The need for new antimicrobial treatments that work alternatively or synergistically with antibiotics to address the problem of the emergence and transmission of antimicrobial resistance has increased interest in the use of minority therapies such as phage therapy. For safe and widespread application of this therapy, it is necessary to establish the pharmacokinetic and pharmacodynamic parameters for its use in humans. This systematic review analyzes the criteria necessary to establish the PK/PD of this therapy, as well as its current application, based on a review of 66 clinical cases that catch diverse infections and phage administration routes.
La necesidad de obtener nuevos tratamientos antimicrobianos que funcionen alternativamente o sinérgicamente con los antibióticos con el objetivo de solventar el problema derivado de la aparición y transmisión de resistencias antimicrobianas, ha aumentado el interés por la aplicación de terapias minoritarias como es la terapia con fagos. Para poder aplicar de manera segura y generalizada esta terapia, es necesario establecer los parámetros farmacocinéticos y farmacodinámicos para su uso en humanos. En esta revisión sistemática se analizan los criterios necesarios para poder establecer la PK/PD de esta terapia, así como la forma en que esta se está aplicando actualmente, a partir de la revisión de 66 casos clínicos que recogen diversas infecciones y vías de administración de los fagos.
Since the introduction of the first antibiotic into clinical practice, the usefulness of these antimicrobial compounds has been widely demonstrated. However, in recent decades, the development of antimicrobial resistance and the emergence and spread of multidrug-resistant microorganisms have made the search for new antimicrobial agents a necessity.
The need to develop alternatives to antibiotics has led to increased interest in using bacteriophages as antimicrobial agents. Bacteriophages are viruses that infect bacteria. They were discovered by Frederik Twort in 1915 and first used as antimicrobials by Félix d'Hérelle in 1917 until antibiotics were introduced in the 1930s. Compared to antibiotics, phage therapy offers the following advantages: greater specificity, protecting the microbiota; low toxicity, since phages are part of the microbiome, do not infect eukaryotic cells, and are well tolerated; self-amplification, as phages replicate at the site of infection, exponentially increasing their numbers; and synergy when used in combination with antibiotics, enhancing the activity of both and often reducing resistance.1
The European Pharmacopoeia Commission has drawn up a list of priorities for the period 2023 to 2025, which includes the development of medicinal products for phage therapy or Phage Therapy Medicinal Products (PTMPs). These products are preparations of natural or genetically modified phages that are used to treat or prevent bacterial infections in animals or humans. They may consist of a single phage or a mixture of several, also known as a ‘phage cocktail’, which is combined with excipients.2
As with other types of pharmacotherapy, the application of phage therapy must be guided by fundamental pharmacological and clinical considerations, and requires pharmacokinetic (PK) and pharmacodynamic (PD) studies. Both new treatments and those requiring optimisation demand an understanding of the mechanisms involved in the evolution of their concentration in the human body (PK), as well as the effects they may have on it (PD).3 When an antimicrobial agent, such as a bacteriophage, is administered to a patient, determining the correct dose is essential. To achieve this, its PK behaviour must be considered in relation to its PD characteristics.4
To understand the PK of phage therapy, it is important to consider how phages interact within the body; therefore, the absorption, distribution, metabolism, and elimination (ADME) of PTMPs must be evaluated, along with the titration of PTMPs throughout the treatment period.5
Understanding PD requires conducting both primary and secondary PD studies. In the case of phage therapy, primary studies analyse the relationship between phage concentration and bacterial elimination, while secondary studies examine the adverse effects of this therapy.6 In primary studies of phage therapy, the effective phage concentration must be established—one that can eliminate or at least reduce the bacterial count in infections—in order to restore health to its pre-disease state or alleviate certain symptoms.
This article reviews the principles of PK and PD in phage therapy and summarises the available information on the phage administration routes and doses for different types of infection.
MethodsA systematic review was conducted to examine the current status of PK and PD in phage therapy.
