To resume the available literature about digoxin population pharmacokinetic studies in elderly patients. To identify the pathophysiological changes in this subpopulation with clinical implications on digoxin pharmacokinetics.
MethodA systematic review was performed regarding the population pharmacokinetic studies in elderly patients receiving digoxin. PubMed, ISI Web of Science, SCOPUS and Science Direct were used to identify the articles with the descriptors (“Digoxin”[Mesh]) AND (“Pharmacokinetics”[Mesh]) AND (“Aged”[Mesh] OR “Elderly”[Mesh]), followed by a manual search.
ResultsNine studies were found and reviewed, five of them carried out in Asian patients. NONMEM was used for pharmacokinetic analysis of digoxin blood levels, being mostly described by a one-compartment model. Serum creatinine, body weight and concomitant administration of calcium channel blockers are the covariates that most frequently influence digoxin pharmacokinetics in elderly patients.
ConclusionsElderly people present pathophysiological changes with influence on the pharmacokinetics of many drugs. The covariates with most influence on digoxin pharmacokinetics should be considered when adjusting this drug dosage in elder patients to achieve optimum health benefits and prevent possible side effects.
Resumir la literatura disponible sobre los estudios de farmacocinética poblacional de digoxina en pacientes de edad avanzada e identificar los cambios fisiopatológicos en esta subpoblación, que conllevan implicaciones clínicas en la farmacocinética de la digoxina.
MétodoSe realizó una revisión sistemática de los estudios de farmacocinética poblacional en pacientes ancianos que recibían digoxina. Se utilizaron PubMed, ISI Web of Science, SCOPUS y Science Direct para identificar los artículos con los descriptores (“Digoxin”[Mesh]) AND (“Pharmacokinetics”[Mesh]) AND (“Aged”[Mesh] OR “Elderly”[Mesh]), seguido de una búsqueda manual.
ResultadosSe encontraron y revisaron nueve estudios, cinco de los cuales de desarrollaron en pacientes asiáticos. Se utilizó NONMEM para el análisis farmacocinético de los niveles plasmáticos de la digoxina, mayoritariamente descrita como un modelo monocompartimental.
ConclusionesLos pacientes ancianos presentan cambios fisiopatológicos con gran influencia en la farmacocinética de muchos fármacos. Las covariables con un mayor impacto en la farmacocinética de la digoxina deben tenerse en cuenta al ajustar la dosis de este medicamento en pacientes de edad avanzada con el fin de lograr beneficios óptimos para la salud y prevenir posibles efectos adversos en esta subpoblación.
Digoxin is a cardiac glycoside indicated for congestive heart failure (CHF) and atrial fibrillation (AF) treatment, diseases that are more prevalent in elderly people1. Digoxin exerts both a direct and indirect mechanism of action. As a direct effect, digoxin inhibits the heart Na/K-ATPase, decreasing the sodium output and increasing intracellular calcium levels, which leads to enhance the positive inotropic effect and the force of contraction of the cardiac muscle. As an indirect effect, digoxin inhibits the neurons Na/K-ATPase, which interferes sodium and potassium active transport across the cell membrane, decreasing sympathetic action and heart rate. Even at therapeutic doses, digoxin prolongs the PR interval and decreases the ST segment due to its effects on the myocardial cells and on the cardiac conduction system2.
In terms of pharmacokinetics, digoxin has 50–90% oral bioavailability, low plasma protein binding (20%) and a volume of distribution of 5–10 L/kg, mainly related to lean body mass, which decreases about 20% in patients between 20 and 70 years3,4. Digoxin is mostly excreted unaltered by passive glomerular filtration and active tubular secretion (30–50%) and a small percentage suffers hepatic metabolism (10–20%) or intestinal secretion via P-glycoprotein. Enterohepatic circulation is insignificant5,6) The plasma elimination half-life of digoxin ranges between 26 and 48 hours in patients with normal renal function but it may be doubled in renal impairment or elderly patients7,8.
