To identify and summarize the processes implemented and the activities performed by community and hospital-based pharmacists during the COVID-19 pandemic.
MethodA scoping review was carried out of the PubMed/Medline database with the aim of identifying articles published until 30 June 2021. The PRISMA recommendations for this type of review were followed. The articles included were reviewed and classified according to their main characteristics and outcomes, according to population, concept and context. The processes and activities identified were grouped into three categories: those performed in community and hospital pharmacies, those performed essentially in community pharmacies, and those performed essentially in hospital pharmacies.
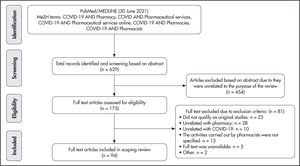
ResultsA total of 629 articles were identified, of which 454 were excluded because they were unrelated to the object of the review and 81 due to meeting the exclusion criteria. So, 94 articles were included in the analysis. Most studies were conducted in Europe and the United States. During the COVID-19 pandemic, the processes implemented and the activities carried out in both community and hospital-based pharmacies included pharmaceutical care, efficient and timely management of services, information and education, psychological support, pharmacovigilance and telepharmacy. Processes implemented and activities carried out essentially in community pharmacies were those related to the detection and referral of COVID-19 patients, testing and immunization, home care recommendations, and drug indications. Finally, processes and activities essentially occurring in hospital pharmacies included those related to participation in drug treatment research, drug evaluation and guidelines development, and to managing off-label drugs.
ConclusionsDuring the COVID-19 crisis, pharmacists have led and implemented processes aimed at mitigating the impact of the pandemic on the population’s health. Pharmaceutical care, efficient and timely management of services, information and education, psychological support, pharmacovigilance and telepharmacy, both in community and hospital pharmacies, are the main processes implemented by pharmacists during the COVID-19 pandemic. These processes and activities, in addition to contributing to the control, prevention and effective and safe treatment of COVID-19; have ensured the implementation of biosecurity measures, proper dispensing of medication, the drug rational use, and the provision of evidence-based information and education.
Identificar y sintetizar los procesos y actividades realizados por el farmacéutico en la farmacia comunitaria y hospitalaria durante la pandemia por COVID-19.
MétodoRevisión sistemática exploratoria en PubMed/Medline de artículos publicados hasta el 30 de junio de 2021, siguiendo las recomendaciones PRISMA para este tipo de revisiones. Los artículos incluidos se clasificaron según sus principales características y resultados, acorde con la estructura: población, concepto y contexto. Los procesos y las actividades identificados se agruparon en tres categorías: realizados en farmacia comunitaria y hospitalaria, llevados a cabo esencialmente en farmacia comunitaria y realizados esencialmente en farmacia hospitalaria.
ResultadosSe identificaron 629 artículos, de los cuales se excluyeron 454 por no estar en relación con el objeto de la revisión y 81 por los criterios de exclusión; por tanto, se incluyeron 94 en la revisión y análisis. La mayoría de los estudios se desarrollaron en Europa y Estados Unidos. Entre los procesos y actividades llevados a cabo por el farmacéutico durante la pandemia, tanto en farmacia comunitaria como en hospitalaria, destacaron: atención farmacéutica, gestión eficiente y oportuna de los servicios, información y educación, apoyo psicológico, farmacovigilancia y telefarmacia. En farmacia comunitaria destacaron también los relacionados con la detección de COVID-19 y derivación de pacientes, inmunización en farmacias, recomendaciones de cuidados en el hogar e indicación farmacéutica. Entre los procesos realizados esencialmente en farmacia hospitalaria destacaron los relacionados con la participación en investigaciones de tratamientos farmacológicos, desarrollo de guías de utilización de medicamentos basadas en evidencia y manejo de medicamentos en indicaciones no aprobadas.