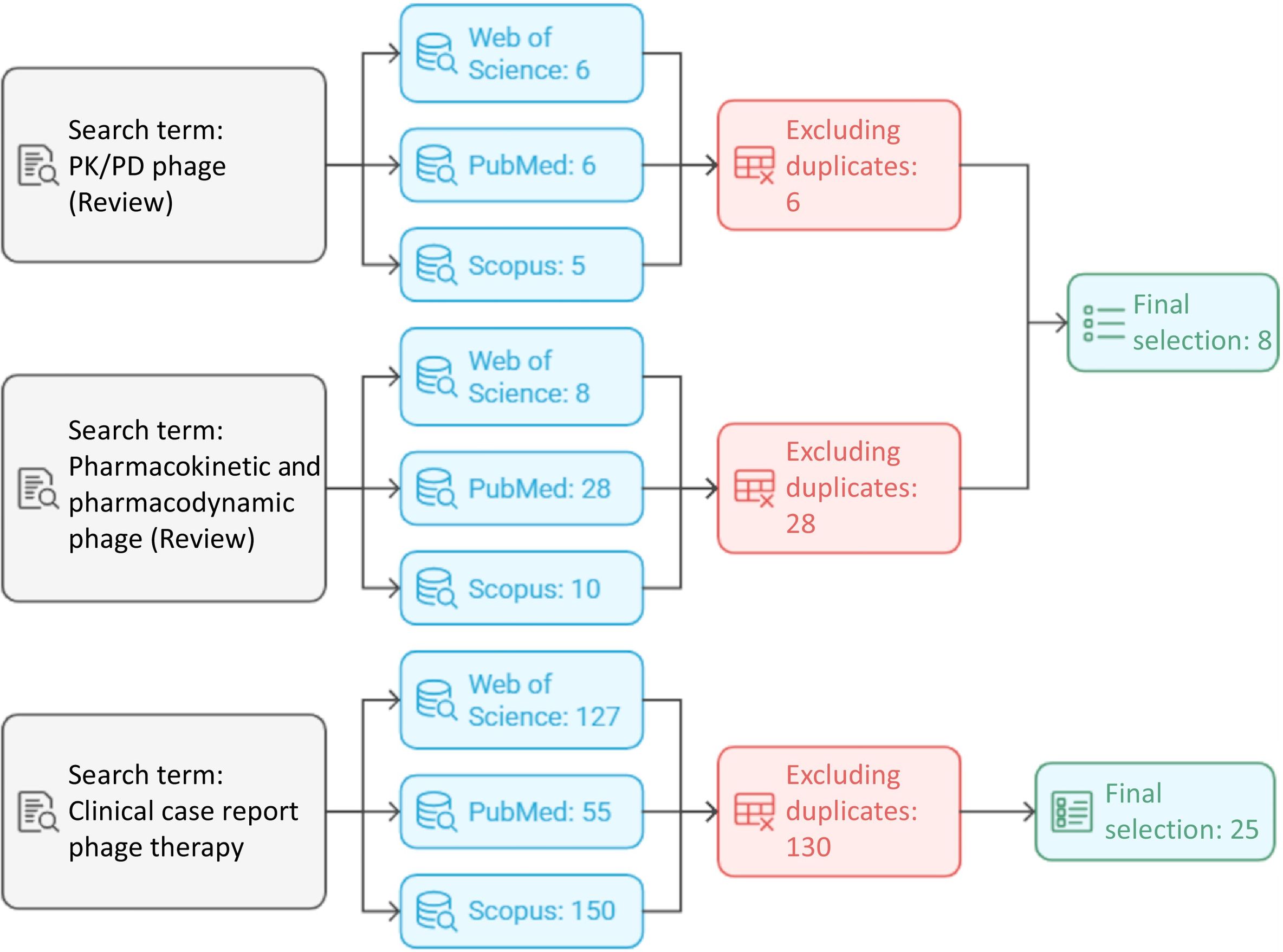
Inclusion and exclusion criteriaThe aim of this review was to identify studies related to PD and PK in phage therapy, as well as clinical cases involving this therapy. It was based on scientific articles published between 2020 and 2025 in journals indexed in Journal Citation Reports and Scimago Journal Rank and retrieved from the Web of Science7 and Scopus databases.8 Inclusion criteria include review articles containing the terms ‘PK/PD’ and ‘pharmacokinetic and pharmacodynamic.’ Another search term was ‘clinical case report’. In this case, priority was given to articles that reviewed multiple clinical cases. Exclusion criteria include articles published before 2020 and duplicate articles across the different databases used.
