Pharmacy service is to provide individualized pharmaceutical care for patients, which should follow the current evidence-based pharmacy, and constantly verify the evidence and then produce new evidence. In pharmaceutical care, differences are often found in the efficacy and adverse reactions of drugs among individuals, even within individuals, which are closely related to patient's genetics, liver and kidney functions, disease states, and drug interactions. Back in the 1980s, therapeutic drug monitoring (TDM) has been applied to routinely monitor the blood drug concentration of patients taking antiepileptic drugs or immunosuppressants after transplantation to provide individualized dosage recommendations and accumulate a large amount of pharmacokinetic (PK)/pharmacodynamic (PD) data. As individualized pharmaceutical care proceeds, the concept of precision medicine was introduced into pharmacy services in combination with evidence-based pharmacy, PK/PD theories and big data to further promote the TDM technology and drugs, and carry out pharmacogenomics analysis. The TDM and pharmacogenomics have been applied gradually to the fields of antimicrobial, antitumor and antipsychotic drugs and immunosuppressants. Based on the concept of precision pharmacy, we adpoted approaches including PK/PD, quantitative pharmacology, population pharmacokinetics, and big data machine learning to provide more personalized pharmacy services, which is mainly for special patients, such as critical patients, patients with interaction risk of multiple drugs, patients with liver and renal insufficiency, pregnant women, children and elderly patients. As the service pattern of precision pharmacy has been constructed and constantly improved, better evidence in clinical practice will be produced to provide patients with better precision pharmacy service.
El servicio de farmacia se encarga de prestar atención farmacéutica personalizada a los pacientes. Los servicios de farmacia deben utilizar aquellas prácticas con mayor nivel de evidencia, y realizar una continua validación de dicha evidencia antes de elaborar nuevas prácticas. En la terapia farmacológica, se observan diferencias inter e intra individuales respecto a los efectos terapéuticos y a las reacciones adversas de los medicamentos, lo que está estrechamente relacionado con las variaciones genéticas, la función hepática y renal, el estado de la enfermedad y la interacción entre medicamentos. Desde la década de los 80 del siglo pasado, se utiliza la monitorización terapéutica de fármacos (MTF) de forma rutinaria para controlar las concentraciones sanguíneas de fármacos antiepilépticos o de inmunosupresores postrasplante y elaborar recomendaciones de dosis personalizadas y recoger una gran cantidad de datos farmacocinéticos (PC)/farmacodinámicos (PD). Con el desarrollo de la atención farmacéutica personalizada, el concepto de medicina de precisión se introduce en la atención farmacéutica, combinando la farmacia basada en evidencias, los enfoques PC/PD y los macrodatos (big data), promover técnicas de MTF en medicamentos, y la realización de análisis farmacogenómicos. La MTF y la farmacogenómica se están aplicando de forma gradual en el tratamiento con antimicrobianos, antitumorales, antipsicóticos e inmunosupresores. Sobre la base del concepto de farmacia de precisión, utilizamos métodos de PC/PD, farmacología cuantitativa, farmacocinética poblacional y aprendizaje automático con big data para ofrecer una atención farmacéutica más personalizada, principalmente a pacientes con necesidades especiales, como los pacientes en estado crítico, con riesgo de interacciones farmacológicas múltiples, pacientes con insuficiencia hepática y renal, mujeres embarazadas, niños y ancianos. Con la construcción y mejora continua del modelo de servicio de farmacia de precisión, la generación de evidencia científica sobre la práctica clínica mejorará y eso redundará en un mejor servicio de farmacia de precisión para los pacientes.
