Recent systematic reviews and meta-analyses suggest that pharmacists' interventions in asthma patients have a positive impact on health-related outcomes. Nevertheless, the association is not well established and the role of clinical pharmacists is poorly represented, as well as severe asthma patients.
The aim of this overview of systematic reviews is to identify published systematic reviews assessing the impact of pharmacists' interventions on health-related outcomes measured in asthma patients, as well as to describe key components of the interventions, the outcomes assessed and any associations between pharmacists' interventions and health-related outcomes.
MethodsPubMed, Embase, Scopus and the Cochrane Library will be searched from inception to December 2022. Systematic reviews of all study designs, severity of asthma and level of care that measured health-related outcomes will be considered. Methodological quality will be assessed using A Measurement Tool to Assess Systematic Reviews 2. Two independent investigators will perform the study selection, quality assessment and data collection, any discrepancy will be solved by a third investigator. Both narrative findings and meta-analysis of primary study data included in the systematic reviews will be synthesised. If data are appropriate for quantitative synthesis, the measures of association will be expressed as the risk ratio and difference in means.
DiscussionThe first results on the establishment of a multidisciplinary network for the management of asthmatic patients have shown the benefits of integrating different levels of care in disease control and morbidity reduction. Further studies showed benefits in hospital admissions, patients' basal oral corticosteroid dose, exacerbations and quality of life of asthma patients.
A systematic review is the most appropriate design in order to summarise the literature and identify the evidence of the benefits of interventions performed by clinical pharmacists in asthma patients, especially those with severe uncontrolled asthma, and encourage future studies to stablish the role of clinical pharmacists in asthma units.
Registration detailsSystematic review registration number: CRD42022372100.
Las revisiones sistemáticas y metaanálisis recientes sugieren que las intervenciones por parte de farmacéuticos en pacientes asmáticos tienen un impacto positivo en resultados en salud. Sin embargo, la asociación no está bien establecida y el papel de los farmacéuticos clínicos está pobremente representado, así como el de los pacientes con asma grave.
El objetivo de esta revisión de revisiones es identificar revisiones sistemáticas publicadas que evalúen el impacto de las intervenciones farmacéuticas en resultados en salud medidos en pacientes asmáticos, así como describir los componentes clave de las intervenciones, los resultados medidos y cualquier asociación entre las intervenciones farmacéuticas y los resultados en salud medidos.
MétodosSe hará una busqueda en Pubmed, Embase, Scopus y la Cochrane Library desde el primer registro hasta diciembre de 2022. Se considerará la inclusión de revisiones sistemáticas de todo tipo de estudios primarios, severidad del asma o nivel asistencial que midan resultados en salud. La calidad metodológica se medirá usando “A Measurement Tool to Assess Systematic Reviews 2”. Dos investigadores independientes realizarán la selección de los estudios, la evaluación de la calidad y la extracción de datos. Cualquier discrepancia será solventada por un tercer investigador. Ambos resultados, narrativos y metaanálisis de los estudios primarios incluidos en las revisiones sistemáticas serán sintetizados. Si los datos son apropiados para un análisis cuantitativo, las medidas de asociación se expresarán como cociente de riesgos y diferencia de medias.
DiscusiónLos primeros resultados del establecimiento de una red multidisciplinar para el manejo de los pacientes asmáticos mostraron beneficios en integrar los diferentes niveles asistenciales en el control de la enfermedad y la reducción de la morbilidad. Estudios posteriores mostraron beneficios en los ingresos hospitalarios, la dosis de corticosteroides orales basal, exacerbaciones y la calidad de vida de los pacientes.
La revisión sistemática es el diseño mas apropiado para resumir la literatura e identificar la evidencia de los beneficios de las intervenciones llevadas a cabo por farmacéuticos clínicos en pacientes asmáticos, especialmente aquellos con asma grave no controlada, y alentar futuros estudios para establecer el papel de los farmacéuticos clínicos en las unidades de asma.
RegistroNumero de registro de revision sistemática: CRD42022372100.
Asthma is one of the main non-communicable diseases, which are the leading cause of death worldwide and represent an emerging global health threat.1 It is a common, chronic, and heterogeneous inflammatory disease of the airways, affecting over 300 million people worldwide.2
The severity of the pathology can be very variable, being most of the cases mild or moderate. However, approximately 5% of the patients with asthma are affected by the severe form of the disease, which is accountable for a large component of the overall disease burden,3 including economic4 and psychological burden,5 as well as impact on the quality of life.6
Over the last 10 years, the management of asthma has deeply changed towards precision medicine. Different monoclonal antibodies targeting distinctive molecules with a key role in asthma were developed and are indicated for severe uncontrolled asthma. The use of these new drugs has brought patients with severe uncontrolled asthma to the outpatient consultation of clinical pharmacists at the hospitals, giving them an important role in the multidisciplinary teams that form the Asthma Units.
