To assess the pharmacist's contributions by analyzing potentially inappropriate prescription and home treatment reconciliation in the complex chronic patient unit of a tertiary hospital.
MethodObservational, prospective, multidisciplinary study of patients in the complex chronic patient unit of a hospital during February 2019–June 2020. Multidisciplinary team of the complex chronic developed a checklist with a selection of non-recommended drugs based on STOPP/START, Beers and Priscus criteria, and drugs susceptible to deprescription according to LESS-CHRON criteria. The pharmacist applied the checklist daily in patients admitted to the unit, in addition to reconciling home treatment by reviewing the prescribed treatment with that detailed in the electronic home prescription. Therefore, the following variables were collected: age, sex and number of drugs on admission as independent variables, and dependent variables: number of drugs at discharge, type of potentially inappropriate prescription, reasons for reconciliation, drugs involved and degree of acceptance of the recommendation by the prescribing physician to assess the pharmaceutical contribution. The statistical analysis was performed with IBM® SPSS® Statistics22.
ResultsWe reviewed 621 patients with a median age of 84 years (56.4% women), and intervention was performed in 218 (35.1%). The median number of drugs was 11 (2–26) at admission and 10 (0–25) at discharge.
373 interventions were performed: 235 for medication reconciliation (78.3% accepted), 71 for non-recommended drugs (57.7% accepted), 42 for deprescription (61.9% accepted) and 25 for other reasons.
Statistically significant differences were observed between the number of drugs at discharge and at admission in both intervention patients (n = 218) and complex chronic patients (n = 114) (p < 0.001 in both cases).
Moreover, statistically significant differences were observed in the number of drugs at admission between patients included in the complex chronic programme and those not included (p = 0.001), and in the number of drugs at discharge (p = 0.006).
ConclusionsThe integration of the pharmacist in the multidisciplinary team of the complex chronic patient unit improves patient safety and quality of care. The selected criteria were useful for detecting inappropriate drugs in this population and favored deprescription.
Evaluar la contribución del farmacéutico mediante el análisis de la prescripción potencialmente inapropiada y la conciliación del tratamiento domiciliario en la unidad de pacientes crónicos complejos de un hospital terciario.
MétodosEstudio observacional, prospectivo y multidisciplinar de los pacientes de la unidad de pacientes crónicos complejos de un hospital durante febrero de 2019-junio de 2020. El equipo multidisciplicar del crónico complejo elaboró un checklist con una selección de fármacos no recomendados basado en los criterios STOPP/START, Beers y Priscus, y fármacos susceptibles de desprescripción según los criterios LESS-CHRON. El farmacéutico aplicaba el checklist diariamente en los pacientes que ingresaban en la unidad, además de realizar la conciliación del tratamiento domiciliario revisando el tratamiento prescrito con el detallado en receta electrónica domiciliaria. Por eso, se recogieron las siguientes variables: edad, sexo y número de fármacos al ingreso como variables independientes, y variables dependientes: número de fármacos al alta, tipo de prescripción potencialmente inapropiada, motivos de conciliación, fármacos implicados y grado de aceptación de la recomendación por parte del médico prescriptor para evaluar la contribución farmacéutica. El análisis estadístico se realizó con IBM® SPSS® Statistics22.
ResultadosSe revisaron 621 pacientes con una mediana de edad de 84 años (56,4% mujeres), y se intervino en 218 (35,1%). La mediana del número de fármacos fue de 11 (2–26) al ingreso y de 10 al alta (0–25) al alta.
Se realizaron 373 intervenciones: 235 por conciliación de la medicación (78,3% aceptadas), 71 por medicamentos no recomendados (57,7% aceptadas), 42 por deprescripción (61,9% aceptadas) y 25 por otros motivos.
Se observaron diferencias estadísticamente significativas entre el número de fármacos al alta y al ingreso tanto en los pacientes intervenidos (n = 218) como en los crónicos complejos (n = 114) (p < 0,001 en ambos casos).
Además, se observaron diferencias estadísticamente significativas en el número de fármacos al ingreso entre los pacientes incluidos en el programa de crónicos complejos y los no incluidos (p = 0,001), y en el número de fármacos al alta (p = 0,006).