The elderly is often related to a chronological age of ≥65 years-old, with this portion of the world population steadily increasing. According to the World Population Prospects 2019, by 2050, 1 in 6 people in the world will be over 65 years-old9. Several age-related physiological changes may modify the pharmacokinetics and pharmacodynamics parameters of many drugs in elderly patients10 because of decreased pharmacokinetic processes: absorption, distribution, metabolism and excretion. Regarding gastrointestinal tract physiology, age-related changes such as increased gastric pH, delayed gastric emptying, reduced splanchnic blood flow and decreased gastrointestinal absorption surface, may potentially reduce drugs absorption. However, as the degree of absorption of most drugs is not usually affected in elder people, these physiological changes do not seem to lead to clinical consequences10,11.
With aging, total body weight, lean mass, and body water content decrease, while fat mass tends to increase. Elder people reduce their water content by up to 10–15%12–14, which modifies the pharmacokinetics of watersoluble drugs such as digoxin: a dose reduction is required due to the diminished volume of distribution and the increased plasma concentrations15.
Although the concentration of plasma proteins and hepatic metabolism are reduced, these changes seem to have little clinical relevance with respect to digoxin16,17.
Regarding renal function, most kidney functions are decreased18–20 which may influence the elimination of digoxin, since this drug is mainly excreted unchanged in the urine.
Furthermore, this functional declining can be strongly influenced by concomitant renal disease11, therefore, older patients should be managed as if they were affected by renal insufficiency21.
All these factors must be taken into consideration when prescribing drugs to older patients due to the clinical implications of the pathophysiological changes that occur with aging: reduced absorption, metabolism and elimination, comorbidity, sensitivity to drugs, polymedication and limited life expectancy3,22,23.
Digoxin has a narrow therapeutic range, severe side effects and high toxicity. Although a therapeutic range for digoxin has traditionally been established between 0.8–2.0 ng/mL, these values are under discussion nowadays. The post hoc analysis by Rathore et al. demonstrated that serum digoxin concentrations (SDC) between 0.5–0.8 ng/mL allowed to optimize the therapeutic effect, while reducing the rate of mortality and duration of hospitalizations24.
Clinical manifestations of its toxicity include gastrointestinal and neurological symptoms, as well as cardiac dysrhythmia25. Digoxin is also involved in multiple pharmacodynamic-pharmacokinetic drug interactions, which may predispose to its toxicity, especially in the elderly26. For instance, pharmacodynamics interactions in the elderly can be related to increased sympathetic tone in which reserpine, β-agonists, theophylline and cyclopropane are involved or related to electrolyte unbalance as hypokalemia in which diuretics and corticosteroids are involved.
Regarding pharmacokinetic interactions predisposing to digoxin toxicity, some increase absorption as those caused by propantheline, atropine, erythromycin, and tetracycline. Other drugs may decrease its volume of distribution as quinidine, some result in decreased renal clearance as occurs with quinine and amiodarone, while thiazide diuretics, hydralazine, furosemide or spironolactone increase the renal elimination.
All these circumstances make it essential for digoxin to be monitored in both adults and elderly patients as well as in the presence of renal impairment1,3,8,27. Several studies found in the literature analysed the population pharmacokinetics (PopPK) of digoxin in pediatric and adult populations (28–54). The purpose of this paper is to conduct a systematic review to summarize the available knowledge regarding digoxin PopPK in patients ≥65 years-old and to identify the sources of variability in its disposition.
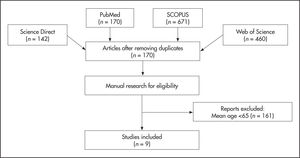
MethodsA systematic review was conducted through PubMed, ISI Web of Science, SCOPUS and Science Direct databases. The following search terms were used: (“Digoxin”[Mesh]) AND (“Pharmacokinetics”[Mesh]) AND (“Aged”[Mesh]) OR (“Elderly”[Mesh]). These descriptors provided 170 nonduplicated articles. Since authors may not specify the mean age of the patients in the title or abstract, a manual search was performed to find articles with patients ≥6 5 years-old. Figure 1 shows the process followed to select the articles included in this systematic review.
