To determine the effectiveness in terms of quality of life perceived by adult patients with moderate/severe plaque psoriasis treated with interleukin 17 or 23 inhibitors and to identify associated factors.
MethodCross-sectional observational study including adult patients diagnosed with moderate/severe plaque psoriasis treated with interleukin 17 or 23 inhibitors for at least 12 or 16 weeks in follow-up, respectively.
ResultsForty-one patients were included: 65% male, median age 54 years (SD=13). The included patients were treated with ixekizumab 35%, guselkumab 25%, secukinumab 17.5%, brodalumab 15%, and risankizumab 7.5%.
Psoariasis area severity index (PASI) reduction was 94.6% (RIC 76.8–100%), DLQI of 1 (RIC 0–2.75), DLQI≤1 60%. The most affected health dimensions were symptoms and perceptions (57.5%), activities of daily living (27.5%), and discomfort caused with treatment (17.5%). No association was found between DLQI score <1 and demographic, comorbidities, and treatment-related variables. The median PASI reduction in patients with DLQI<1 was superior to patients with DLQI>1 (100% vs 90.2%, p=.025).
ConclusionsPatients with moderate/severe plaque psoriasis treated with interleukin 17 or 23 inhibitors achieve adequate therapeutic targets achieving the target set according to clinical practice guideline recommendations (score ≤1 on the DLQI questionnaire and 90–100% reduction in the PASI index) and in accordance with the results of recent meta-analyses and real-life studies.
A greater reduction of the PASI index is observed in the group reaching the quality of life target, there being the possibility of using patient-reported outcomes in the evaluation of treatment effectiveness.
Determinar la efectividad en términos de calidad de vida percibida por pacientes adultos con psoriasis en placas moderada/grave tratados con inhibidores de interleucinas 17 o 23 e identificar factores asociados.
MétodoEstudio observacional transversal que incluyó pacientes adultos diagnosticados de psoriasis en placa moderada/grave en tratamiento con inhibidores de interleucinas 17 o 23 durante al menos 12 o 16 semanas en seguimiento, respectivamente.
ResultadosSe incluyeron 41 pacientes: 65% hombres, edad media 54 años (DE = 13). Los pacientes incluidos fueron tratados con ixekizumab 35%, guselkumab 25%, secukinumab 17,5%, brodalumab 15% y risankizumab 7,5%.
La reducción del índice de gravedad y área de la psoriasis, PASI, fue del 94,6% (RIC 76,8–100%), el índice Dermatology Life Quality Index, DLQI, de 1 (RIC 0–2,75), DLQI≤1 60%. Las dimensiones de salud más afectadas fueron síntomas y percepciones (57,5%), actividades de la vida diaria (27,5%) y molestias ocasionadas con el tratamiento (17,5%). No se encontró asociación entre la puntuación DLQI≤1 y variables demográficas, comorbilidades y relacionadas con el tratamiento. La mediana de reducción de PASI en los pacientes con DLQI≤1 fue superior a los pacientes con DLQI>1 (100% vs 90,2%, p = 0,025).
ConclusionesLos pacientes con psoriasis en placa moderada/grave tratados con inhibidores de interleucinas 17 o 23 consiguen objetivos terapéuticos adecuados logrando el objetivo fijado según las recomendaciones de las guías de práctica clínica (puntuación ≤1 en el cuestionario DLQI y reducción del 90–100% del índice PASI) y de acuerdo con los resultados de recientes metaanálisis y estudios de vida real. Se observa una mayor reducción del índice PASI en el grupo que alcanza el objetivo de calidad de vida, existiendo la posibilidad de utilizar los resultados reportados por el paciente en la evaluación de la efectividad de los tratamientos.