ConclusionesLa integración del farmacéutico en el equipo multidisciplinar de la unidad del paciente crónico complejo mejora la seguridad del paciente y la calidad asistencial. Los criterios seleccionados fueron útiles para detectar fármacos inapropiados en esta población y favorecieron la desprescripción.
Polypharmacy and inappropriate prescribing of drugs in elderly patients threaten the quality of life of these patients, producing adverse events and associated complications. There is evidence that the pharmacist's participation and contribution within multidisciplinary teams through medication review and the establishment of tools on deprescription and non-recommended drugs, improves prescribing in this type of patients.
Our study shows the results of pharmaceutical care in real clinical practice in polymedicated patients admitted to the complex chronic patient unit. The pharmaceutical intervention performed has been accepted by the physicians in more than 50% of interventions related to non-recommended drugs or deprescription, and more than 70% in the reconciliation of home treatment. In addition, it has been shown that the integration of the pharmacist in the multidisciplinary team of the complex chronic patient unit has led to an improvement in safety in more than a third of the patients reviewed.
IntroductionThe care of complex chronic patients (CCPs), who present greater complexity in management due to changing needs that require continuous re-evaluations and require the orderly use of different levels of care and in some cases health and social services, is an objective in current health systems.1
These patients are characterized by chronic diseases, pluripathology and polypharmacy, the prevalence of which in our environment currently poses major challenges.
Polymedication or polypharmacy is associated with great complexity of therapeutic management and increases the risk of suffering adverse effects (35% of polymedicated elderly patients develop them), making errors in taking drugs, decreasing adherence to treatment and suffering falls.2,3 Elderly patients are especially vulnerable to adverse reactions due to physiological reasons, comorbidities, polymedication or different pharmacokinetic/pharmacodynamic behavior of the drugs.2 Specifically, adverse drug reactions (ADRs) are the cause of up to 30% of hospital admissions in the elderly.4,5
One of the main causes of adverse reactions in the elderly patient is Potentially Inappropriate Prescribing (PPI) of drugs, which is a situation in which the risk of adverse effects is higher than the expected clinical benefit, especially when safer and/or more effective alternatives are available.5 Active monitoring of prescriptions in polymedicated elderly patients makes it possible to reduce polypharmacy in more than half of prescribed drugs, resulting in improvement in patients' cognitive status and overall health.2 Currently, there are several validated tools for assessing the appropriateness of prescribing in the elderly, among which the most-used are explicit methods, such as the Beers criteria,6 STOPP/START,7 the French Consensus8 and the PRISCUS list.9
CCPs are susceptible to deprescription, understood as a process of dismantling drug prescriptions through a review that concludes with the modification of doses, substitution or elimination of some drugs and addition of others.4,10,11 Priority should be given to the following patients: polymedicated patients, especially the elderly; patients for whom drugs produce adverse effects; patients for whose health issues drugs have not been shown to be effective in clinical trials or for whom drugs are ineffective; terminal, fragile or advanced dementia patients; patients for whom, upon routine medication review, drugs are found that are unnecessary or inappropriate; patients for whom duplicity, relevant interactions, prescription errors, inappropriate medication or non-compliance are detected.4,5
Another activity related to patient safety is medication reconciliation, which is currently one of the main strategies for reducing medication errors related to the transition of care. Basically, medication reconciliation consists of obtaining the complete pharmacotherapeutic history of a patient in the outpatient setting and using it as a reference to compare with the prescriptions made on admission, transfer of service or hospital discharge and thus detect and correct existing divergences. Different studies have shown that failure to perform a correct home medication reconciliation is responsible for approximately 50% of medication errors that occur during care transitions and up to 20% of adverse events caused by medications in the hospital setting.12–14 For a medication reconciliation program to influence the quality of pharmacotherapy, a multidisciplinary approach is essential, involving sharing responsibility among different involved health professionals and maintaining continuity over time.12–14
The objective of pharmaceutical care (PC) in these patients is to optimize the effectiveness, safety and efficiency of treatment to maintain or improve patients' quality of life.
