparison to the prefilled syringe. The mean VAS score (SD) was 4.4±1.9 compared with 2.3±2.1 after 2 months of One-Press autoinjector pen use and 2.1±1.9 after 6 months of One-Press autoinjector pen use (p<.05) (Table 2). No safety-related findings were identified in relation to the administration of GUS using the One-Press device pen.
DiscussionThe present study was conducted in 40 patients with dermatology and rheumatology chronic conditions who were receiving IL23 therapy with GUS. The results of the study demonstrate that the One-Press autoinjector pen was well-received and preferred as the delivery system by the patients. They found the One-Press autoinjector pen easier to use compared to the prefilled syringe and expressed a high level of confidence, along with a great degree of satisfaction. This could help reduce non-adherence due to forgetfulness and facilitate patient self-administration empowerment. After 6 months of using the One-Press autoinjector pen, 98% of the patients preferred it over their previous prefilled syringe, resulting in increased patient confidence and satisfaction with both the pen and the GUS treatment.
There were no mechanical problems with One-Press autoinjector pen occurred during the study, there were no safety-related findings related to GUS One-Press autoinjector pen administration Throughout the study period, there were no mechanical issues reported with the One-Press autoinjector pen, this indicates its reliability, use, functional and structural integrity. Furthermore, no safety-related findings were identified in relation to the administration of GUS using the One-Press autoinjector pen.
This study is the first to report the switch and use of the GUS One-Press autoinjector pen, comparing ease of use, usability, and preference for the pen among dermatology and rheumatology GUS patients with previous experience with the prefilled syringe in real-world standard practice during a 6-month follow-up.
These results are comparable to a previously published in a Phase 3, multicentric, double-blind, placebo-controlled study (ORION, Clinicaltrials.gov identifier-NCT02905331) randomized adults with moderate-to-severe Ps that evaluated the One-Press usability/acceptability using the SIAQ and Patient-Controlled Injection Device Questionnaire. The SIAQ questionnaire evaluated the patient experience at weeks 0, 4, and 12 on a scale of 0 (worst) to 10 (best) across 6 domains (feelings about injections, self-image, self-confidence, pain, skin reactions during or after the injection, the ease of use of the self-injector device, and satisfaction with self-injection). The mean score for SIAQ was 9.2 and the mean score for “easy-to-use” was 9.2 (with 10 indicating “very satisfied”). SIAQ results demonstrated 99% (68/69) of patients were satisfied/very satisfied with One-Press at week 28. Most patients rated the One-Press autoinjector pen as easy/very easy to use (87%–100%) and were satisfied/very satisfied with their One-Press experience (81%–100%).4
PsA can affect the joints of the hands, making it difficult for patients to use a pen to administer medications. In our study, patients with PsA have not present problems related to pen use and reported a high level of satisfaction with the One-Press pen. Patients found the pen to be easy to use, convenient, and painless. Probably because of the educational support provided by hospital pharmacists on how to use and manage the One-Press pen. Although our study included a small sample of only 6 patients with PsA, further research with a larger cohort is warranted to confirm these findings.
In a study carried out in Spain in daily practice conditions, 66 patients were interviewed regarding preferences and injection-site pain after switching to the adalimumab autoinjector pen, and mean VAS score was significantly reduced from 3.5 for the prefilled syringe compared with 2.0 for the autoinjector pen (p<.001) and 96.1% chose the pen as their preferred delivery system.9
Pain is one of the main factors that can influence patients' satisfaction with self-administration devices and can be driven by characteristics such as needle diameter and sharpness, speed of injection, and injection volume.8 The training and patient education on a new device by a hospital pharmacist is associated with less pain during anti-TNF drug administration.4,9,10 In addition, GUS is dispensed through the hospital pharmacy outpatient clinic in our country. The hospital pharmacist has a very important role in educating and training the patient who is going to self-inject GUS at home, as well as in solving problems associated with use of injection devices. Patients in this study reported a significantly less pain VAS scores during GUS administration with the One-Press autoinjector pen was shown in comparison to the prefilled syringe, indicating that injection site pain was low and self-injection was well tolerated using One-Press autoinjector pen. These results confirmed the value of the participation of the hospital pharmacist in patient training device administration when a switch from administration devices.
All domain subscores from the post-injection SIAQ (‘feelings about self-injection’, ‘self-image’, ‘self-confidence’, ‘injection site reactions’, ‘ease of use’, and ‘satisfaction with self-injection’) were also high with mean scores above 8 for both the One-Press autoinjector pen at month 2 and 6. The high post-injection SIAQ scores with the One-Press autoinjector pen at both visits indicate that patients had an overall positive self-injection experience. The One-Press autoinjector pen's convenience and ease of use may improve adherence and, therefore outcomes, in patients with Ps and PsA receiving GUS by subcutaneous route.
The present study was conducted under conditions of daily clinical practice. However, it is important to interpret the results considering some limitations: the study had an open-label and single-arm design, a relatively small number of patients [n=40], and absence of a control group to evaluate the GUS administration by the prefilled syringe administration. We planned perform a new real world study with a cross-over design, involving a larger number of patients and a longer follow-up period to confirm the findings obtained from the current open-label, cross-sectional, and prospective study.
In conclusion, patients with Ps and PsA, who were receiving subcutaneous GUS treatment via the prefilled syringe, reported a positive response, satisfaction, less pain, and preference after switching to the One-Press autoinjector pen.The autoinjector pen rapidly became the preferred device and may improve the overall patient experience. Based on our results, we believe that the One-Press autoinjector pen is an advantageous, safe, and effective delivery option for GUS. This study provides further evidence to support that the One-Press autoinjector pen is a valid method for switching GUS from syringe with high preference in patients with Ps and PsA. Further real-world studies with more patients and long-term follow-up are needed to confirm the clinical practice value of the One-Press autoinjector pen in GUS Ps and PsA patients.
FundingThe authors received no financial support for the research, authorship, and/or publication of this article.
Ethical disclosureNo ethical issues.
CRediT authorship contribution statementJoaquin Borrás-Blasco: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Rebeca Alcalá García: Visualization, Methodology, Investigation, Data curation, Conceptualization. Silvia Cornejo-Uixeda: Writing – review & editing, Writing – original draft, Project administration, Investigation, Formal analysis. Maria Matellanes-Palacios: Validation, Methodology, Conceptualization. Elvira Casterá-Melchor: Validation, Supervision.