Inclusion criteria were human studies, mean age ≥65 years-old, patients treated with digoxin and analysis developed with nonlinear mixed effect modelling software (NONMEM). We excluded articles performed in animals, with patients <65 years-old and papers not written in English. As quality criteria, we considered articles published in indexed journals and the limited list of items reported by Dartois et al.55: number and characteristics of the patients, dosage and route of administration, number of observations, model selection, structural model, interindividual variability, error models, method of estimation and software.
This systematic review was developed according to PRISMA guidelines to improve the quality of this kind of articles56.
ResultsNine articles were found and reviewed according to the inclusion, exclusion and quality criteria: : Konishi et al.41, Hornestam et al.42, Williams et al.43, Choi et al.44, Chen et al.45, Komatsu et al.46, Bauer et al.47, Yukawa et al.48 and Zhou et al.49. The reviewed studies were carried out in populations between 94 and 294 patients, mostly conducted in Asian patients from China, Japan and Korea (66.7%). All authors used the software NONMEM to develop the PopPK analysis. However, the final models varied between studies: Williams43, Choi44, Chen45, Yukawa48 and Zhou49 used a one-compartment model to describe digoxin pharmacokinetics. Hornestam42 applied a two-compartment model while Konishi41 used the hyperbolic model to develop a PopPK model for digoxin. This approach, different from the rest authors, implied the estimation of two rate constants (b and g) to calculate digoxin clearance. Komatsu46 and Bauer47 used a steady-state model by means of trough serum concentrations of digoxin. The pharmacokinetic model was the following:
where Css is the steady-state serum digoxin concentration (ng/mL), D is the dose of digoxin (µg), Cl is the total body clearance of digoxin (L/h), and T is the dosing interval (h).The information evaluated in the studies included age, gender, body weight, digoxin dose, levels and sampling time, renal function, measured as serum creatinine (Scr) or creatinine clearance (CrCl) and concomitant treatments. The characteristics of the patients included in the papers reviewed are summarized in Table 1. Only one study investigated the serum potassium, which proved to increase digoxin clearance44. In this study, the nutritional status of the patients was also estimated using the nutritional risk index (NRI) by collecting the following data: serum albumin, cholesterol, percentage of ideal body weight and total lymphocyte count. NRI was calculated as follows:
Patient data included in the studies
| Konishi41 | Homestam42 | Williams43 | Choi44 | Chen45 | Komatsu46 | Bauer47 | Yukawa48 | Zhou49 | |
|---|---|---|---|---|---|---|---|---|---|
| Number of patients | M: 82 V: 99 | 105 | 94 | M: 106 | M: 122 | 192 | 294 | M: 94 | M: 119 |
| V: 16 | V: 20 | V: 28 | V: 8 | ||||||
| Number of samples | M: 107 V: 128 | 154 | 230 | M: 255 | M: 405 | 287 | 366 | M: 140 | M: 173 |
| V: 43 | V: 43 | V: 28 | V: − | ||||||
| Samples per patient | − | − | − | M: 2.40 (1-10) | M: 3.4 ± 0.8 (1-5) | − | − | − | − |
| V: 2.68 (1-8) | V: 2.2 ± 0.6 (1-3) | ||||||||
| Gender | |||||||||