In line with this, the aim of our study is to assess the pharmacist's contribution within the multidisciplinary team by analyzing potentially inappropriate prescriptions (PIPs) and the reconciliation of home treatment in the Complex Chronic Patient Unit (CCPU) of a tertiary hospital.
MethodsThis research comprises an observational, prospective, single-centre study that included all patients admitted to the CCPU of a third-level university hospital from February 2019 to June 2020.
In our hospital, based on the care program for CCPs of the autonomous community, a multidisciplinary group was formed that consisted of internal medicine physicians responsible for the CCPU, nursing staff, social worker and pharmacist in charge of the ward. This group was responsible for performing comprehensive patient care and optimizing patients' pharmacological treatment.
In the care program for CCPs of the autonomous community, the identification of the complex chronic patient can be done automatically or according to clinical criteria. Automatically based on the classification by Adjusted Morbidity Groups (AMG), which are a morbidity grouping developed for primary care that is structured considering morbidity and complexity, with patients with a 99th percentile or higher being susceptible to being incorporated into the programme. According to clinical criteria, it is carried out in those patients with an AMG between 97–99, the primary care physician checks that the patient meets at least three of the following criteria: the patient has had 3 or more hospital admissions in the last 12 months, the patient is taking five or more different active ingredients, there is a positive response to the first two questions of the Barber questionnaire (do you live alone and do you have no one to turn to if you need help?) and Barthel index ≤60. Clinical identification can also be carried out by the responsible team in an internal medicine or geriatrics unit, if they consider that a patient should be included in the programme, they will proceed to check that they meet the criteria of a multi-pathological patient according to the clinical categories of care for multi-pathological patients of the Ministry of Health of the Government of Andalusia. The internal medicine or geriatrics physician will make the proposal for inclusion in the programme to the primary care physician, as in all cases (clinical or automatic identification) inclusion in the programme can only be made effective by the primary care team of reference, after reviewing the patients.15
To implement the project, the pharmacist and physicians responsible for the CCPU developed a strategy for adjusting the medication of patients admitted to the unit. This strategy used a checklist based on a selection of non-recommended drugs, according to the STOPP/START7, Beers6 and Priscus9 criteria and criteria defining certain drugs as susceptible to deprescription (LESS-CHRON16 criteria) in elderly patients (Annex 1).
Daily, the pharmacist responsible for the CCPU applies the checklist to new patients admitted to the ward, reconciles home medication based on the information provided by the electronic medical record and validates prescribed treatments.
Regarding reconciliation, the medication included in the emergency report was reviewed with the medication prescribed in hospitalization and compared with the patient's home treatment prescribed in the electronic prescription. In addition, to ensure that the patient was taking all the medication prescribed in the prescription, the dispensing of the medicines picked up by the patient in the community pharmacy was accessed through the prescription module.
If we observed any discrepancy in the reconciliation, or if we carried out any intervention on non-recommended drugs or deprescription, we communicated with the responsible physician by telephone or in writing through the prescription, depending on the severity, so the responsible physician assesses their appropriateness.
It should be noted that all patients admitted to the CCPU were reviewed, but only those patients on whom an intervention was performed were included in the study. These patients on whom an intervention was performed could be elderly patients or patients included in the complex chronic program of the autonomous community, since due to hospital overload issues, elderly patients could be admitted to this unit without being in the complex chronic program, but all patients who were admitted were reviewed.
The following variables were collected: age, sex, reason for discharge, whether the patient was included in the complex chronic disease program, number of chronic drugs on admission, polypharmacy (over 5 chronic drugs), extreme polypharmacy (over 10 chronic drugs), number of drugs on discharge, detected type of PIP (non-recommended drugs or drugs susceptible to deprescription), type of reconciliation error, drug involved in intervention and therapeutic group it belongs to, and degree of acceptance of the conducted pharmaceutical interventions.
Age, sex, and number of chronic drugs on admission were considered independent variables; the remaining variables were dependent on the objective of the study.
The variables were collected in Microsoft® Excel®, and the statistical analysis was performed with IBM® SPSS® Statistics22. The qualitative variables were expressed as percentages, and the quantitative variables were expressed as measures of central tendency (median and interquartile range or mean and standard deviation). For quantitative variables, we analyzed statistically whether they followed a normal distribution (Kolgomorov-Smirnov test) and performed tests for independent samples (T-student or U-Mann Whitney according to normality) or paired samples (T-student or Wilcoxon) based on the variables studied.