Previous primary research studies have evaluated the effect of pharmaceutical care for asthma patients on patient-related outcomes and health-related problems. However, interpreting the evidence related to pharmacists' interventions can be a challenge due to the variation in study designs, patients included, interventions, and settings. There are also systematic reviews and meta-analyses published in recent years that suggest that pharmacists' interventions have a positive impact on asthma control, severity and symptoms and medication adherence.7,8 Nevertheless, the role of clinical pharmacists is poorly represented, most of the patients included present mild to moderate asthma, and with the commercialization of the monoclonal antibodies the treatment has become much more complex.
An overview of systematic reviews summarising existing research and highlighting the absence of evidence can add value by improving access to specific information and supporting decision-making by clinicians, policy makers and developers of clinical guidelines. In addition, the Cochrane Collaboration recommends an overview of systematic reviews to summarise the evidence of existing systematic reviews that address different outcomes for a single intervention.9
The main objective of this umbrella review is to identify published systematic reviews assessing the impact of pharmacists' interventions on health-related outcomes measured in asthma patients, as well as to describe key components of the interventions, the outcomes assessed and any associations between pharmacists' interventions and health-related outcomes in asthma patients.
Secondary objectives are to assess the participation of clinical pharmacists in the pharmacists' interventions reported and the presence of severe asthma patients in the primary studies.
MethodsThis protocol was developed following the Preferred Reported Items for Systematic Review and Meta-analysis Protocols (PRISMA-P),10 and the review will be reported in accordance with the PRISMA statement.11
The inclusion criteria for the systematic review according to PICOS (Population, Intervention, Comparison, Outcome and Study design) will be the following: adult patients with asthma; pharmaceutical care provided at any level of care (hospital, primary care); usual practice or without comparator; patient health-related outcomes, for example: quality of life (QoL), asthma control, therapeutic adherence, lung capacity or inhaler technique; and systematic review with/without meta-analysis.
On the other hand, systematic reviews that do not include patients with asthma, systematic reviews that only report the impact of drugs and systematic reviews that report results of interventions in which pharmacists do not participate will be excluded.
In addition, there will be no date or language restriction, but in order to be included the articles will have to be accessible in full text.
The search strategy was carefully designed by the authors and critically revised by an experienced librarian. The searches will be updated before the review is ready for publication.
A comprehensive search will be performed by two authors including all available articles from inception until December 31st 2022 in databases of peer-reviewed articles and sources of grey literature. Sources of peer-reviewed literature to be searched include: PubMed, Embase, Scopus (Elsevier Science) and Cochrane Library. A combination of Medical Subject Headings (MeSH) and free terms will be used. An example of our search strategy for PubMed is reported in online supplementary appendix 1. Grey literature will be included using Google Scholar, as well as the reference lists of identified relevant articles.
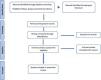
The PRISMA literature search and study selection flowchart is included as Fig. 1.
A peer-review of the literature selected will be performed by two independent investigators and the results will be uploaded to EndNote X9 software. Once the first search results are obtained, duplicate articles will be discarded. Both title and abstract of the selected articles will be reviewed taking into account the previously defined inclusion and exclusion criteria, thus eliminating those that do not meet them. In the case of discrepancies or uncertainty in any review, the full text will be checked and will be resolved by discussion or further consultation with a third reviewer.
With the remaining articles, a reading of the full text will be made in order to analyse them exhaustively, making a table where the excluded articles will be exposed and the reasons for this non-selection will be explained.
Every systematic review included will be assessed for its quality and risk of bias. Two independent reviewers will carry out the assessment using a critical appraisal tool designed for this purpose, A Measurement Tool to Assess Systematic Reviews 2 (AMSTAR 2).12 In case of discrepancies on quality ratings, a common consensus will be reached, with a third reviewer intervening if necessary.
This quality-assessment tool consists of 16 items whose answers can be “yes”, “no” or “partial yes”. The overall quality can be rated as high, moderate, low, and critically low.
Two reviewers will independently extract data from the systematic reviews included and any discrepancies will be solved by discussion or further consultation with a third author. For each systematic review, both general and specific variables will be registered. General variables are author and year of publication, aim of the systematic review, number and design of primary studies, number and type of participants (adult and/or paediatric), asthma severity (mild, moderate or severe), setting (hospital or primary care), funding statements and conflict of interest. Specific variables are QoL, both general or asthma specific questionnaires, asthma control (Asthma control questionnaire (ACQ), asthma control test (ACT) or other questionnaires), therapeutic adherence, lung capacity (measured as Forced expiratory volume in 1 s (FEV1), FEV1/forced vital capacity, Peak expiratory flow volume rate) or inhaler technique.
The data synthesis phase will involve summarising the results in a table indicating the core characteristics of the systematic reviews included: Author and publication year, aim of the systematic review, number and design of the studies, number and type of participants, asthma severity, setting (hospital, primary care) and type of intervention.
Both narrative findings and meta-analysis of primary study data included in the systematic reviews will be synthesised. If data are appropriate for quantitative synthesis, the measures of association between pharmaceutical care interventions and health outcomes will be expressed as the risk ratio (RR) and difference in means (MD), with consistency (I2) reported by individual reviews and meta-analyses.