ConclusionesDurante la pandemia por COVID-19, los farmacéuticos han liderado e implantado procesos orientados a mitigar su impacto en la salud de la población. Atención farmacéutica, gestión eficiente y oportuna de los servicios, información y educación, apoyo psicológico, farmacovigilancia y telefarmacia fueron los principales procesos y actividades realizados en farmacia comunitaria y hospitalaria durante la pandemia por COVID-19. Dichos procesos y actividades buscaron, además de contribuir al control, prevención y tratamiento efectivo y seguro de la COVID-19, asegurar la implantación de medidas de bioseguridad, la dispensación y uso adecuado de los medicamentos y la información y educación basadas en la mejor evidencia disponible.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the causative agent of COVID-19, was initially reported in Wuhan (China) in December 2019 and rapidly spread worldwide until it reached pandemic status1. The resulting health crisis added to the problems related to prevention and appropriate treatment, drove society to look for urgent solutions. During that period, pharmacists rose to the occasion and contributed to minimizing the risk and to making rational, effective and safe decisions2.
Some publications describe how, in addition to interventions and activities related to acquiring and dispensing medications and medical devices, pharmacists have carried out important functions in the realms of research into and prevention and mitigation of COVID-19. They also took on the responsibility to inform and educate the general public on the risks and preventive and pharmacotherapeutic measures to be adopted in the fight against COVID-19, and to identify and refer persons suspected to be infected with the disease3,4. For that reason, the structured identification and the summarization of the information available on a global scale about the processes implemented and the activities carried out by pharmacists could favor the dissemination and practical implementation of the lessons learned, and the development of plans and strategies allowing a more effective adaptation and response to similar situations in the future. Against this background, the purpose of this review was to identify and summarize the main processes implemented and the activities carried out by community and hospital pharmacists during the COVID-19 pandemic.
MethodsA scoping review was carried out following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines extension for scoping reviews (PRISMA-ScR) checklist5.
Inclusion and exclusion criteriaThe systematic review was directed at identifying studies containing information on the processes implemented and the activities carried out by community and hospital pharmacists during the COVID-19 pandemic. Studies of all kinds were selected, published in any country and pertaining to any healthcare domain. Inclusion criteria were as follows: 1) original studies analyzing the activities performed by pharmacists in connection with the COVID-19 pandemic; and 2) articles describing processes, activities, strategies or approaches related with the role of pharmacists during the pandemic. All articles included were reviewed and classified according to their main characteristics and results, following the recommendations for scoping reviews5.
The exclusion criteria were as follows:
- –
Studies focused on describing comorbidities or the diagnosis and treatment of COVID-19.
- –
Studies unrelated with COVID-19.
- –
Studies unrelated with pharmaceutical matters.
- –
Studies where full text was unavailable.
A search of the literature was performed in the PubMed/Medline database of articles in English and Spanish published up to 30 June 2021, using the following MeSH search terms: “COVID-19” AND (“Pharmacy” OR “Pharmaceutical Services” OR “Pharmaceutical Services Online” OR “Community Pharmacy Services” OR “Pharmacists” OR “Pharmacies”). Three independent reviewers examined the titles and abstracts of all the articles found to determine whether they met the above-mentioned eligibility criteria. If the abstract of an article met the inclusion criteria, the full text was read and analyzed. Disagreements between the reviewers were resolved by discussion and consensus or, alternatively, by consultation with a fourth reviewer.
The following general and detailed information was recorded and tabulated in a specifically designed Microsoft Excel template:
- –
General information: language, name of first author, year of publication, continent, country, and publication type.
- –
Specific information of the processes implemented and the activities carried out by pharmacists in the community, hospital or community and hospital domains (processes and related activities).
The information identified in the course of this scoping review is presented following a narrative synthesis both in text and tables. Processes and activities were grouped into three categories depending on the area where they were carried out: community pharmacy, hospital-based pharmacy, and community and hospital-based pharmacy. In addition, a series of perspectives and challenges were included, which could serve as orientation for the processes implemented and activities performed in the community and hospital-based pharmacy settings.