ResultsDuring the follow-up period (14 months), a total of 621 patients were reviewed, and a pharmaceutical intervention was performed in 218 of them (35.1%), these being the patients included in the study.
The 218 patients in the study had a median age at admission of 84 years (42–99 years), 56.4% of whom were women. Of the patients who underwent intervention (n = 218), 114 patients (52.3%) were included in the complex chronic disease program of our autonomous community. The median age of the complex chronic patients was 85 years (58–94), with 52.6% being women.
The median number of drugs at admission of the patients included in the study (n = 218) was 11 (with a range of 2–26 drugs; 96.8% of patients were involved in polypharmacy, and 64.7% were involved in extreme polypharmacy), and at discharge, the median number of drugs was 10 (with a range of 0–25 drugs). Statistically significant differences were observed between the number of drugs at discharge and at admission (p < 0.001).
Regarding complex chronic patients (n = 114), the median number of drugs at admission was 11 (4–26) and 98.2% of complex chronic patients were involved in polypharmacy and 75.4% extreme polypharmacy. The median number of drugs at discharge was 11 (with a range of 2–25 drugs). In this group of patients, statistically significant differences were observed between the number of drugs at discharge and at admission (p < 0.001).
In addition, statistically significant differences (p = 0.001) were observed in the number of drugs at admission between patients included in the complex chronic programme (median 11 (range 4–26)) and those not (10 (2–23)), and in the number of drugs at discharge (complex chronic patient 11 (2–25) and complex non-chronic patient 10 (0–19), p = 0.006).
A total of 373 interventions were performed: 235 (63.0%) for medication reconciliation, 71 (19.0%) for non-recommended drugs, 42 (11.3%) for deprescription and 25 (6.7%) for other reasons (adjustment for renal failure, therapeutic duplication, lack of adherence, etc.).
In 61 patients non-recommended drugs were detected (9.8% of the patients reviewed (n = 621) and 28.0% of the patients intervened (n = 218)); of the 71 interventions performed for this reason, 57.7% were accepted, 32.4% were not accepted and 9.9% were not accepted but justified by the prescriber. Of the total number of interventions for non-recommended drugs, 29.6% involved long-acting benzodiazepines, 16.9% involved antispasmodics, 11.3% involved short-acting benzodiazepines at high doses, 8.5% involved escitalopram at doses higher than 10 mg and 8.50% involved zolpidem at doses higher than 5 mg per day (see Table 1).
Analysis of non-recommended drugs and drugs not prescribed according to Annex I.
| Drug not recommend | Number of interventions | % (n = 71) |
|---|---|---|
| Long-acting benzodiazepine | 21 | 29,6 |
| Antiespasmodics | 12 | 16,9 |
| Short-acting benzodiazepine | 8 | 11,3 |
| Escitalopram | 6 | 8,5 |
| Zolpidem | 6 | 8,5 |
| Alpha beta-blockers | 5 | 7,0 |
| Antihistamines | 2 | 2,8 |
| Digoxin | 2 | 2,8 |
| Fluoxetine | 2 | 2,8 |
| Hydroxyzine | 2 | 2,8 |
| Tricyclic antidepressants | 1 | 1,4 |
| Spironolactone | 1 | 1,4 |
| Megestrol | 1 | 1,4 |
| Nifedipine | 1 | 1,4 |
| Sulfonylurea | 1 | 1,4 |
| Total | 71 | 100 |
| Deprescribed drug | Number of interventions | % (n = 42) |
| Statins | 16 | 38,1 |
| Allopurinol | 10 | 23,8 |
| Bisphosphonates | 8 | 19,0 |
| Citicoline | 3 | 7,1 |
| Calcium/vitamin D | 2 | 4,8 |
| Antidementia drugs | 1 | 2,4 |
| No indication | 2 | 4,8 |
| Total | 42 | 100 |
Deprescription was performed in 37 patients (6.0% of patients reviewed (n = 621) and 17.0% of patients intervened (n = 218)). Regarding interventions for deprescription (n = 42), 61.9% were accepted by the physician, 28.6% were not accepted, and 9.5% were not accepted but justified. Moreover, these interventions were mainly on statins (38.2%), allopurinol (23.8%) and bisphosphonates (19.0%) (Table 1).