The following subgroup analyses will be performed if feasible: severe asthma patients, hospital setting and high/moderate quality systematic reviews.
DiscussionAdvances in knowledge and research in asthma have led us to a better understanding of the physiological causes of this disease and its treatment, but it has also shown us many unknowns that still need to be addressed. Given the complexity of the pathology, in the last decade there has been an emerging role of specialised asthma units, integrating multidisciplinary teams, for the management of patients suffering from the severe form of the disease.
The first results on the establishment of a multidisciplinary network for the management of asthmatic patients were published in 2006 and reported the benefits of the implementation of the National Asthma Program in Finland. This paper shows the benefits of integrating different levels of care (pulmonologist, primary care physician, and primary care pharmacist) in disease control and morbidity reduction.13
One of the countries with the greatest experience in the management of asthmatic patients in specialised asthma units is the United Kingdom. A prospective follow-up study was published in 2005, the main objective was to assess the benefit of managing patients with severe asthma in asthma units of the country on QoL and other health variables. This study, which included 346 patients with severe asthma, showed a benefit in terms of reduced visits in primary care and emergency departments, in hospital admissions, in the patients' basal OCS dose, in the number of exacerbations that required short OCS cycles, on the QoL and asthma control of the patients.14
There are several studies showing the benefits of pharmaceutical care in asthma patients, with different endpoints such as QoL, adherence or asthma control. The majority of the studies take place in community pharmacies and involve patients with mild, moderate or severe asthma. However, severe uncontrolled asthma is almost a different entity itself, and these patients do need a higher level of care.
A systematic review is the most appropriate design in order to summarise the literature and identify evidence. This is the protocol of the first umbrella review designed with the aim of identifying published systematic reviews assessing the impact of pharmacists' interventions on health-related outcomes measured in asthma patients. Furthermore, to assess the participation of clinical pharmacists in the pharmacists' interventions reported and the presence of severe asthma patients in the primary studies will encourage future studies for the purpose of stablishing the role of clinical pharmacists in Asthma Units.
However, this review of systematic reviews might not go without limitations, which will be given by the limitations of the systematic reviews included. We expect the results given by the systematic reviews and meta-analysis to be highly heterogeneous, since pharmaceutical care can be very variable. Another limitation could be the quality of the reviews included, nonetheless, in order to minimise bias, a subgroup analysis will be performed with those reviews with high and moderate quality.
Registration detailsSystematic review registration number in PROSPERO: CRD42022372100.
Funding statementThis work was funded by the Fundación Andaluza de Farmacia Hospitalaria. However, the fund was not involved in the design of this protocol, nor in the writing of this manuscript.
Ethics and disseminationEthical approval was not sought for this study because the data to be collected are not linked to individuals. Data will be presented at international conferences and published in peer reviewed journals.
Presentation letterThis manuscript is the protocol of a review of systematic reviews.
It is an original and relevant work, since it summarizes existing research on the effect of pharmaceutical care in asthmatic patients on patients' health-related outcomes, which can add value by improving access to specific information and supporting decision making by physicians, policy makers and guideline developers. It is also designed to highlight the absence of evidence and encourage the design of additional researches. The role of clinical pharmacists in the management of patients with severe asthma is not well established and more studies need to be conducted to generate evidence on their benefits in patients' health-related outcomes, as well as the added value of including clinical pharmacists in the multidisciplinary teams that conform Asthma Units.
Contributions| Author 1 | Author 2 | Author 3 | Author 4 | |
| Concepts | X | X | X | X |
| Design | X | X | X | X |
| Definition of intellectual content | X | X | X | X |
| Literature Search | X | X | - | - |
| Clinical studies | NA | NA | NA | NA |
| Experimental studies | NA | NA | NA | NA |
| Data collection | NA | NA | NA | NA |
| Data analysis | NA | NA | NA | NA |
| Statistical analysis | NA | NA | NA | NA |
| Manuscript preparation | X | X | - | - |
| Editing the manuscript | X | X | X | X |
| Manuscript review | X | X | X | X |
| Warantor | X | X | X | X |
OMP designed the study, led the development of the protocol, and provided oversight and mentorship to FSG, which wrote the first draft, coordinated and integrated the comments of the other authors. ESG and CPG contributed to the development of the search strategy and provided specific expertise. All authors contributed to the selection of variables. All authors critically reviewed successive drafts of the manuscript, provided notes, and approved the final version for the publication.
Responsibility and copyright assignAll authors accept the responsibility defined by the International Committee of Medical Journal Editors (available at http://www.icmje.org/).
The authors assing, in the event of publication, exclusively the rights of reproduction, distribution, translation and public communication (by any means or sound, audiovisual or electronic support) of our work to Farmacia Hospitalaria and by extension to the SEFH. For this purpose, a letter of assignment of rights will be signed at the time of sending the work through the online manuscript management system.