Psoriasis is an immune-mediated dermatological disease with an irregular and chronic course. In Spain, it has an estimated prevalence of 2.3%.1
The most common type is plaque psoriasis, and may be considered moderate or severe in 30% of cases.2
Psoriasis often co-occurs with other conditions such as metabolic syndrome, arthritis, and psychiatric disorders, putting these patients at increased physical and psychological risk.3 As a result, there is a significant impact on quality of life (QoL). An estimated 75% of patients feel that the disease has a negative impact on their QoL.4,5 Several studies have found associations between QoL and factors such as the Psoriasis Area Severity Index (PASI), age, gender, presence of psoriatic arthritis, or substance abuse.6–8
Non-adherence to treatment is another factor that may influence health outcomes and therefore QoL. Adherence refers to the extent to which patients follow the prescribed dosing interval and dosage of a treatment regimen. Good adherence is typically defined as achieving a Medication Possession Ratio (MPR) of 80% or higher. Psoriasis studies have found that adherence rates are suboptimal (around 60%).9,10
In recent years, Patient Reported Outcomes (PRO) have been incorporated into healthcare practice. These are defined as outcomes reported by the patient, reflecting their perception of the disease and/or its treatment. Health-related QoL has been identified as a specific PRO measure in the context of psoriasis. This factor is used to assess several dimensions of patients' lives, encompassing the effects of disease and treatment on activities of daily living (ADLs), well-being, as well as physiological, physical, and social function.11 In the field of dermatology, the Dermatology Life Quality Index (DLQI) is the most widely used tool for assessing QoL.12,13
This index is used in clinical practice to assess the severity of psoriasis in combination with other indices such as the Psoriasis Area and Severity Index (PASI). Scores equal to or greater than 10 on these indexes suggest the presence of moderate-to-severe psoriasis (MSPP).14
Interleukin 17 (IL-17) inhibitors (secukinumab, ixekizumab, and brodalumab) and interleukin 23 (IL-23) inhibitors (guselkumab, risankizumab, and tildrakizumab) are the most recent additions to the therapeutic options for the treatment of MSPP. They have been shown to have good efficacy and safety profiles.14
The main objective of this study was to determine the effectiveness of IL-17 and IL-23 inhibitors in adult patients with MSPP using patient-reported QoL and disease severity as assessed by the PASI after at least 12 or 16 weeks of treatment, respectively. The secondary objective was to identify factors related to patient demographics, comorbidities, substance abuse, previous and current treatment, and disease severity that affect patient-reported QoL.
MethodWe conducted a cross-sectional study between November 2021 and April 2022. Inclusion criteria were as follows: adult patients (minimum age 18 years), diagnosed with MSPP, and on treatment with IL-17 inhibitors (secukinumab, ixekizumab, and brodalumab) or IL-23 inhibitors (guselkumab and risankizumab), on maintenance therapy (at least 12 and 16 weeks of treatment, respectively), and being followed up in the dermatology and pharmacy care clinics of a tertiary care hospital. Patients in social and medical centres were excluded because they could not be interviewed.
Data were collected on the following variables: demographic, comorbidities (obesity, metabolic syndrome, psychiatric comorbidities, substance abuse, psoriatic arthritis), treatment history (previous biologic treatment, dosage according to technical data sheets, adherence, and time on treatment with IL-17 or IL-23 inhibitors), and effectiveness in terms of QoL (DLQI score and dimensions affected), and reduction in PASI scores. All these variables, except for the DLQI score, were obtained from the electronic medical record.
Metabolic syndrome was defined as meeting at least three criteria (obesity, hypertension, dyslipidaemia, or diabetes) according to the National Cholesterol Education Program Adult Treatment Panel-III.15
Adherence to treatment was measured as the MPR calculated as the number of days for which medication was dispensed compared to the number of days in that period.
The DLQI (Appendix A) scores were obtained through interviews with patients during the dispensing process at the pharmacy care clinic. Patients who did not collect their medication in person during the study period were interviewed by telephone.
The DLQI comprises 10 questions on the patients' perceptions of the impact of psoriasis over the last week.14,15 The 10 items are scored on a 4-point Likert scale (“very much”, “a lot”, “a little”, “not at all”) with scores ranging from 3 to 0. There is a “not applicable” option. The index includes the following dimensions distributed across the various questions: symptoms and perceptions (1–2), ADLs (3–4), leisure (5–6), work/study (7), interpersonal relationships including sexuality (8–9), and treatment (10). The final score is obtained by adding the scores for each question, ranging from 0 (minimum impact on QoL) to 30 points (maximum impact). We also assessed the response rate to the questionnaire.
The PASI6,7 was used to assess the severity of lesions based on 4 clinical parameters (itching, erythema, scaling, and induration), each related on a severity scale ranging from 0 to 4, as well as the percentage of skin involved on the four body region (head, arms, trunk, legs) using a scale ranging from 0 to 6. The sum of the 4 severity parameters for each body region is multiplied by the area estimate for the respective region and its weight factor (0.1 head, 0.2 arms, 0.3 trunk, and 0.4 legs). The sum of scores for each body region provides a total score ranging from 0 (no disease) to 72 (the greatest severity).