Search strategiesA literature review was conducted in the PubMed (NCBI), Web of Science, and Scopus databases for English-language articles published between 2020 and 2025 (Fig. 1).7–9 The database search focused on PK/PD-related phage therapy studies; articles based on clinical trials with phages were also included in the search. Articles that were duplicates across databases, or that did not offer new insights, were excluded from the review. In the case of clinical trials, studies that did not provide information on any of the criteria used to calculate PK/PD were also excluded. The terms used for this search were ‘PK/PD phage’, ‘Pharmacokinetic/Pharmacodynamic phage’, and ‘Clinical case report phage’. The term ‘PK/PD’ yielded 15 articles in Web of Science, 11 in PubMed, and 14 in Scopus. After removing duplicates, 14 scientific articles remained. ‘Pharmacokinetic and Pharmacodynamic phage’ produced 23, 103, and 27 articles, respectively, with 105 selected after deduplication. ‘Clinical case report phage’ yielded 127, 55, and 150 articles, respectively, leaving 130 unique articles. Finally, 8 scientific publications with the terms ‘PK/PD phage’ and ‘Pharmacokinetic/Pharmacodynamic phage’ and 25 scientific publications with the search term ‘Clinical case report phage’ were used to prepare the review.
ResultsPharmacokinetic principles in phage therapyTo elucidate the interactions between phages and the human body, it is essential to understand the ADME processes and dose adjustment of PTMPs throughout treatment. The physicochemical parameters that ensure stability during storage must also be known in advance.5,10
Determining the PK of phages in the body requires knowledge of the initial phage count administered during treatment. This is done using the indirect method of counting lysis plaques in a culture of the host bacterial strain. It should be noted that the number of plaques does not always represent the total number of phage particles present in a system, as only those capable of lysing and forming visible plaques are counted. It is important to select the appropriate host bacterium, as the number or activity of phages may be underestimated depending on the phage's ability to lyse it. Therefore, the efficiency of the plaque formation in the target strain must be calculated.10
The stability of PTMPs can vary, so they must be monitored during both storage and administration. For this reason, establishing and maintaining appropriate pH, temperature, and ionic strength values is essential for each PTMP used in phage therapy.6,10
Once the initial concentration of phages to be administered has been determined, the route of administration must be carefully selected, as the ADME of the phages will depend on it. Phages can be administered via the intravenous (IV), intraperitoneal, intramuscular, subcutaneous, oral, inhalation, intranasal, endotracheal, intrauterine, rectal, vaginal, and topical routes.11
The metabolism is responsible for inactivating and eliminating PTMPs. Phages mainly accumulate in the liver and spleen, which are the organs responsible for these processes. Kupffer cells in the liver are more effective than splenic macrophages at phagocytosing phages. However, the spleen contributes to adaptive immunity by stimulating antibody production to neutralise phages rather than eliminating them through phagocytosis.10,11 Although the vast majority of drugs are excreted in urine, phage elimination via this route is low and varies according to age, disease, renal function, and variability in phage transcytosis.10,11
Pharmacodynamic principles in phage therapyTo establish the phage concentration required for primary studies, the multiplicity of infection (MOI)10 value must be calculated, defined as the ratio between the infectious agent and its target. In phage therapy, the MOI represents the number of phages per bacterial cell, although in this case, the number of phages added does not always correspond to the number of phages that actually interact with the bacteria. To overcome this discrepancy, the following formula was proposed:
where k is the constant adsorption rate and Ct is the bacterial concentration.There are two models of phage therapy, depending on the MOI applied for treatment: passive and active. In passive therapy, the concentration of phages administered must greatly exceed the bacterial load so that the PD does not depend on the production of bacterial progeny. In active therapy, the bacterial load exceeds the proliferation threshold (the minimum bacterial concentration needed to increase the phage concentration), and the phages exceed the flooding threshold (the minimum phage concentration required to reduce the bacterial load); this ensures that the administered phage product eliminates the bacteria.1
Secondary studies of phage therapy must establish the toxicity levels of PTMPs, which are determined by the presence of endotoxins derived from the bacterial culture used to propagate the phage. Therefore, all PTMPs have to undergo purification processes using chromatography. In addition to toxicity prevention studies, the effects of PTMPs on the microbiota, their interaction with the immune system, and their impact on eukaryotic cells must also be assessed.11
Studies on phage therapy administration: routes and dosagePhage therapy involves the administration of phages as an antimicrobial treatment for bacterial infections. Although phages possess inherent antimicrobial activity, they are often combined with antibiotics, producing a synergistic effect known as phage-antibiotic synergy, which reduces the emergence of resistance to both agents.