ResultsArticles included in the reviewA total of 629 articles were identified, of which 454 were excluded on account of not being related with the purpose of the review and 81 because they did not meet the inclusion criteria. This means that the study sample was made of 94 articles, which contained relevant information on the processes implemented and the activities carried out by pharmacists during the pandemic (Figure 1). Of the 94 articles, 31 (33%) had been published in Europe and 25 (27%) in North America. A total of 78 (83%) articles had been published in English and 31 (33%) were commentary-type publications (Table 1).
Characteristics of the studies included in this scoping review
| Characteristic | Category | Number (n = 94) | % |
|---|---|---|---|
| Language | English | 78 | 83 |
| Spanish | 16 | 17 | |
| Europe | 31 | 33 | |
| North America | 25 | 27 | |
| Continent | Asia | 23 | 24 |
| Africa | 8 | 9 | |
| South America | 4 | 4 | |
| Oceania | 3 | 3 | |
| Type of publication | Commentary | 31 | 33 |
| Case report | 20 | 22 | |
| Cross-sectional study | 17 | 18 | |
| Review | 14 | 15 | |
| Cohort study | 4 | 4 | |
| Editorial | 4 | 4 | |
| Letter to the editor | 3 | 3 | |
| Cases and controls | 1 | 1 |
The review revealed that, during the study period, pharmacists made contributions related to the control, prevention, and treatment of COVID-19. The information concerning the processes implemented and the activities performed in each pharmacy domain is described below.
1Processes implemented and activities carried out by pharmacists in the settings of community and hospital pharmacyProcesses and activities in the community and hospital pharmacy setting were mainly concerned with: 1) pharmaceutical care; 2) efficient and timely management of services: effective and safe dispensing and management of medicines and medical devices; 3) information and education; 4) psychological support; 5) pharmacovigilance; and 6) telepharmacy. These processes and activities are described in Table 2.
Processes implemented and activities carried out by community and hospital-based pharmacists
| Author (country, year) | PROCESSES: ASSOCIATED ACTIVITIES |
|---|---|
| Aruru et al.4 (United States, 2021); Al-Quteimat et al.6 (Jordan, 2021); Amariles et al.7 (Colombia, 2021); Adunlin et al.8 (United States, 2021); Hedima et al.9 (Nigeria, 2021); Hussain et al.10 (United States, 2021); Kretchy et al.11 (Ghana, 2021); Li et al.12 (China, 2021); Merks et al.13 (Poland, 2021); Okoro14 (Nigeria, 2021); Song et al.15 (China, 2021); Surapat et al.16 Thailand, 2021); Ta et al.17 (Pakistan, 2021); Visacri et al.18 (Brazil, 2021); Ying et al.19 (China, 2021); Zheng et al.20 (China, 2021); Lemtiri et al.21 (United States 2020); Mallhi et al.22 (Pakistan, 2020); Strand et al.23 (United States, 2020); Ung24 (China, 2020). | Pharmaceutical care:
|
| Al-Quteimat et al.6 (Jordan, 2021); Amariles et al.7 (Colombia, 2021); Hussain et al.10 (United States, 2021); Merks et al.13 (Poland, 2021); Mallhi et al.22 (Pakistan, 2020); Ung24 (China, 2020); Adam et al.25 Canada, 2021); Badreldin et al.26 (Saudi Arabia, 2021); Nigro et al.27 (Brazil, 2021); Paul et al.28 (United States, 2021); Schiller et al.29 (United States, 2021); Sousa-Pinto et al.30 (the Netherlands, 2021); Warr et al.31 (United States, 2021); Alexander et al.32 (United States, 2020); Alonso-Herreros et al.33 (Spain, 2020); Siddiqui et al.34 (UAE, 