All statistical calculations were performed using SPSS statistical software (v. 25). A p-value of <.05 was used as a cut-off for statistical significance. Confidence intervals are presented at the 95% level (CI95%). Normally distributed quantitative variables are expressed as mean and standard deviation (SD), while the remaining variables are expressed as interquartile range (IQR). Depending on the sample size, the normality of each distribution was assessed using the Kolmogorov–Smirnov test or the Shapiro–Wilk test.
Quantitative and qualitative variables were compared using the t test for independent samples (normal distribution), or the Mann–Whitney U test (non-normal distribution). Categorical variables were compared using the chi-squared test.
The estimated prevalence of psoriasis in Spain is 2.3%,1 30% of cases are moderate to severe,2 and approximately 32% of patients are treated with interleukin inhibitors. These data were extrapolated to the population of the study area, resulting in an estimated sample size of 233 patients for a confidence level of 95% and a 5% margin of error.
All patients signed informed consent and the study was approved by the Hospital's Ethics and Research Committee (internal protocol code B-275).
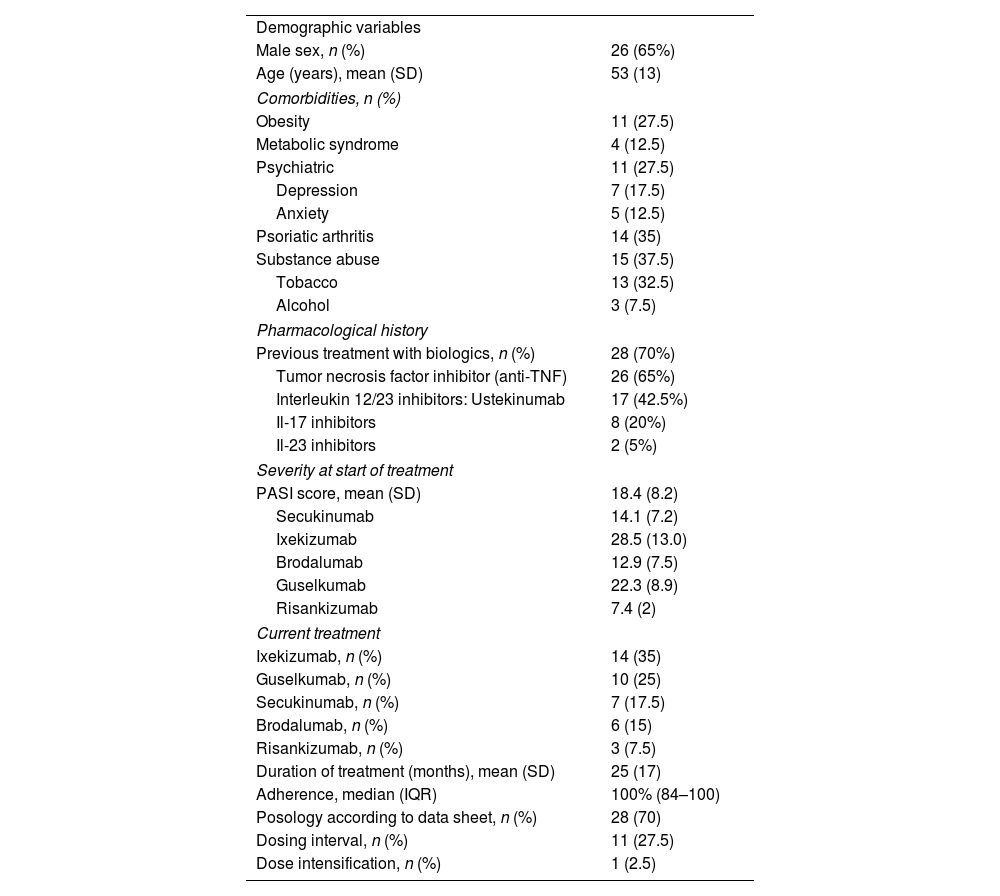
ResultsOf the 41 candidates, 40 patients met the inclusion criteria and the questionnaire was administered to all of them. Three interviews were conducted by telephone and 37 in person resulting in a response rate of 97.6%. Table 1 shows demographic variables, comorbidities, and treatment-related variables.