Precision pharmacy service focuses on the differences among individuals or even within individuals in the efficacy and adverse reactions of important therapeutic drugs. Pharmacists select drugs and their dosage for specific populations according to the patient's specialist examination results, liver and kidney function,with technologies in therapeutic drug monitoring(TDM) and pharmacogenomics(PGx), as well as approaches including pharmacokinetic (PK)/pharmacodynamic (PD), quantitative pharmacology, population pharmacokinetics, big data machine learning, and evidence-based pharmacy, so that to provide comprehensive pharmaceutical services.1 Precision pharmacy service is expected to improve treatment efficacy, reduce adverse reactions and save medical resources. Back in the 1980s2, the TDM technology has been used in treating epilepsy and kidney transplant patients. As pharmacy service continues to improve, the TDM technology and PGx have been applied gradually to the fields of antimicrobial, antitumor and antipsychotic drugs and immunosuppressants, and the service pattern of individualized precision pharmacy has been constructed.3
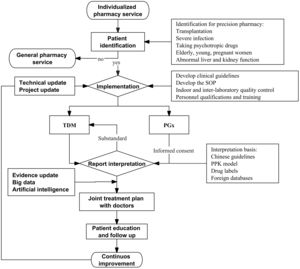
Precision pharmacy service pattern in ChinaService objectsPatients in need of individualized precision pharmacy service often use drugs with narrow therapeutic windows, individual differences in pharmacokinetics and/or nonlinear pharmacokinetics, risk of serious adverse reactions, and genetic differences that may affect the efficacy or safety of drugs (Fig. 1). In addition, polypharmacy increases the risk of drug interactions, and the pharmacokinetics of drugs often change significantly in critically ill patients, pregnant women, children, and patients with hepatic and renal insufficiency.4–7 Patients in need of precision pharmacy service mainly include:
a. Patients with organ or bone marrow/stem cell transplantation: They need long-term use of immunosuppressants with narrow therapeutic window and obvious adverse reactions, and most immunosuppressants are not only substrates of liver drug enzymes and/or transporters, but also inhibit liver drug enzymes and/or transporters, which are prone to drug interactions.8
b. Patients with severe infection, including ICU patients, patients with sepsis, and patients infected with multi-drug resistant bacteria. With the rapid change of the patients' PK/PD data and their condition, the dosage and frequency of medication should be determined according to the PK/PD data.9
c. Patients taking psychotropic drugs for a long time, which can cause multiple adverse reactions and interactions. The efficacy of such drugs varies greatly among individuals with uncertain adherence.10
d. Patients taking antineoplastic drugs, which can cause severe adverse reactions with high treatment costs, and may lead to drug interactions.11
e. Patients taking multiple drugs may have drug interactions, which significantly affects the efficacy of drugs and increase adverse reactions.12
f. Others including elderly patients, children, pregnant women, and patients with abnormal liver and kidney function who may have changes in pharmacokinetics and are prone to adverse reactions.
Service procedureGeneral pharmacy serviceGeneral pharmacy service mainly refers to medication therapy management (MTM), through the patient interview, and collecting information such as demographics and pharmacogenetics, as well as medication, allergy, adverse drug reaction, personal and, family history and patient problem list; evaluating the problems related to medication of patients, such as adverse events, inappropriate dosage, misuse, repeated medication, medication ineffectiveness, unnecessary medication, the need for additional medication, and medication adherence; following evidence-based pharmacy to solve the drug-related problems of patients alone or together with doctors/nurses through medication reorganization, simplification and education.13 General pharmacy service helps solve the drug-related problems of most patients. For others, it is necessary to further provide individualized and precise pharmacy service,
Identification of target patients in need of precision pharmacy serviceDifferent from general pharmacy service, pharmacists draw up a list of drugs for individualized and precise service, and reach a consensus with doctors to negotiate specific implementation rules in precision pharmacy service. In particular, they will provide some personalized drug testing services, like TDM or PGx, which the doctor will issue medical orders or prescriptions. The identification of target patients in need of individualized precision pharmacy service includes active or passive identification by pharmacists.
a. Active identification: Specialist clinical pharmacists identify target patients in pharmacy clinic or pharmaceutical care; Identification with the help of Hospital Information System (HIS), such as multiple medication risks; and those with liver and kidney insufficiency in need of technical support for dose adjustment.
b. Passive identification: Physicians send out consultation applications and invite clinical pharmacists to provide precision pharmacy service for difficult cases, or patients with adverse reactions and poor treatment effects and those who make a request for such service.
TDM and/or PGx assaysTDM should comply with the principles of safety, effectivity and economy for clinical drug treatment, and the goal of individualized drug treatment. PGx is mainly based on clinical testing standards in Technical Guidelines for Gene Detection of Drug Metabolizing Enzymes and Drug Action Targets (Trial) and Technical Guidelines for Individualized Treatment of Tumors (Trial) issued by the National Health Commission of China (http://www.nhc.gov.cn/yzygj/s3593/201507/fca7d0216fed429cac797cdafa2ba466.shtml).
a. Establish quality control standards, including standard operating procedures (SOP), indoor and inter-laboratory quality control, qualification certification, personnel training, design and environment of laboratory, and repair and maintenance of instruments and equipment.
b. Set up a clinical pathway involving multiple disciplines such as medicine, pharmacy, nursing and information, as well as application, charging, sample collection and acceptance, and reporting.
c. Make special guidelines for the hospital, including the time period for different drugs to reach steady blood concentration, blood collection time, blood collection tubes, sample storage and transportation, application and charging.
d. Accept the samples and report the test results in time.