| Male | M: 49. V: 59 | − | − | M: 58. V: 8 | M: [251]. V: [26] | 121 | 160 | M: 60. V: 16 | M: 69. V: − |
| Female | M: 33. V: 40 | − | − | M: 48. V: 8 | M: [154]. V: [17] | 71 | 134 | M: 34. V: 12 | M: 50. V: − |
| Digoxin dose (mg/day) | M: 0.120 ± 0.051 (0.0625-0.2500) | Variable | 0.189 ± 0.075 (0.058-0.500) | M: 0.133 ± 0.053 | 0.0625-0.2500 once or twice daily | 0.125 mg/3 days-0.25 mg/día | 0.235 ± 0.100 | M: 0.21 ± 0.07 (0.087-0.410)a | M: − |
| V: 0.128 ± 0.047 (0.0625-0.2500) | V: 0.113 ± 0.066 | V: 0.259 ± 0.050 (0.19-0.48)a | V: 0.125 | ||||||
| Serum digoxin concentration (μg/l) | M: 0.79 ± 0.32 (0.12-2.19) | Early sampling: 7.5 ± 2.5 (2.8-13.7) | − | M: 0.866 ± 0.341 | M: 1.21 ± 0.41 (0.31-3.66) | 0.90 ± 0.56 | 1.5 ± 1.1 | M: 0.88 ± 0.35 (0.35-2.10) | M: 1.11 (0.07-4.45) |
| V: 0.76 ± 0.38 (0.21-2.86) | Late sampling: 1.25 ± 0.78 (0.30-6.17) | V: 0.693 ± 0.380 | V: 1.08 ± 0.33 (0.36-1.81) | V: 1.22 ± 0.44 (0.59-2.00) | V: 1.43 (0.51-2.56) | ||||
| Sampling time (h post-dose) | 21-24 | Early: 0.35 ± 0.06 (0.25-0.50) | ≥ 7 | Trough | Variable | − | ≥ 8 | Trough | M: 22.9 (6-192) |
| Late: 16.10 ± 0.37 (15.6-18.6) | V: − | ||||||||
| Age (years) | M: 67.8 ± 12.6 V: 67.1 ± 13.0 | 67 ± 12 (21-89) | 69.2 ± 10.3 (36-88) | M: 72.8 ± 13.0 | M: 75.5 ± 8.3 (65-82) | 71 ± 12 | 68 ± 12 | M: 73.7 ± 6.0 (65.0-91.0) | M: 71 (60-88) |
| V: 70.1 ± 12.5 | V: 74.1 ± 5.0 (66-80) | V: 71.9 ± 5.7 (65.1-83.7) | V: − | ||||||
| Total body weight (kg) | M: 54.1 ± 11.5 (35-82) | 77 ± 15 (47-139) | 72.0 ± 15.6 (45-111) | M: 57.3 ± 12.0 | M: 62 ± 18 (31-99) | 55.47 ± 11.94 | 66 ± 16 | M: 54.1 ± 10.4 (26.1-87.3) | M: 62.9 (34-91) |
| V: 54.5 ± 11.7 (28-94) | V: 54.2 ± 12.9 | V: 52.1 ± 9.0 (42-70) | V: 51.5 ± 9.4 (30.0-68.0) | V: 65.4 (55.0-86.5) | |||||
| Serum creatinine (mg/dL) | M: 0.99 ± 0.44 (0.28-3.02) | − | − | − | M: 1.19 ± 0.49 (0.46-3.25) | − | 1.7 ± 1.4 | M: 0.86 ± 0.28 (0.46-1.78) | M: 1.43 (0.41-7.75) |
| V: 0.84 ± 0.29 (0.28-1.99) | V: 1.38 ± 0.66 (0.49-3.04) | V: 0.98 ± 0.25 (0.60-1.50) | V: 1.27 (0.64-2.83) | ||||||
| Creatinine clearance (mL/min) | M: 56.8 ± 26.8 (8.7-140.5) | 71 ± 27 (20-140) | 59.8 ± 25,9 (6.5-127,4) | M: 51.5 ± 24.6 | − | 56.17 ± 33.76 | − | − | − |
| V: 71.0 ± 28.0 (20.4-130.0) | V: 42.9 ± 27.6 | ||||||||
| Indications n (%) | |||||||||
| Atrial fibrillation | − | 105 | − | M: 55 (51.9) | − | − | − | − | − |
| V: 13 (81.2) | |||||||||
| CHF | 82 | 0 | 48 | M: 12 (11.3) | M: [312] (77) | − | 153 | M: 41 | M: 113 |
| V: 2 (12.5) | V: [35] (81) | V: 6 | V: 8 | ||||||
| Other | − | 0 | − | M: 39 (36.8) | − | − | − | − | − |
| V: 1 (6.3) | |||||||||
| Concomitant drugs n (%) | |||||||||
| ACEIs | − | 17d | − | − | M: [247] (61) | − | − | − | − |
| V: [30] (70) | |||||||||
| CCBs | − | 9d | − | M: 19 (17.9) | M: [89] (22) | − | − | M: 40 V: 2 | M: 54 |
| V: − | |||||||||
| V: 3 (18.7) | V: [10] (23.3) | ||||||||
| Diltiazem | − | − | − | − | b | c | − | b | M: 1 |
| V: − | |||||||||
| Spironolactone | 31 | − | − | M: 3 (2.8) V: 0 | M: [232] (57.3) | 35 | − | M: 62 V: 6 | M: 32 |
| V: [22] (51.1) | V: 4 | ||||||||
| Verapamil | − | − | 18 | − | b | c | 21 | b | − |
Values reported as mean ± standard deviation (range); [n]: results reported as number of samples; (−): data not reported.