Demographic, comorbidity, and treatment-related variables.
| Demographic variables | |
| Male sex, n (%) | 26 (65%) |
| Age (years), mean (SD) | 53 (13) |
| Comorbidities, n (%) | |
| Obesity | 11 (27.5) |
| Metabolic syndrome | 4 (12.5) |
| Psychiatric | 11 (27.5) |
| Depression | 7 (17.5) |
| Anxiety | 5 (12.5) |
| Psoriatic arthritis | 14 (35) |
| Substance abuse | 15 (37.5) |
| Tobacco | 13 (32.5) |
| Alcohol | 3 (7.5) |
| Pharmacological history | |
| Previous treatment with biologics, n (%) | 28 (70%) |
| Tumor necrosis factor inhibitor (anti-TNF) | 26 (65%) |
| Interleukin 12/23 inhibitors: Ustekinumab | 17 (42.5%) |
| Il-17 inhibitors | 8 (20%) |
| Il-23 inhibitors | 2 (5%) |
| Severity at start of treatment | |
| PASI score, mean (SD) | 18.4 (8.2) |
| Secukinumab | 14.1 (7.2) |
| Ixekizumab | 28.5 (13.0) |
| Brodalumab | 12.9 (7.5) |
| Guselkumab | 22.3 (8.9) |
| Risankizumab | 7.4 (2) |
| Current treatment | |
| Ixekizumab, n (%) | 14 (35) |
| Guselkumab, n (%) | 10 (25) |
| Secukinumab, n (%) | 7 (17.5) |
| Brodalumab, n (%) | 6 (15) |
| Risankizumab, n (%) | 3 (7.5) |
| Duration of treatment (months), mean (SD) | 25 (17) |
| Adherence, median (IQR) | 100% (84–100) |
| Posology according to data sheet, n (%) | 28 (70) |
| Dosing interval, n (%) | 11 (27.5) |
| Dose intensification, n (%) | 1 (2.5) |
Abbreviation: IQR, interquartile range; PASI, Psoriasis Area Severity Index.
Study patients were predominantly male (65%) with a mean age of 53 years (SD=13). Comorbidities were tobacco and/or alcohol abuse (37.5%), psoriatic arthritis (35%), obesity (27.5%), anxiety and/or depression (27.5%), and metabolic syndrome (12.5%). Overall, 70% of the patients had previously been treated with biologics and 65% had received an anti-TNF drug. The baseline PASI severity score was 18.4 (SD 8.2). Biologic treatments included ixekizumab (35%), guselkumab (25%), secukinumab (17.5%), brodalumab (15%), and risankizumab (7.5%). Median treatment time was 25 months (SD=17) and median adherence was 100% (IQR 84–100).
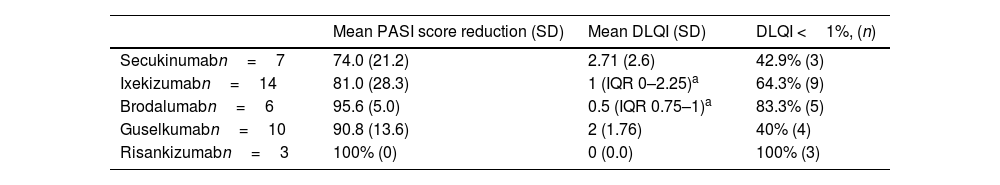
We observed a 94.6% reduction in PASI scores (IQR 76.8–100%) and a median DLQI score of 1 (IQR 0–2.75). Overall, DLQI scores were less than 1 in 60% of patients. Table 2 shows the effectiveness parameters grouped by drug.
Effectiveness of IL-17 and IL-23 inhibitors in the treatment of moderate-to-severe psoriasis.
| Mean PASI score reduction (SD) | Mean DLQI (SD) | DLQI <1%, (n) | |
|---|---|---|---|
| Secukinumabn=7 | 74.0 (21.2) | 2.71 (2.6) | 42.9% (3) |
| Ixekizumabn=14 | 81.0 (28.3) | 1 (IQR 0–2.25)a | 64.3% (9) |
| Brodalumabn=6 | 95.6 (5.0) | 0.5 (IQR 0.75–1)a | 83.3% (5) |
| Guselkumabn=10 | 90.8 (13.6) | 2 (1.76) | 40% (4) |
| Risankizumabn=3 | 100% (0) | 0 (0.0) | 100% (3) |
Abbreviations: IQR, interquartile range; PASI, Psoriasis Area Severity Index; DLQI, Dermatology Life Quality Index.