Analysis of the test resultsStandard operating procedures and flow charts are formulated for interpreting the test results. First of all, factors such as inappropriate sampling method and/or time, improper storage and transportation of samples, and incorrect laboratory test results should be excluded. The interpretation should take into account factors such as the patient's disease, efficacy and adverse reactions, pathophysiology, drug use and adherence, and genetics. The comprehensive analysis and evaluation are performed on the causes and the impact of the test results on drug treatment effects and safety.
Individualized and precise medication recommendationsPharmacists should provide individualized and precise medication recommendations according to the test results, as well as approaches including evidence-based pharmacy, pharmacotherapeutics, PK/PD, quantitative pharmacology, population pharmacokinetics, and big data machine learning. It should be noted that PGx testing is different from TDM, and patients only need to be tested once in their lifetime. Therefore, when giving recommendations based on the results of pharmacogenetic testing, it is necessary to take into account PGx and the results of database review, combined with non-genetic factors such as the patient's age, gender, weight, drug interactions, and diseases.14 The genetic test report should strictly protect patients' privacy.
The recommendations should include but not limited to:
a. Medication recommendations: Pharmacists give medication recommendations and inform the patient of the recommended dose according to the best available evidence, and the monitoring purpose and result analysis.
b. Suggestions on self-management of patients: Pharmacists provide patients with suggestions on drug adherence, medication methods, the monitoring of efficacy and adverse reactions, and daily diet.
Follow-up of patientsIndividualized monitoring and follow-up plans should be made for the patients, including the adjustment of the next treatment plan, and the treatment effect and adverse reactions. If necessary, precision pharmacy services should be provided for the second time.
If the TDM results exceed the target range with ineffective treatment and an increased risk of adverse reactions, follow-up should be conducted to ensure that recommendations are followed until the TDM results reach the standard to prove effective and safe treatment.
If the TDM results are within the target range with ineffective treatment or adverse reactions, follow-up should be conducted to ensure that recommendations are followed until the drug is proved safe and effective. Genetic marker detection can also be adopted to guide clinical medication if needed. Pharmacists should communicate with patients for medication education and monitoring.
Technical support for precision pharmacy serviceTDM technologyTDM is based on individualized drug treatment with a customized drug administration plan for the patient made by measuring the patient's exposure to drugs, and pharmacological markers or pharmacodynamic indicators, applying the models of quantitative pharmacology and population pharmacokinetics, and using the drug therapeutic window as the baseline.15,16
The basic conditions for precision pharmacy service through TDM include qualified testing technology, professional pharmacists, patients who meet the monitoring indications, and customized and precise drug treatment plans.17
The analytical techniques to determine the drug concentration in biological samples (blood drug level, urine drug level, other tissue fluid or homogenate drug level) mainly include spectral analysis, chromatographic analysis, liquid chromatography-mass spectrometry, and immunological detection technology. Liquid chromatography-mass spectrometry (LC–MS) and high performance liquid chromatography (HPLC) are recommended according to the specificity of drugs. Chromatography-mass spectrometry is used more and more to conduct TDM in Chinese medical institutions.
Currently, the drugs for TDM mainly include cyclosporine A, tacrolimus, sirolimus, mycophenolic acid, carbamazepine, phenytoin, valproic acid, levetiracetam, methotrexate, vancomycin, teicoplanin, voriconazole and its metabolites, itraconazole and its metabolites, posaconazole, amikacin, meropenem, imipenem, tigecycline, linezolid, Imatinib and its metabolites, dasatinib, and nilotinib.