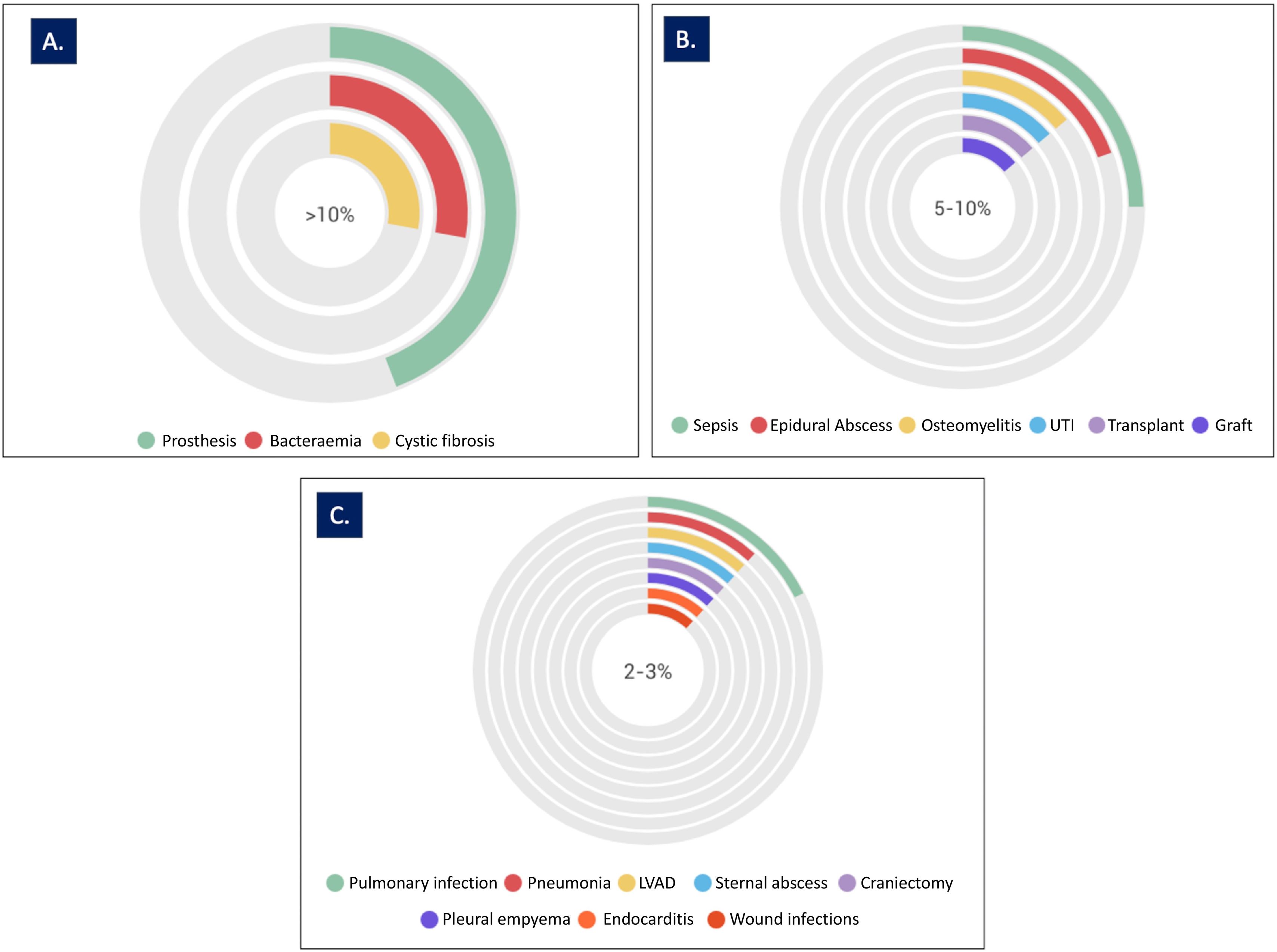
This review analysed 66 clinical cases, drawn from 25 scientific publications, in which patients were treated with phage-antibiotic combination therapy (Table 1S. Supplementary Material). In total, 25 different types of infections were analysed (Fig. 2). Among the studies analysed, 69% addressed specific clinical conditions, with bacteraemia and prosthetic infections each accounting for 14%, followed by septic shock (8%), and infections related to left ventricular assist devices and epidural abscesses (7% each). Pneumonia, osteomyelitis, graft infections, and urinary tract infections (UTIs) each