Of the 218 included patients, errors related to medication reconciliation were detected in 23.2% of the patients, with 235 interventions: 52.3% of errors were due to omission of the drug, 17.4% were due to initiation of medication (discrepancy by commission), 16.2% were due to errors in dosage, route or frequency of administration, 12.3% were due to a wrong drug and 1.7% were due to incomplete prescription. Table 2 details the reasons for reconciliation and the acceptance of the performed interventions.
Pharmacist's intervention in reconciliation process.
| Reasons for reconciliation | Number of interventions | Accepted(%) | Rejected (%) | Justified (%) |
|---|---|---|---|---|
| Omission of the drug | 123 | 79,7 | 8,9 | 11,4 |
| Discrepancy by commission | 41 | 80,5 | 4,9 | 14,6 |
| Errors in dosage, route or frequency of administration | 38 | 73,7 | 13,2 | 13,1 |
| Wrong medication | 29 | 75,9 | 24,1 | 0 |
| Incomplete prescription | 4 | 75,0 | 25,0 | 0 |
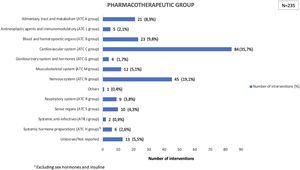
Drugs acting on the cardiovascular system presented a greater number of reconciliation errors (35.7%), followed by those acting on the nervous system (19.1%) and those acting on blood and sense organs (9.8%). Fig. 1 shows the reconciled drugs according to their pharmacotherapeutic groups.
DiscussionThe prevalence of polypharmacy in elderly patients is extremely high, which increases the risk of PIP. In our study, 96.8% of the patients in whom a pharmaceutical intervention was carried out had polypharmacy, a result higher than that of Zavala et al17 research, which revealed a polypharmacy prevalence of 89%. In the study by López Sáez et al,18 the mean numbers of drugs at admission were 6.1 (SD 3.8) and 5.7 (3.4) among the two populations included in their study, and at discharge, the means were 7 (3.9) and 7 (3.4), respectively, in their populations. Both means are lower than the mean number of drugs detected in our study, but the decreases from admission to discharge are the same. It is possible that the differences are due to the fact that our study includes patients included in the complex chronic unit, so that, in general, they are more complicated patients and of very advanced age since we have a median age of 84 years, so that we observed greater polypharmacy and drugs on admission than the aforementioned studies.
A total of 9.8% of the patients who underwent medication review had PIP according to our checklist based on the STOPP (STOPP/START), Beers and Priscus criteria.6,7,9 Our overall detected percentage of PIP is lower than those found in other studies, which could be due to the fact that our checklist does not include all the criteria, but rather only a selection of criteria. Cruz Esteve et al19 found a PIP prevalence of 39.0%, whereas Galvin et al20 found a percentage of 14.6% in a population of 3454 patients, both based on the STOPP/START criteria.7 In the study by López-Saez et al,18 carried out in hospitalized patients over 65 years of age, 19.5% of the patients had at least one inappropriate drug prescription according to the Beers criteria.6
The therapeutic groups most frequently implicated were long-acting benzodiazepines, urinary antispasmodics and short-acting benzodiazepines at high doses. The studies by Zavala et al17 and Cruz-Esteve et al19 included benzodiazepines with long half-lives used for more than one month of treatment and antiplatelet agents in primary prevention as the most frequently implicated drugs according to the STOPP criteria,7 but the latter were not included in our checklist. The potentially inappropriate drugs detected in the study by López-Sáez et al18 include ferrous sulfate, digoxin, meperidine and doxazosin as drugs implicated in 70% of the inappropriate prescriptions detected. According to the Beers criteria, Oscanoa et al21 found diazepam, digoxin, ferrous sulphate, chlorpheniramine and amitriptyline as the most frequently implicated drugs. In the study of Monzón-Kenneke et al,22 the most common potentially inappropriate medication was a proton-pump inhibitor (38.5%), followed by aspirin (24%), tramadol (15.6%), a benzodiazepine (13.5%) or an opioid (8.3%). As we can see, in most of the studies, benzodiazepines are drugs implicated in these processes of not recommended, as in our study.