In addition to perform therapeutic drug monitoring of small molecule agents, we are trying to monitor macromolecule targeted agents and biosimilar agents in recent years.18
PGx technologyPGx is to explore the influence of genetic variation on the safety and efficacy of drugs from the perspective of genome. PGx detection helps to better explain individual differences in drug efficacy and adverse reactions,19 which brings the following advantages in terms of drug therapy:
a. Select drugs before treatment to avoid ineffective treatments, saving time for patient.
b. Predict the initial dose for each patient to help quickly determine the drug efficacy and ease the symptoms.
c. Avoid serious adverse drug reactions, such as the detection of genes related to severe skin adverse reactions of allopurinol and carbamazepine to reduce such reactions and improve medication safety of patients.20
d. Provide a scientific basis for the development and post-marketing evaluation of new drugs in clinical trials, which based on PGx.21
More commonly used methods for detecting drug-related gene mutations include fluorescence quantitative PCR, in situ hybridization, gene chip and direct sequencing, and some popular second-and third-generation sequencing methods are also used in genetic testing of drugs, especially tumor-targeted therapy.22
The drugs involved in PGx testing mainly include warfarin, clopidogrel, aspirin, methotrexate, allopurinol, tacrolimus, glucocorticoids, azathioprine, paclitaxel, antidepressants, voriconazole, proton pump inhibitors, tamoxifen, cyclophosphamide, and folic acid.23
Research based on real data and data analysis technologyIn current medical research, real-world data from clinic is an important source of big data. Compared with clinical trials, real-world research is closer to clinical practice. TDM and PGx data are used in the analysis of patient-related information through HIS system, and an individualized medication model can be built with artificial intelligence (AI) algorithms such as machine learning. For example, the vancomycin dose prediction model has been built with the support of XGBoost machine learning algorithm to solve the problem that the dose demand varies greatly among individuals and to optimize the vancomycin dosing schedule.24 The prediction model of warfarin maintenance dose based on LightGBM machine learning algorithm can help solve the problem of missing values in the data set, and recommend the accurate dosing for patients in the case of a narrow warfarin treatment window, thus increasing the success rate of treatment.25 In addition, deep learning algorithms are increasingly used to process high-dimensional, complex and interactive clinical data. For example, the use of TabNet deep learning algorithm helps to establish the dose prediction model of lapatinib, and dig deeply into the relevant variables that affect the drug dose, establish a concise and accurate individualized medication model, and recommend the accurate dosage in clinic.26 Establishing an individualized medication model of tacrolimus with TabNet deep learning algorithm can effectively solve the problems of narrow therapeutic window of tacrolimus and individual differences, and improve the prediction accuracy of tacrolimus dosage in kidney transplant patients.27 Dose prediction models relying on machine learning and deep learning technology demonstrate good prediction performance and robustness. With the increase of sample size of input data, the prediction model will be continuously optimized to achieve better performance and practicability to support clinical decision-making and precision pharmacy service.
More elaborate pharmacy service can be provided through post-marketing observation and real-world studies. For example, the post-marketing observation clinical research of enalapril plus folic acid, showed that the progress rate of chronic kidney disease (CKD) and the decline rate of estimated glomerular filtration rate (GFR) of patients treated with enalapril plus folic acid are lower than those of patients treated with enalapril alone. Enalapril plus folic acid can reduce the risk of chronic kidney disease progression in CKD patients by 56%, the risk of eGFR decline rate by 44%, and the serum homocysteine level by 21% compared with baseline. The study showed that enalapril plus folic acid have a greater protective effect on kidney and has higher safety and lower cost. At the same time, this study also provided a basis for the management of refined pharmacy service of CKD patients with serum homocysteinemia.28
Risk assessment and early warning of polypharmacy in the elderlyPatients with chronic diseases, especially elderly patients, often suffer from several diseases and need multiple medication treatments, which can lead to drug interactions and adverse events. Therefore, a risk assessment and early warning model for polypharmacy in the elderly is under construction through information technology to help identify such risks and provide precision pharmacy services to elderly patients.
In addition to the prescription drugs, the evaluation and management of polypharmacy should also consider the over-the-counter drugs, traditional medical drugs, herbs and health care products taken by patients at the same time.29
Pharmaceutical monitoring technology for high-alert drugs in the hospitalHigh-alert drugs refer to drugs that can lead to serious adverse reactions when used improperly. In order to improve medication safety, the monitoring and management of high-alert drugs through information systems are being explored in the hospital, including the management of blood sugar, pain, anticoagulants, antimicrobials and anti-tumor drugs. The high-risk groups identified by the information system are provided with precision pharmacy service. For example, for targeted anti-tumor preparations, the suitability of targeted drugs is screened through pharmacogenetic testing technology with the drug dosage for each patient clarified and possible adverse reactions predicted and monitored. For drugs with therapeutic window, the TDM technology can be used to make fine dose adjustment during the medication process, and help identify the risk of adverse reactions and adherence.