accounted for 5% of the total cases analysed. The remaining 31% of studies analysed 16 different types of infection (Fig. 2). In some cases, the studies analysed more than 1 type of infection in the same patient.12–18
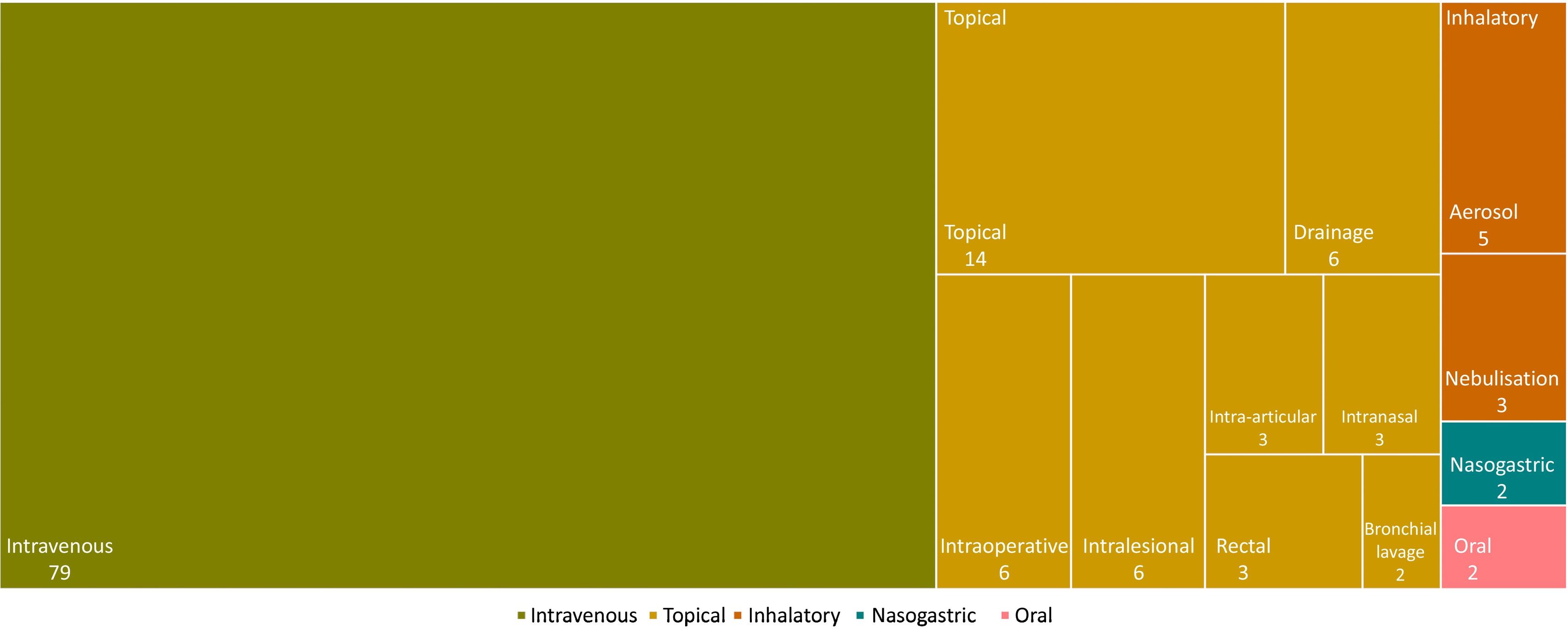
Phages were administered via 5 main routes: IV, topical (drainage, intraoperative, intra-articular, intralesional, intranasal, rectal, or bronchial lavage), inhalational (nebulisation or aerosol), nasogastric, and oral. The IV route was the most commonly used (in 79% of cases), followed by the topical route (42%); in some cases, more than 1 route of administration was used (Fig. 3).