In our study, we observed a higher percentage of interventions, based on LESS-CHRON criteria,16 due to deprescription of statins for primary prevention in patients over 80 years of age, allopurinol in patients without gout attacks in the past 5 years and bisphosphonates in primary prevention (treatment using these drugs for over 5 years is non-recommended) and in secondary prevention in patients who do not walk. Given the recent publication of these criteria, there is currently no literature with which to compare our results.
In relation to the interventions performed, statistically significant differences were observed in the number of drugs on admission and at discharge in both our study sample and in the group of complex chronic patients, with the pharmacist's intervention being a key factor in this difference due to the deprescription and review of non-recommended drugs. Furthermore, statistically significant differences were also observed in the number of drugs on admission between complex chronic patients and those who were not, and in the number of drugs on discharge, with a greater number being observed in complex chronic patients, which confirms the multimorbidity and complexity of their treatment, which is why it makes sense to have a special care process for this group of patients.
Treatment reconciliation and review are strategies that contribute to adjusting patients' medication and are key processes for expanding patient information, controlling polymedication and trying to reduce inappropriate medications.10,11 In our study, the percentage of patients with medication reconciliation errors was similar to that obtained in the study by Rubio-Cebrian et al,23 developed for patients over 75 years of age in the hospital setting, but lower than those reported by other studies in the literature, as was the case in the study by Cascone et al,25 71% of conciliation errors occurred, with a percentage of errors that is three times the value obtained in our study.
In the study by Rubio-Cebrian et al,23 carried out in patients over 75 years of age in the hospital setting, most of the reconciliation errors were due to the omission of a chronic medication of the patient (75.4%), followed by the prescription of the wrong dose, route or frequency (18.3%) and the wrong medication (3.7%). In the study by Taladriz-Sender et al,24 49.7% of the errors were due to omission, 23.3% were due to dose change, 12.3% were due to commission and the rest (14.7%) were due to error in the frequency or route of administration or incomplete prescriptions. In another study by Rogado-Vegas et al,26 58% of patients were reconciled for medication omission. In our study, 52.3% of errors were reconciled by omission, similar results to the study by Cascone et al25 and Rogado Vegas et al.26 Errors of omission were followed by errors of commission with 17.4% of the cases, this rate being slightly higher than that of Taladriz et al.24 In contrast, our results regarding reconciliation due to dose, route or frequency of administration error, wrong medication and incomplete prescription were much higher.
Regarding the therapeutic groups most involved in reconciliation, in the study by Rogado-Vegas et al26 carried out in an emergency department, the therapeutic groups most involved were hypnotics and sedatives, followed by lipid-lowering agents. In our case, the results obtained could be said to be similar since in our case the drugs reconciled belong to the cardiovascular system group followed by the central nervous system.
In our study, the prospective nature has favored the collection of information because it is part of the daily clinical practice of the pharmacist responsible for the CCPU. The selection of a limited number of drugs has facilitated the checklist's applicability to all patients admitted to the CCPU. However, our study has limitations, specifically, the main limitation of this study is that our prepared checklist does not include all the criteria for non-recommended drugs, according to the STOPP/START, Beers and Priscus criteria nor all the criteria susceptible to deprescription in elderly patients according to LESS-CHRON, but rather includes a selection of the drugs considered of greatest clinical relevance by the multidisciplinary CCPU team, which has made comparison with other observational studies difficult. Another limitation is the fact that we were unable to perform a complete and detailed statistical study, since we did not know which interventions were performed on complex chronic patients or on patients not included in the complex chronic group, so that in the future we should consider including this data in the database collected during clinical practice in order to compare both groups, thus enriching the study. It would also be interesting to be able to compare with patients in whom we have not performed an intervention, but who were reviewed, to find out if there are differences between the two groups, as this could further support the pharmacist's contribution. The study was initially designed with the patients on whom we performed intervention in order to know our current situation and how we could improve, so it will be taken into account for future studies. Moreover, in the future, it would be interesting to carry out new prospective studies to evaluate the degree of project implementation by comparing future results with those obtained in the described study.