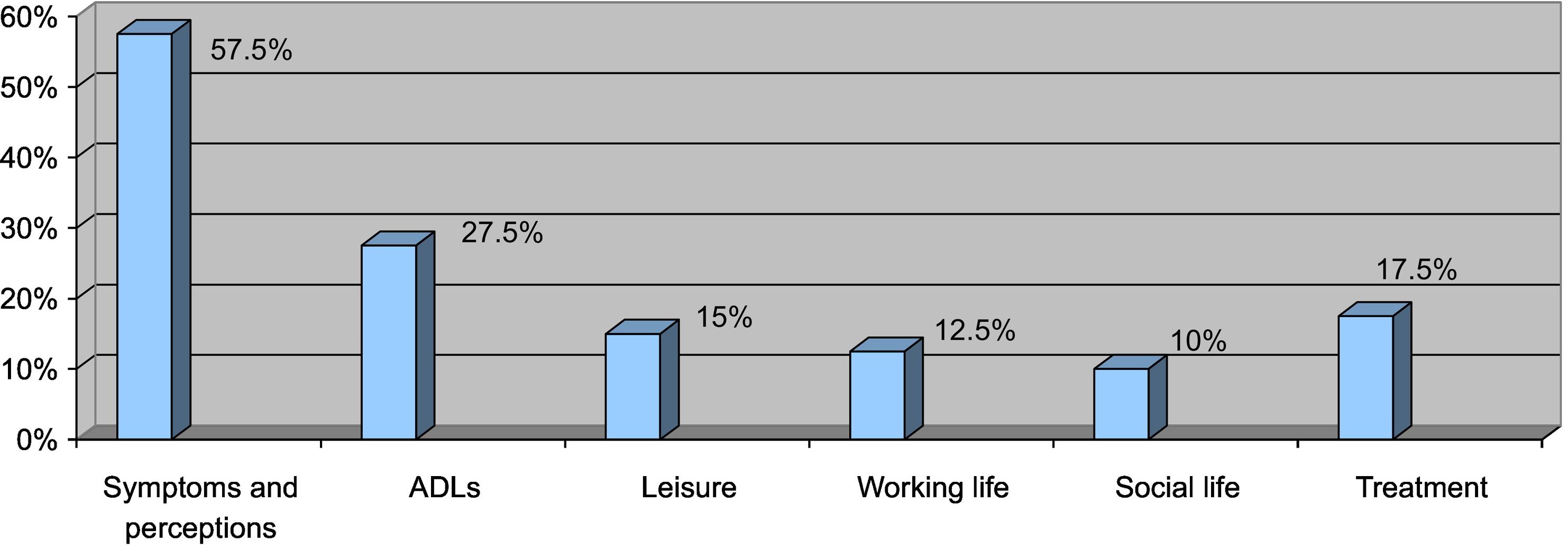
The most-affected health dimensions were symptoms and perceptions (57.5%), ADLs (27.5%), and discomfort caused by treatment (17.5%) (Fig. 1).
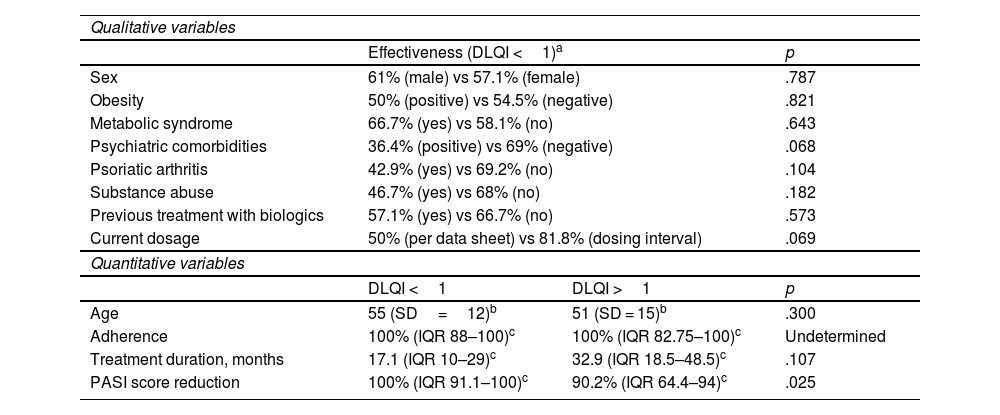
No association was found between a DLQI score <1 and demographic, comorbidities, or treatment-related variables.