In cases of bacteraemia, sepsis, and osteomyelitis, phage therapy was administered exclusively via the IV route.13,16 In other situations, such as endocarditis, the IV route was combined with administration via drainage, whereas for abscesses, it was combined with local administration at the site of infection.13,16,19,20 In pulmonary infection studies, treatments were administered via both the IV and nasogastric routes, and via nebulisation.18,19,21,22 For UTIs, phage treatments were given via intravenous, vaginal, and intrarectal routes.15,16,23 In prosthetic joint infection studies, treatment was administered topically by applying the phages via the intra-articular route at the site of infection.16,17,24 Ear infections were treated via the intravenous and intra-auricular routes.17
In these studies, the PTMP doses varied depending on the clinical case, the type of infection, and the type of PTMP used. The doses analysed ranged from 106 to 1011 CFU/mL (colony-forming units/ml), although in most cases studied, a dose of 109 CFU/mL was used.12,13,15–19,21–29 Typically, PMPT treatments are prepared in a 0.9% NaCl buffer and administered over periods ranging from 2 to 24 h, with 6 h being the most common.30,31
DiscussionThe Law on Guarantees and Rational Use of Medicines and Health Products (29/2006, 26 July) defines an active ingredient as “any active substance or mixture of substances intended for the manufacture of a medicinal product and which, when used in its production, becomes an active component of said medicinal product intended to exert a pharmacological, immunological, or metabolic action with the aim of restoring, correcting, or modifying physiological functions, or to establish a diagnosis'. An active substance is defined as ‘any substance or mixture of substances intended for the manufacture of a medicinal product and which, when used in its production, becomes an active component of said medicinal product intended to exert a pharmacological, immunological, or metabolic action with the aim of restoring, correcting or modifying physiological functions, or to establish a diagnosis’. A medicinal product for human use is defined as ‘any substance or combination of substances presented as possessing properties for treating or preventing disease in humans, or which may be used in humans or administered to humans with the purpose of restoring, correcting, or modifying physiological functions by exerting a pharmacological, immunological or metabolic action, or establishing a diagnosis” (BOE-A-2015-8343).32
Phages are biological entities that can be used to treat or prevent infections and diagnose disease. They therefore fit the definition of medicines33 and can be considered to fall within the remit of pharmacology.