Continuous improvement and development of precision pharmacy serviceThe Chinese Pharmacological Society (CNPHARS) initiated the formulation of the Consensus of Pharmaceutical Experts on Drug Monitoring of Anti-tumor Biosimilars (2020),30 marking the progress of Chinese pharmacists in providing precision pharmacy service based on TDM. In the days ahead, targeted therapy of tumor guided by high-throughput sequencing and precision pharmacy for biopharmaceuticals/biosimilars will be further explored.
An increasing number of studies led by pharmacists have been initiated with the support of big data and TDM, and artificial intelligence technologies such as deep machine learning and models of quantitative pharmacology and population pharmacokinetics have been applied to promote precision pharmacy service.31 From drug selection, initial dose recommendation to dose adjustment during treatment, advanced technologies and methods can be used to provide scientific and reliable pharmaceutical support for patients.
Choosing appropriate drugs for patients according to their genetics, and providing suggestions on the dosage and frequency of drugs are the foundation of precision pharmacy.32 However, drug-related genetics cannot fully reflect the differences of patients' efficacy and adverse reactions, so it is necessary to integrate PGx with proteomics, transcriptomics, and metabolomics. Moreover, big data can help to integrate patients' physiological and pathological information, genetic information, environmental information, and living habit information, to use bioinformatics to describe individual differences, to establish a clinical database of patient, and to form a feedback mechanism to guide precision medicine service.
ConclusionChina's individualized precision pharmacy service pattern has been constructed and gradually improved. As precision pharmacy technologies continue to thrive, new clinical research evidence is emerging, evidence-based pharmacy evidence and big data are updated, the population enjoying precision pharmacy service is growing, and the service range is expanded to help more and more patients to benefit from precision pharmacy.
ResourcesNone.
Presentación en Congresos: No.
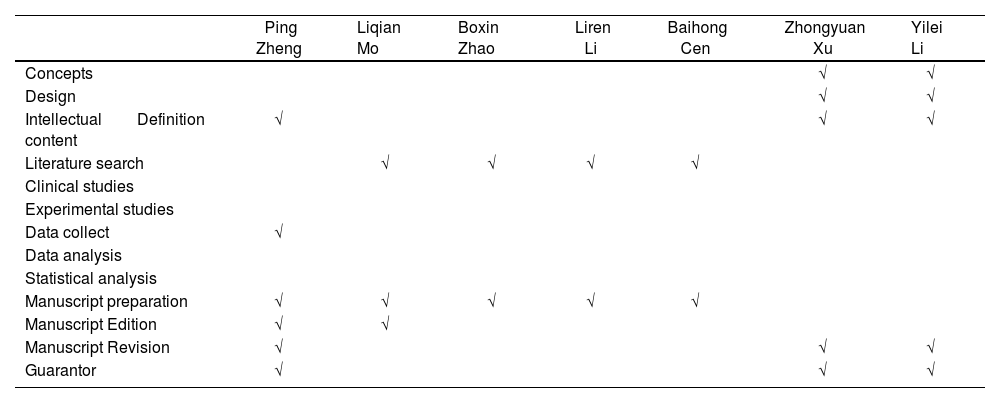
Contributions| Ping Zheng | Liqian Mo | Boxin Zhao | Liren Li | Baihong Cen | Zhongyuan Xu | Yilei Li | |
| Concepts | √ | √ | |||||
| Design | √ | √ | |||||
| Intellectual Definition content | √ | √ | √ | ||||
| Literature search | √ | √ | √ | √ | |||
| Clinical studies | |||||||
| Experimental studies | |||||||
| Data collect | √ | ||||||
| Data analysis | |||||||
| Statistical analysis | |||||||
| Manuscript preparation | √ | √ | √ | √ | √ | ||
| Manuscript Edition | √ | √ | |||||
| Manuscript Revision | √ | √ | √ | ||||
| Guarantor | √ | √ | √ |
All of the authors accept the responsibility defined by the International Committee of Medical Journals Editors (Available at: http://www.icmje.org/).
All of the authors assign, in the event of publication, exclusively all the reproduction, distribution, translation, public communication (by any sound, audiovisual or electronic support) our work to Farmacia Hospitalaria and by extension to SEFH. For this, a session letter of rights will be signed at the time of sending the work through the Online system of manuscript management.
FoundationNo foundation.