2020); Brey et al.35 (South Africa, 2020); Cabañas et al.36 (Spain, 2020); Chahine37 (United States, 2020); Choo et al.38 (United States, 2020); Climent-Ballester et al.39 (Spain, 2020); Cochran et al.40 (United States, 2020); Corregidor-Luna et al.41 (Spain, 2020); Czech et al.42 (Poland, 2020); David et al.43 (Nigeria, 2020); Ding et al.44 (China, 2020); García-Gil et al.45 (Spain, 2020); Hashimoto et al.46 (Japan, 2020); Hua et al.47 (China, 2020); Larrouquere et al.48 (Spain, 2020); Liu et al.49 (China, 2020); Meng et al.50 (China, 2020); Nguy et al.51 (Australia, 2020); Palomar-Fernández et al.52 (Spain, 2020); Peris-Martí et al.53 (France, 2020); Shuman et al.54 (United States, 2020); Sin et al.55 (United States, 2020); Stergachis et al.56 China, 2020); Strand et al.23 (United States, 2020); Zaidi et al.57 (UK, 2020); Wu et al.58 (China, 2020). | Effective and timely management of services: effective and safe dispensing and use of medicines and medical devices:
|
| Al-Quteimat et al.6 (Jordan, 2021); Amariles et al.7 (Colombia, 2021); Merks et al.13 (Poland, 2021); Mallhi et al.22 (Pakistan, 2020); Ung24 (China, 2020); Chahine37 (United States, 2020); Alhamad et al.59 (Jordan, 2021); Bahlol et al.60 (Egypt, 2021); Erku et al.61 (Australia, 2021); Alderman et al.62 (United States, 2020); Bhat et al.63 (United States, 2020); Cerbin-Koczorowska et al.64 (Poland, 2020); Fan et al.65 (United States, 2020); Kasahun et al.66 (Ethiopia, 2020); Herranz-Alonso et al.67 (Spain, 2020); Mahmoudjafari et al.68 (United States, 2020). | Information and education:
|
| Amariles et al.7 (Colombia, 2021); Ta et al.17 (Pakistan, 2021); Zheng et al.20 (China, 2021); David et al.43 (Nigeria, 2020); Kasahun et al.66 (Ethiopia, 2020); Hayden et al.69 (Ireland, 2020); Luykx et al.70 (the Netherlands, 2020). | Psychological support:
|
| Al-Quteimat et al.6 (Jordan, 2021); Amariles et al.7 (Colombia, 2021); Adunlin et al.8 (United States, 2021); Ta et al.17 (Pakistan, 2021); Mallhi et al.22 (Pakistan, 2020); Alonso-Herreros et al.33 (Spain, 2020); Larrouquere et al.48 (France, 2020); Wu et al.58 (China, 2020); Diaby et al.71 (United States, 2021); Alshamrani et al.72 (Saudi Arabia, 2020); Aranguren-Oyarzábal et al.73 (Spain, 2020); Brandt et al.74 (United States, 2020); Gérard et al.75 (France, 2020); Gil-Navarro et al.76 (Spain, 2020); Harrigan et al.77 (United States, 2020); Lund et al.78 (Denmark, 2020); Santolaya-Perrin et al.79 (Spain, 2020); Sevilla-Sánchez et al.80 (Spain, 2020); Slimano et al.81 (France, 2020); Sun et al.82 (China, 2020). | Pharmacovigilance:
|
| Adunlin et al.,8 United States (2021); Merks et al.13 (Poland, 2021); Sousa-Pinto et al.30 (the Netherlands, 2021); Mohamed-Ibrahim et al.83 (UAE, 2021); Como et al.84 (United States, 2020); Elson et al.85 (UK, 2020); Hoti et al.86 (Kosovo, 2020); Ma87 (United States, 2020); Mallhi et al.22 (Pakistan, 2020); Margusino-Framiñán et al.88 (Spain, 2020); Thiessen et al.89 (United States, 2020); Tortajada-Goitia et al.90 (Spain, 2020); Yemm et al.91 (Ireland, 2020). | Telepharmacy:
|
In their role as first-line care institutions, community pharmacies have acted as suppliers of medicines, personal protection equipment (PPE) and hygiene and disinfection products. They have also provided information on the drug therapy indicated for persons with suspected COVID-19, detecting suspect cases and referring them to healthcare centers2'20'23'35'70.