As with other medicines, PK/PD values must be established for PTMPs to ensure uniform and safe application in patients. One of the main challenges in establishing PK/PD parameters is determining the appropriate phage dose to be administered. Therefore, the greatest obstacle to phage therapy is establishing PK, as the MOI is a key factor in this calculation. This requires knowledge of the number of phages to be administered per bacterium, which is difficult to determine due to the variability in both bacteria and phage numbers at the site of infection, given their capacity to proliferate. It should also be noted that bacteria develop resistance to phages more rapidly at higher MOI levels. Thus, it is important to strike a balance between bacterial load and phage concentration. The difference compared to antibiotic administration lies in the nature of drug exposure: for antibiotics, exposure is an independent variable in the exposure-response relationship; for phages, however, exposure is not an independent variable due to the predator–prey relationship.10
However, the studies reviewed in this article show that phage therapy was effective in 68% of cases, achieving bacterial eradication (negative cultures), and that 77% of patients showed clinical improvement. These figures are similar to those reported in a study reviewing 100 clinical cases treated with phage therapy.34 The doses used in the cases analysed in this review were also consistent with those reported in that study, with the most frequently administered dose being 109 CFU/mL every 12 h. The cases analysed involved multiple routes of PTMP administration. In some instances, different routes were combined—most commonly IV in combination with others—particularly when infections involved multiple organs or tissues. PTMPs were also combined with antimicrobials in 69% of cases, demonstrating a synergistic effect. However, in 1 clinical case involving rifampicin in combination with phages, an antagonistic effect was observed.
Although the oral route is the most convenient way to administer drugs to humans, the bioavailability of PTMPs given by this route is usually low. Nevertheless, oral administration remains a promising strategy for treating bacterial gastrointestinal infections. However, further preclinical studies are needed to analyse the sensitivity of PTMPs to pH, their adsorption to intestinal contents, and inactivation of phages by serum and blood.6,35 Similarly, despite their low bioavailability, PTMPs administered nasally have shown good efficacy against respiratory infections. Data on the systemic delivery of PTMPs following topical application to intact skin are limited, but show that the phages have poor penetration and low bioavailability. Nonetheless, the topical application of PTMPs has been shown to be effective against local wound infections.10 Intravenous administration has been shown to effectively deliver PTMPs systemically, as it enables good tissue penetration, particularly in tissues affected by inflammation, where endothelial barrier permeability is increased.35 Rapid absorption is also achieved via the intraperitoneal, intramuscular, and subcutaneous routes.10
Studies on the distribution of PTMPs in different organs have shown that the highest number of phages is found in the liver and spleen after IV administration. These organs are responsible for filtering foreign bodies from the blood. It has also been demonstrated that it is important to take the microbiota into account, as bacterial strains with receptors for the administered phages may act as chemoattractants, thereby affecting phage distribution.10,11
The rapid increase in infections caused by multidrug-resistant bacteria means that there is a growing need to develop alternative approaches, such as phage therapy. It should be noted that the pace of acquiring knowledge on the PK/PD of phages is lagging behind the growing need for their use as therapeutic agents. The main PK and PD elements to consider regarding phages are as follows: the pharmacological characteristics of the phages; the route of administration; the site of bacterial infection; the respective concentrations of phages and bacteria at various locations and sites (including the site of infection, if possible); and the involvement of the host's immune system. In general, the standardisation of PK/PD parameters for phages would represent a crucial first step towards the successful clinical introduction of phage therapy.
CRediT authorship contribution statementLucía Blasco: Data curation, Methodology, Writing – original draft. Inés Bleriot: Formal analysis, Methodology, Writing – original draft. Patricia Fernández-Grela: Investigation, Methodology, Visualization. José Ramón Paño-Pardo: Formal analysis, Visualization. Jesús Oteo-Iglesias: Formal analysis, Visualization, Writing – review & editing. María Tomás: Formal analysis, Funding acquisition, Supervision, Validation, Writing – review & editing.
FundingThis study has been funded through grants PI19/00878 and PI22/00323, awarded to principal investigator M. Tomás within the Plan Estatal de I+D+i 2013–2016 (Plan Nacional de Investigación Científica, Desarrollo Tecnológico e Innovación 2008–2011), and co-financed by the ISCIII-Dirección General Adjunta de Evaluación y Promoción de la Investigación – Fondo Europeo de Desarrollo Regional “A Way of Making Europe”, Instituto de Salud Carlos III FEDER, together with a grant from the Instituto de Salud Carlos III (MePRAM Project, PMP22/00092), Ministerio de Ciencia e Innovación, financed by NextGeneration European Union funds within the framework of the Resilience and Recovery Fund.
None declared. This work has not been submitted to any conference or journal for publication.