ACEIs: angiotensin converting enzyme inhibitors; AF: atrial fibrillation; CCBs: calcium channel blockers; CHF: congestive heart failure; M: modeling group; V: validation group.
An NRI score of 100 indicates no risk; 97.5 to 100.0, mild risk; 83.5 to 97.5, moderate risk; and <83.5, severe risk. However, this parameter did not show any influence on the pharmacokinetic model44. Two authors analysed the pharmacokinetics of digoxin based on two drug interactions: digoxinquinidine43,47 and digoxin-verapamil47. Both interactions proved to decrease digoxin clearance. Large discrepancies were found when analyzing concomitant medications. Spironolactone was the most studied drug (five out of nine reviewed articles), however, only two equations included this drug in the final PopPK model. In both studies spironolactone decreased digoxin clearance45,49. Three authors, Williams43, Choi44 and Chen45, analysed the effect of different covariates on the volume of distribution. In these studies, patients with higher TBW showed an increased volume of distribution. The final equation developed by Chen45 also included CHF, which decreased the volume of distribution of digoxin.
Zhou49 conducted a PopPK study of digoxin in Chinese elderly patients. In this article, and unlike the other ones reviewed, the authors established as inclusion criteria patients with oral digoxin for more than 7 days and without serious hepatic or renal dysfunction. As digoxin was given orally, the parameters Cl and Vd were interpreted as Cl/F and Vd/F, respectively.
Concerning the evaluation methods, Konishi41, Hornestam42, Williams43 and Bauer47 used exclusively a basic internal evaluation. Chen45 and Komatsu46 employed both basic and advance evaluation. Yukawa48 evaluated the model by basic internal and external evaluation. Only Choi44 and Zhou49 analysed the PopPK model of digoxin by basic internal, advanced internal and external evaluation with appropriate metrics.
DiscussionThe objective of this review is to analyse the PopPK studies of digoxin in patients ≥65 years-old, a frail and usually multipathological and polymedicated group. Pathophysiological changes in elderly people may have an important effect on the pharmacokinetics of many drugs. Thus, PopPK studies are of high interest, especially when dealing with digoxin. Due to its narrow therapeutic index, strong side effects and large interindividual and intraindividual variability, an individualized dosing must be considered. The information provided by PopPK studies may increase the safety of its treatment, achieve optimal therapeutic efficacy and reduce its adverse events.
Regardless the age of the patients, most of the studies described digoxin PopPK as a one-compartment open model, with just a few authors using a two-compartment model42,57,58. Kramer et al.59 compared the fit of a twoand three-compartment pharmacokinetic model to determine SDC-time data following rapid intravenous injection of 1.0 mg of the drug. The results showed that the three-compartment fit resulted in better performance. Yukawa54 observed that oral and intravenous digoxin showed a different evolution of the serum concentrations with the absorption phase not being significant. Thus, to simplify the variability of digoxin disposition, the authors applied a two-compartment open model, which explained better the pharmacokinetic profiles. The analysis showed that total body weight (TBW), CrCl and the co-administration of spironolactone and calcium channel blockers (CCBs) were the variables with most influence on digoxin pharmacokinetics. Therefore, they stated that these variables must be considered when establishing optimal therapeutic regimens for digoxin.