Despite these limitations, it is worth mentioning once again that the fact of showing the daily clinical practice of our center is a strength of the work we present, as it is the result of the work carried out as part of a multidisciplinary team.
In conclusion, the integration of the pharmacist into the multidisciplinary team of the CCPU has led to a safety improvement in more than one-third of the reviewed patients, contributing to an improvement in the quality of care.
The selected criteria have been useful for detecting inappropriate drug use in this population and for facilitating deprescription.
This study shows that reconciliation errors are frequent. The implementation of reconciliation programs in the CCPU is a strategy for improving the adequacy of prescribing that should be routinely incorporated in health care practice.
Declaration of authorshipAll authors have contributed intellectually to the work, meet the conditions of authorization and have approved the final version of the work. The contribution of each of them is detailed below:
▪Conception and design of the work: Arantxa Magallón Martínez, Andrea Pinilla Rello. Pilar Casajús Lagranja, Alfonso García Aranda, María del Carmen Bueno Castel.
▪Data collection: Arantxa Magallón Martínez and Andrea Pinilla Rello.
▪Data analysis and interpretation: Arantxa Magallón, Martínez, Andrea Pinilla Rello, Pilar Casajús Lagranja, Ruth Caballero Asensio, María Sevil Puras
▪Article writing: Arantxa Magallón, Martínez, Andrea Pinilla Rello, Pilar Casajús Lagranja, Abad Sazatornil María Reyes
▪Revision of the article: Arantxa Magallón Martínez, Andrea Pinilla Rello. Pilar Casajús Lagranja, Alfonso García Aranda, María del Carmen Bueno Castel, Ruth Caballero Asensio, María Sevil Puras, Abad Sazatornil María Reyes
▪Approval for publication: Arantxa Magallón Martínez, Andrea Pinilla Rello. Pilar Casajús Lagranja, Alfonso García Aranda, María del Carmen Bueno Castel, Ruth Caballero Asensio, María Sevil Puras, Abad Sazatornil María Reyes
No funding was obtained for this study.
Not applicable.
DATE:
NAME:
EMR
ROOM:
PHARMACOTHERAPEUTIC ASSESSMENT
DRUGS NOT RECOMMENDED FOR THE ELDERLY.
| DRUG | HOME TREATMENT | OBSERVATION |
|---|---|---|
| Tricyclic antidepressants(Amitriptyline, Imipramine…) | ||
| Fluoxetine | ||
| Escitalopram >10 mg/d | ||
| Citalopram >20 mg/d | ||
| Long-acting benzodiazepines (diazepam, clorazepate, bromazepam) | ||
| Short and intermediate-acting benzodiazepines at high doses | ||
| Zolpidem >5 mg/d | ||
| Long-acting sulfonylureas (glibenclamide, glimepiride, glipizide and glicazide) | ||
| Thiazolidinedione in heart failure | ||
| Digoxin >0.125 mg/d | ||
| Spironolactone >25 mg/d + ACE inhibitors and ARBs II | ||
| Delayed-release nifedipine | ||
| Alfa-blockers: doxazosin, prazosin, terazosin | ||
| Nonsteroidal anti-inflammatory drugs | ||
| Urinary antispasmodics (tolterodine, fesoterodine, solifenacin) | ||
| Megestrol | ||
| Metoclopramide | ||
| Antihistamines (hydroxyzine..) |
DRUGS AND SITUATIONS SUBJECT TO DEPRESCRIPTION
| DRUG | SITUATION | HOME TREATMENT |
|---|---|---|
| Calcium/vitamin D | Fracture prevention in non walking patients | |
| Statins | Primary prevention in patients >80 years of age | |
| Allopurinol | Patients without gout attack in the previous 5 years | |
| Bisphosphonates | Primary prevention treatments not recommended for more than 5 years and in secondary prevention in patients who do not walk | |
| Antidementia drugs | Patients with advanced disease or if not responding to treatment | |
| Citicoline | Vascular dementia (drug of low therapeutic usefulness) |