- •
COVID-19 detection and patient referral. A significant number of community pharmacies have been authorized and trained to sell and perform rapid (particularly antigen) COVID-19 detection tests and to send samples to labs for analysis. Pharmacists have advised persons with suspected COVID-19 on how to take care of themselves at home and, in cases where symptoms persisted, referred consultations to a healthcare center2,7,9,20,23,43,92,93.
- •
Pharmacy-based immunization. Community pharmacists have played a key role in the large-scale administration of vaccines against COVID-19 expanding access to immunization23,94, and making available information on the safety and efficacy of vaccines15,95. The involvement of pharmacists has contributed to optimizing visits to health institutions, to a wider availability of care and information61 and, ultimately, to wider accessibility, higher immunization rates, and greater amounts of information on the progress of vaccination23,42,69.
- •
Home care. Community pharmacists have advised patients on the measures they must adopt at home if they suspect they may be infected with COVID-19, including proper hygiene and disinfection of surfaces and frequently used objects and utensils in order to decrease the spread of the virus7,20.
- •
Pharmaceutical indication. When someone walks into a community pharmacy and asks “what can I take to resolve this minor ailment I have?” the pharmacist is allowed to provide the guidance requested. When consulted about minor ailments (self-limiting or uncomplicated conditions which can be identified and treated without medical intervention), community pharmacists indicate non-pharmacological interventions or over-the-counter drugs, or they may refer the person to a physician7. During the COVID-19 pandemic, consultations at community pharmacies increased due to a higher incidence of skin rashes, cough, colds, and gastrointestinal symptoms95. In this role, pharmacists have contributed to reducing the risk of medicine stock-outs11,95 and the number of unnecessary health care consultations6.
Within hospitals, pharmacists have been instrumental in guaranteeing the supply of medicines and other products required for the management of COVID-1920'53. They have also implemented the technological tools needed for monitoring and sending out prescribed medicines and they have participated in the development of care protocols for COVID-19 patients41.
- •
Participation in research into pharmacological treatments. Hospital pharmacists have participated in the development of clinical trials geared toward evaluating the efficacy and safety of the drugs used for treating patients hospitalized for COVID-197. As regards clinical trials on COVID-19 vaccines, they have been part of programs aimed at monitoring adverse events associated to the vaccines7,15,49.
- •
Development of evidence-based guidelines for medication use. In an endeavor to mitigate the effects of COVID-19, pharmacists have worked hand in hand with physicians to improve the quality of care provided to patients by making dose adjustments for medications in accordance with pharmacokinetic, pharmacodynamics and clinical criterions. This has resulted in a more appropriate, effective and safe use of medications and has freed up staff time for taking care of other patients74.
- •
Off-label use of medicines for COVID-19. The search of alternatives for the treatment of COVID-19 has led to use the some medicines that have not been approved for COVID-1968,74,86. Such was the case of azithromycin, hydroxychloroquine, lopinavir-ritonavir and nonsteroidal anti-inflammatory drugs80, among others78. This has resulted in the need for stricter surveillance to identify drug-related adverse events and closer monitoring of the positive and negative outcomes of the use of those medicines75.
The articles reviewed underscore the existence of several challenges in connection with the development and strengthening of pharmacists’ competencies for mitigating, controlling, and treating COVID-19 and other emerging infections. Such challenges include:
- –
Preserving the pharmacy staff’s physical and mental health; adopting the required biosecurity measures and modifying workflows accordingly trying to minimize the risk of that patients or the pharmacy staff may get infected7,20,23,34,35,43,70.
- –
Monitoring the stock of essential medications and other medical devices, guarantying medicines supply chain, and therefore that they can be dispensed as required7,20,23,35,43,54,26,70.
- –
Improving healthcare coverage and ensuring that the patients’ needs are met, upholding the principles of high-quality pharmaceutical care and the recognition of the role of pharmacists as primary healthcare providers11,17,23.
- –
Optimizing the regulatory processes associated with the evaluation and presentation of the best available evidence for the inclusion of medicines and other pharmaceutical products in clinical guidelines65,68,96.
- –
Managing and optimizing hospital bed occupancy and participating in disaster recovery plans at an institutional level29,50,58.