The median PASI score reduction in patients with a DLQI score <1 was greater than in patients with a DLQI score >1 (100% vs 90.2%, p=.025). Table 3 presents the results.
Association between DLQI and each variable.
| Qualitative variables | |||
| Effectiveness (DLQI <1)a | p | ||
| Sex | 61% (male) vs 57.1% (female) | .787 | |
| Obesity | 50% (positive) vs 54.5% (negative) | .821 | |
| Metabolic syndrome | 66.7% (yes) vs 58.1% (no) | .643 | |
| Psychiatric comorbidities | 36.4% (positive) vs 69% (negative) | .068 | |
| Psoriatic arthritis | 42.9% (yes) vs 69.2% (no) | .104 | |
| Substance abuse | 46.7% (yes) vs 68% (no) | .182 | |
| Previous treatment with biologics | 57.1% (yes) vs 66.7% (no) | .573 | |
| Current dosage | 50% (per data sheet) vs 81.8% (dosing interval) | .069 | |
| Quantitative variables | |||
| DLQI <1 | DLQI >1 | p | |
| Age | 55 (SD=12)b | 51 (SD = 15)b | .300 |
| Adherence | 100% (IQR 88–100)c | 100% (IQR 82.75–100)c | Undetermined |
| Treatment duration, months | 17.1 (IQR 10–29)c | 32.9 (IQR 18.5–48.5)c | .107 |
| PASI score reduction | 100% (IQR 91.1–100)c | 90.2% (IQR 64.4–94)c | .025 |
Abbreviations: DLQI, Dermatology Life Quality Index; IQR, interquartile range; PASI, Psoriasis Area Severity Index.
The results indicate that IL-17 and IL-23 inhibitors are effective treatments for MSPP, meeting the targets set outlined in clinical practice guideline recommendations (DLQI score ≤1 and 90–100% reduction in PASI scores).3,16 These results are in line with those of recent meta-analyses and real-world studies.17–21
More than half of the patients achieved the DLQI target score. These results are consistent with those of other studies in which approximately 60% of patients achieved a DLQI score <1. Patients in pivotal clinical trials achieved DLQI target rates of 59% for secukinumab,22 64% for ixekizumab,23 56–61% for brodalumab,24 51–56% for guselkumab,25 and 66% for risankinzumab.2
DLQI target rates of 60% and 57% were observed in 2 real-world studies involving treatments with secukinumab and guselkumab over 24 and 28 weeks, respectively.17,18 However, lower percentages were observed in these studies at 12 and 16 weeks. Currently, there are no direct comparisons of interleukin IL-17 and IL-23 inhibitors, so we have to rely on network meta-analyses with indirect comparisons. These analyses have shown that these 2 drugs are the most effective in terms of improving QoL. The network meta-analysis conducted by Mahil et al. investigated tildrakizumab, guselkumab, and secukinumab,19 while the analysis by Sbidian et al. focussed on risankizumab and ixekizumab.20 Although the present study was limited by the small sample size, the results show that the top-ranked drugs in terms of QoL were risankizumab and brodalumab.
The high ranking of brodalumab may be related to its rapid onset of action, leading to earlier improvements in QoL.21 However, the pre-treatment PASI score of these patients was lower than that of the other study patients, which could also contribute to the higher QoL ranking observed.
Despite the effectiveness observed in terms of measured QoL, more than half of the participants reported an effect on symptoms and perceptions (57.5%), while just over a quarter of the participants experienced an effect on ADLs (27.5%). Some participants also considered that treatment with IL-17 or IL-23 inhibitors caused them problems.
These results are in line with the conclusions of the Reto 6391 study (2008–2009), which showed that psoriasis negatively affects both physical health and social relationships, and has a strong impact on self-perception.5 Impact on QoL remains an unresolved issue in immune-mediated diseases. Other studies have shown that, despite achieving disease control, QoL is lower than in the general population.26
A wide range of questionnaires have been developed to assess QoL in general dermatology and specifically in psoriasis.5,13 The DLQI is the most widely used because of its ease of use. This index was developed in 1994, hence some of its items may not accurately reflect the current experience of patients with psoriasis treated with biologics. The DLQI mainly focuses on the discomfort caused by topical treatments that are currently indicated for mild forms of psoriasis or as an adjunct to systemic therapy. Furthermore, as the questionnaire refers to the last 7 days, it may not provide a reliable picture of QoL in patients with chronic diseases. In this sense, the results may easily be influenced by seasonal or ad hoc factors. Such bias could be addressed by using this scale in long-term studies assessing the effect of these treatments on patients' QoL. Its routine application in regular clinical practice could also be considered.