According to clinical guidelines60, the drugs with potential interaction with digoxin are amiodarone, antiacids, CCBs, cholestyramine, thiazide and loop diuretics, macrolides, nonsteroidal anti-inflammatory drugs, potassiumsparing diuretics, prokinetic agents, propafenone, proton-pump inhibitors, sulfonylureas and trazodone. However, only Komatsu46 evaluated amiodarone, one of the drugs most associated with an interaction with digoxin. The results proved that this drug increased digoxin trough levels due to inhibition of P-glycoprotein and renal tubular secretion. These results confirm that digoxin and amiodarone combination increase the mortality in patients with AF61. In addition, drugs that inhibit P-glycoprotein (itraconazole, cyclosporine and erythromycin) as well as verapamil and bepridil, were not included as covariates because a few patients were treated with them (46).
Several drugs can exert an important influence on the pharmacokinetics of digoxin: spironolactone and CCBs, which inhibit P-glycoprotein, decrease digoxin clearance around 23%47, up to 20%45, 21.6%54 and 4.3%48.
Patients with CHF evidenced a digoxin clearance reduced by 10%45 and 5.9%48. In fact, patients in treatment with spironolactone and diagnosis of CHF showed a digoxin metabolism decreased by 30%45. Beta-blockers (bisoprolol and carvedilol), atorvastatin and tolvaptan may increase SDC by 1.3-fold, 1.2-fold and 1.3-fold, respectively46. These data demonstrate the importance of considering these covariates when establishing an individualized digoxin regimen in elderly patients. All authors evaluated renal function with different methods: five studies estimated CrCl41–44,46 whereas four papers analysed SCr45,47–49. The covariates evaluated in the studies reviewed are summarized in Table 2.
Population pharmacokinetic parameters, interindividual variability, residual variability and structural models
| Study | Variables evaluated | Pharmacokinetic parameters (IIV) | Residual variability | Structural model |
|---|---|---|---|---|
| Konishi41 | CrCl | − | − | Hyperbolic |
| Co-medications: SPI | ||||
| Hornestam42 | Age, CrCl, TBW, SEX | Cl (L/h): 9.88 (66%) | 0.19 ng/mL | Two-compartment |
| Co-medications: none | Vc (L): 27.8 (−) | |||
| Vp (L): 444 (−) | ||||
| Q (L/h): 71.8 (−) | ||||
| Williams43 | Age, CrCl, TBW, IBW, CHF, BSA | Cl (L/h): 3.1 (44%) | 26% | One-compartment |
| Co-medications: QUIN | V (L): 4.03 (48%) | |||
| Ka (h-1): 0.47 (fixed) | ||||
| F: 0.82 (fixed) | ||||
| Choi44 | Age, CrCl, TBW, PIBW, SEX, K, albumin, cholesterol, TLC, NRI, indication for digoxin | Cl/F (L/h): 1.36 (34.26%) | 0.225 ng/mL | One-compartment |
| Co-medications: none | V/F (L): 735 (56.83%) | |||
| Ka (h−1): 1.63 (fixed) | ||||
| Chen45 | Age, SCr, TBW, BUN, ALT, AST, SEX, CHF, AF | Cl/F (L/h): 8.9 (43.2%) | 31.6% | One-compartment |
| Co-medications: SPI, ACEIs, CCBs, thiazide diuretics | V/F (L): 420 (65.8%) | |||
| Ka (h−1): 3.85 (fixed) | ||||
| Komatsu46 | Age, CrCl, TBW, EF, systolic blood pressure | Cl/F (L/h): 1.21 (32.2%) | 25.5% | Steady-state |
| Co-medications: AMD, bisoprolol, amlodipine, atorvastatin, azelnidipine, carvedilol, nifedipine, SPI, tolvaptan, antiarrhythmic drugs (class I and IV) | ||||
| Bauer47 | Age, SCr, CrCl, TBW, IBW, SEX, BSA, CHF | Model 1: Cl/F (L/h): 2.37 (26%) | Model 1: 61% | Steady-state |
| Co-medications: QUIN, CCBs (VER) | Model 2: Cl/F (L/h): 0.795 (24%) | Model 2: 55% | ||
| Yukawa48 | Age, SCr, TBW, SEX, CHF, digoxin Ctrough | Cl/F (L/h): 0.588 (3.5%) | 13% | One-compartment |
| Co-medications: SPI, CCBs (VER, diltiazem, nifedipine) | V (L/kg) = 7.5 (fixed) | |||
| Ka (h−1): 0.47 (fixed) | ||||
| Zhou49 | Age, SCr, TBW, SEX, ALT, AST, BUN, albumin | Cl/F (L/h): 5.90 (49.0%) | 0.365 ng/mL | One-compartment |
| Co-medications: SPI, CCBs (nifedipine, diltiazem), nitrate, propafenone | V/F (L): 550 (94.3%) | |||
| Ka (h−1): 1.63 (fixed) |
Information reported as (−): data not recorded.