- –
Preparing isolation, hygiene and disinfection protocols for different hospital areas, increasing pharmacists’ competencies in the epidemiological and clinical aspects of COVID-1927,55,72.
- –
Optimizing patient information and education, ensuring that the public and other healthcare providers are kept informed on the emerging evidence on vaccines and treatments against COVID-1915,23,69,95.
- –
Implementing information and education services based on the use of information and communication technologies (telepharmacy, telesupport, and teleguidance)23,33,41,44,86,87,89.
- –
Implementing innovative educational tools to improve patient education and satisfaction60,61,65.
- –
Designing and implementing free-of-charge online training sessions geared towards the pharmaceutical community on COVID-19 and associated prevention and treatment strategies59,63,67.
- –
Optimizing the conditions under which remote work takes place, videoconsultations are conducted, and telehealth is enforced with a view to improving patient access to healthcare and ensuring continuity of pharmaceutical care and activities23,41,47,86,89,91.
- –
Ensuring that pharmacy services are provided 24 hours a day, 7 days a week, in line with the demand arising out of the COVID-19 pandemic67,80,85,88.
- –
Creating spaces for disseminating educational contents and information on COVID-19 and other diseases with a significant impact on public health59,63,67.
- –
Providing psychological support to patients, identifying those with excessive levels of anxiety, concern or fear13,17,66.
- –
Optimizing pharmacovigilance programs, including the monitoring of the adverse effects of medicines used off-label. Such results should be reported so as to minimize the potential risks derived from using the said medications33,73,75,76,78.
- –
Evaluating the inclusion of medicines and medical devices according to the best evidence available2,74,77.
- –
Participating in multidisciplinary teams trough pharmacotherapy follow-up of patients with chronic conditions73,75,76,78,79,82.
- –
Implementing home dispensing systems, promoting the use of telepharmacy as a way of minimizing the risk of infection of both patients and healthcare providers44,48,53,69,74,77.
- –
Managing drug-drug interactions in polymedicated patients and those with comorbidities6,8,17,25,80.
This scoping review identified and summarized the processes implemented and the activities carried out by pharmacists during the COVID-19 pandemic. Such processes and activities have revolved around the control of the disease, the prevention of the spread of infection, and the treatment of the condition in individuals who developed it. In short, the processes implemented and the activities carried out by community and hospital pharmacists were aimed at ensuring continuous and timely access to medicines, medical devices and PPE, pharmaceutical care, health information and education, and at implementing biosecurity measures. Most of the processes and activities identified were carried out both in community and hospital pharmacies and underscore the importance of the work done and the contribution made by the pharmaceutical profession during the pandemic2,23,24,53,64,86.
As regards the processes implemented both by community and hospital pharmacies, special mention should be made of telepharmacy, a strategy that has allowed to provide different kinds of pharmacy services. Several authors, including Ding et al.44 have shown that the implementation of home dispensing systems and the use of telephone-based communication have made it possible to keep drug dispensing services running and ensure that pharmacists could communicate with their patients whenever necessary so that the latter could get appropriate levels of support and advice. Hua et al.47, for their part, proposed an information and education model based on the implementation of a module-based radio station to disseminate information to patients on the rational use of medications.
In the community pharmacies setting, several processes were identified related with detection of COVID-19 and patient referrals, vaccination at the pharmacies, home care recommendations and pharmacist-based indications. Moreover, some publications report on the activities carried out by pharmacists with regard to regulatory and educational matters65,74,96. In this connection, Amariles et al.3 proposed a route that could be followed by pharmacists to contribute to timely detection and to the referral of patients with suspected COVID-19. The route has three possible entry points: antiinfluenza medicines, symptoms compatible with a COVID-19 infection, or a request for disinfectant products or protective products such as antiseptic gels or face masks. Education on selfcare is often accompanied by an assessment of symptoms and signs compatible with the disease and, when a case is identified, it is immediately reported to the health authorities3. In essence, it is paramount that pharmacies advise patients on the symptoms associated with the virus, dispense medicines and PPE, educate patients on selfcare, and report possible cases to the designated telephone lines2,3,23,35,70.