The DLQI is also limited by the significant floor effect (percentage of patients scoring at the lowest extreme values) and its low sensitivity to change. This translates into challenges in detecting changes in QoL when the severity of the clinical condition varies.13,14 It might be of interest to review other QoL questionnaires5 for use in routine clinical practice.
Several factors typically associated with QoL were observed in participants, including obesity and metabolic syndrome, psychiatric comorbidities such as anxiety and depression, psoriatic arthritis, and patterns of tobacco and alcohol use. However, no association was found between these factors and QoL.
Previous studies have found that QoL is lower in women and young patients, individuals with psoriatic arthritis or comorbidities (mainly psychiatric), and in tobacco or alcohol users, whereas QoL is generally better in patients treated with biologics, including IL-17 inhibitors.13,27
Although biologics appear to be more effective in reducing depression, there is a lack of robust comparative data. Secukinumab and ixekizumab have demonstrated improvements in patients' QoL, with a 40% reduction in depressive symptoms. In the case of brodalumab, the available evidence suggests cautious use in patients with depression and its discontinuation if depressive symptoms occur. However, a causal relationship between this drug and suicide risk has not been confirmed.28,29 In this study, although not reaching statistical significance, we found that the percentage of patients with a DLQI <1 was lower in patients with psychiatric comorbidities, consistent with the aforementioned association. However, as there are no previous data on depression before starting treatment with IL inhibitors, it was not possible to establish a direct association between the use of these drugs and an improvement in depressive symptoms.
Patients with obesity had a worse response to the drugs studied.29
Adherence to treatment merits comment. In this study, adherence was higher than that reported in the literature,9,10 and it decreased proportionally with the severity of psoriasis. Thus, lower PASI scores and improved QoL due to interleukin inhibitors may have resulted in increased adherence, and vice versa.
In some patients, the dosing interval was increased without any detectable negative impact on QoL. In fact, although not statistically significant, there was a higher percentage of patients with DLQI <1 in the group of patients in which the dosing interval had been increased.
Previous studies on psoriasis and QoL have found a correlation between improvements in QoL and reductions in PASI scores of at least 75% in patients with MSPP receiving biologic therapies.8 However, there is disagreement regarding the degree of correlation between DLQI and PASI scores.13
In this context, clinical trials with secukinumab have found moderate correlations between improvements in PASI scores and improvements in DLQI scores, specifically in the dimensions of ADLs, leisure activities, and symptoms and perceptions.30
In the present study, we observed greater PASI score reductions in the group that reached the QoL target. This finding suggests that PRO may be useful in assessing treatment effectiveness, although the scores should be compared with the baseline DLQI score to verify that the reduction in PASI scores is related to the reduction in DLQI scores and therefore to the improvement in QoL.
As mentioned above, the limitations of the study include those related to the DLQI and its narrow time frame. Therefore, we recommend that future studies employ a variety of tools to assess effectiveness simultaneously. Furthermore, the responses to certain questions, such as those concerning sexuality, may have been influenced by the method of questionnaire completion (i.e. in person or by telephone). In addition, the DLQI score before starting IL inhibitors was unknown; therefore, although we observed good QoL in the patients, it cannot be directly associated with the use of these drugs. Finally, we must highlight the small sample size of this study, which prevented statistical significance from being achieved. Future research should aim to recruit a larger number of participants by conducting multicentre studies with a larger pool of patients.
Contribution to the scientific literatureDespite the known impact of psoriasis on patients' QoL, there is currently a scarcity of published evidence on this topic, particularly for patients treated with biologics, including IL inhibitors.
By taking advantage of the close relationship between pharmacists and patients with psoriasis, studies such as this one enable us to afford PROs the significance they warrant, thus aiding in the assessment of treatment effectiveness and maximizing clinical benefit.
CRediT authorship contribution statementBárbara Anguita-Montenegro: Writing – original draft. Vera Lucía Areas-del Águila: Writing – original draft, Conceptualization. Elena Palacios-Moya: Writing – original draft, Conceptualization. Mónica García-Arpa: Writing – original draft. María Prado Sánchez-Caminero: Writing – original draft. María Luque-Jiménez: Writing – original draft, Conceptualization.