ACEIs: angiotensin converting enzyme inhibitors; AF: atrial fibrillation; ALT: alanine aminotransferase; AMD: amiodarone; AST: aspartate aminotransferase; BMI: body mass index; BSA: body surface area; BUN: blood urea nitrogen; CCBs: calcium channel blockers; CHF: congestive heart failure; Cl: clearance; CrCl: creatinine clearance; Ctrough: trough digoxin concentration; EF: ejection function; F: bioavailable fraction; IBW: ideal body weight; IIV: interindividual variability; K: serum potassium; Ka: absorption rate constant; NRI nutritional risk index; PIBW: percentage of ideal body weight; Q: intercompartmental clearance; QUIN: quinidine, coded as 1 when quinidine was co-administered and 0 otherwise; SCr: serum creatinine; SEX: gender, coded as 1 for females and 0 otherwise; SPI: spironolactone, coded as 1 when spironolactone was co-administered and 0 otherwise; TBW: total body weight; TLC: total lymphocyte count; V: distribution volume; Vc: central volume of distribution; VER: verapamil; Vp: peripheral volume of distribution.
Another difference in the criteria refers to sampling time. Two authors used digoxin trough levels44,48. The other studies did not specify the sampling time or they did not report this information. These different criteria when collecting and evaluating patient data may explain the discordant pharmacokinetic results.
The authors found that CCBs co-administration diminished digoxin clearance around 20%.
Finally, Yukawa48 developed an equation to achieve the desired therapeutic effect of digoxin in elderly patients:
where MD is maintenance dose, Cav is the average serum concentration of digoxin, CCB is 1 for concomitant administration of calcium channel blockers and 0 otherwise, CHF is 1 for patients with congestive heart failure and 0 otherwise and SEX is 0 for male and 1 for female.Concerning the analytical methodologies employed in the reviewed studies, four different techniques were used to analyze SDC. Hornestam42, Williams43, Choi44, Chen45 and Zhou49 quantified SDC with a fluorescence polarization immunoassay. Komatsu46 and Yukawa48 used a cloned enzyme immunoassay and Bauer47, a radioimmunoassay. All techniques presented a coefficient of variation ≤12%.
Konishi41 used a microparticle enzyme immunoassay. However, Cobo et al.62 reported cross-reactions between SDC estimation with this analytical method and clinical doses of spironolactone and potassium canrenoate, producing falsely high or low concentrations. According to DeFrance et al.63, the use of the particle enhanced turbidimetric inhibition immunoassay (cDig) and chemiluminescent microparticle immunoassay (iDig) avoid this interaction, being an alternative to microparticle enzyme immunoassay. Moreover, based on the Bland-Altman analysis, interchangeability of immunoassays is not valid. Therefore, patients should be monitored with the same analytical technique even if there are no interferences63.
Digoxin is often used to treat CHF and AF, which mainly affect elderly patients. Several clinical, demographics and pharmacological covariates may affect digoxin clearance and volume of distribution, with the need for adjusting this drug dosage. According to the nine studies analysed, the covariates with the highest influence on digoxin pharmacokinetics were renal function (9/9), body weight (6/9), the co-administration of CCBs (3/9) and the presence of CHF (3/9). The authors found other covariates with effect on digoxin PopPK: age (2/9), concomitant spironolactone (2/9), quinidine (2/9) or amiodarone (1/9), sex (1/9) and serum potassium (1/9). However, there are three sources of variation that would explain the difference in the results obtained by the authors: the disparity in the clinical parameters collected, the chosen structural model and the applied analytical method. The final PopPK equations are shown in Table 3.