As regards hospital pharmacies, the most frequently mentioned processes had to do with the participation of pharmacists in research projects on drug treatments, the development of guidelines on the evidence-based use of medicines, and the follow of medicines used off-label48,78,97,98. In addition, hospital pharmacists have actively participated in drug administration programs, in following up on potential drug-drug interactions, and in joining the physicians’ clinical guidance and investigation efforts to enhance the prescription process98.
It should be mentioned that Chinese field hospitals implemented dispensing and administration protocols for antipyretic analgesics, antimicrobials and, generally, the drugs necessary to treat non-communicable chronic conditions and the drugs used in the context of emergencies and resuscitation50,99. In this regard, hospital pharmacists have been made their decisions based on the follow-up and evaluation of the effectiveness and safety of medicines, contributing to achieving the best possible therapeutic results in patients with COVID-19.
The present study identified two similar reviews related to the role played by pharmacists during the COVID-19 pandemic. Visacri et al.18 carried out a scoping review describing and identifying the basic services provided by pharmacists during the pandemic, and Peris-Martí et al.53 described the implementation of pharmaceutical care in a rehabilitation facility, considering the lessons learnt with a view to their future application. Other publications, not identified by our search, also bear some similarities with the present article. Passos et al.97 performed a comprehensive literature review trying to determine the role of pharmacists during the COVID-19 pandemic in the 2019–2020 period; and Sami et al.98 described the contributions of pharmacies in the community, hospital and industrial domains.
The present review covered the period until 30 June 2021 and summarized in text and table format the processes implemented and the activities carried out by pharmacists, broken down into three categories: community and hospital pharmacies, mainly community pharmacies, and mainly hospital pharmacies. In addition, this review presents the challenges that will be faced by community and hospital-based pharmacists in the future in their endeavor to effectively adapt and respond to situations akin to the COVID-19 pandemic.
The COVID-19 pandemic has accelerated the consolidation of some pharmaceutical services, including telepharmacy, pharmacy-based vaccination and the screening of cases with suspected health problems. Further studies must be conducted whose design allows an assessment of the effectiveness of these processes and activities in achieving pharmacists’ long-sought goal of contributing the best possible health outcomes.
This review presents with several limitations, among them restricting the search to articles published in English and Spanish. Another limitation was the use of only one database (PubMed/Medline) as some relevant studies may have been left out as a result of not being indexed in that database. Moreover, the rapid increase in the number of publications on COVID-19 these days means that studies relevant to the research published after the established search period were left out. Finally, due to its exploratory nature, this systematic review did not analyze the quality of the studies included.
In conclusion, this scoping review identified and summarized the processes implemented and the activities carried out by pharmacists during the COVID‑19 pandemic. Although further studies are required whose methodological design allows drawing conclusions with higher levels of evidence, the information identified demonstrates that community and hospital-based pharmacists have played a key role in the prevention and, consequently, the reduction of the spread of the virus around the world. Their efforts have been directed at minimizing the infection risk, ensuring adequate dispensing of medicines and other medical devices, and achieving the best results possible with the drugs used, minimizing the risk of drug-related adverse events and drug-drug interactions. Pharmacists also played a significant role by providing patients with information and education on the drugs administered, particularly on the safety and efficacy of vaccines.
FundingThe Pharmaceutical Promotion and Prevention Group received funding from the 2018–2019 sustainability program of the Research Development Committee of the University of Antioquia and from the #UdeARespondeAl-COVID-19 initiative.
AcknowledgementsThe authors would like to express their gratitude to the #UdeARespondeAlCOVID-19 initiative and to Jaime Alejandro Hincapié García and Johan Granados Vega, professors at the University of Antioquia, for their contributions and suggestions while the manuscript was being prepared.
Conflict of interestsNo conflict of interests.
Presentation at congressesA summary of the article was submitted to and accepted by (in poster format) the VI Colombian Pharmaceutical Care Congress to be held from 11–13 November 2021.
Early Access date (09/28/2022).