Final equations of the models
| Study | Final model |
|---|---|
| Konishi41 | Cl (L/h) = ß · CrCl + Y |
| Hornestam42 |
|
| Williams43 |
|
| Choi44 |
|
| Chen45 |
|
| Komatsu46 | Cl/F (L/h) = (1.21 + 0.0532 · CrCl) · (1 + 0.787. AMD) |
| Bauer47 |
|
| Yukawa48 | Cl/F (L/h) = 0.588 · TBW0189 · SCr−0163 · (AGE/65)−0152 · 0.957CCB · 0.941CHF · 0.965SEX · Ctrough−0.18 |
| Zhou49 | Cl/F (L/h) = 5.9 · [1-0.412 · SPI] · [1-0.0101 · (TBW-62.9)] · [1-0.0012 · (SCr-126.8)] |
| V/F (L) = 550 |
AMD: amiodarone; CCB: calcium channel blocker; CHF: congestive heart failure, coded as 1 if the patient was diagnosed with CHF and 0 otherwise; Cl: clearance; CrCl: creatinine clearance; Ctrough: trough digoxin concentration; F: bioavailable fraction; K: serum potassium; PIBW: percentage of ideal body weight; Q: intercompartmental clearance; QUIN: quinidine, coded as 1 when quinidine was co-administered and 0 otherwise; SCr: serum creatinine; SEX: gender, coded as 1 for females and 0 otherwise; SPI: spironolactone, coded as 1 when spironolactone was co-administered and 0 otherwise; TBW: total body weight; V: distribution volume; Vc: central volume of distribution; VER: verapamil, coded as 1 when verapamil was co-administered and 0 otherwise; Vp: peripheral volume of distribution; ß: constant; Y: constant.
The systematic review performed by Abdel Jalil et al.51 analyzed the PopPK of digoxin in adult patients. Sixteen articles were included in the analysis with only two studies conducted in elderly patients as a separate population. The authors found large dispersion in the age of the participants, without analyzing pathophysiological characteristics of elderly patients and their influence on digoxin pharmacokinetics. Most studies were conducted in East Asian populations (66.8%) with digoxin pharmacokinetics usually described by a one-compartment model. The most identified predictors of digoxin clearance were weight, age, kidney function, presence of CHF and co-administered medications (CCBs). In the present paper, we focus exclusively on elderly patients (≥65 years), the main target population for digoxin treatment, with nine articles found and reviewed. Another possible source of bias is the different sample size (ranged from 94 to 294 patients) and the absence of a validation group in four studies.
A limitation of this review as well as that of Abdel Jalil51 is that most of studies (six out of nine in our article) were carried out in patients from East Asia (China, Japan and Korea).
Therefore, their conclusions may not be extrapolated to patients from other ethnic origins due to differences in genetic and lifestyle characteristics. However, no studies have been found that relate the results obtained with the ethnicity of the patients. Another limitation of our study is that SDC was determined using four analytical techniques. This may lead to different values, with higher variations if interference with spironolactone or potassium canrenoate with the analytical techniques used. Finally, the recorded covariates must be considered as another limitation. A great disparity was found, mainly related to concomitant medications. For instance, amiodarone, a drug with a high influence on SDC according to clinical guidelines, was evaluated in one study46. In addition, serum potassium, a parameter usually related to digoxin pharmacodynamics, was evaluated in one article, proving its influence increasing digoxin clearance44.
Considering the characteristics of digoxin (narrow therapeutic range, severe side effects, high toxicity) we believe that further research is necessary to develop PopPK models in elderly patients (≥65 years-old) to facilitate identifying covariates and how to interpret their likely importance for dose individualization in this population.
FundingNo funding.
Conflict of interestNo conflict of interest.
Early Access date (11/20/2022).