Intensive care units (ICUs) pose challenges in managing critically ill patients with polypharmacy, potentially leading to adverse drug reactions (ADRs), particularly in the elderly.
ObjectiveTo evaluate whether the severity and clinical prognosis scores used in ICUs correlate with the prediction of ADRs in aged patients admitted to an ICU.
MethodsA cohort study was conducted in a Brazilian University Hospital ICU. APACHE II and SAPS 3 assessed clinical prognosis, while GerontoNet ADR Risk Score and BADRI evaluated ADR risk at ICU admission. Severity of the patients' clinical conditions was evaluated daily based on the SOFA score. ADR screening was performed daily through the identification of ADR triggers.
Results1295 triggers were identified (median 30 per patient, IQR=28), with 15 suspected ADRs. No correlation was observed between patient severity and ADRs at admission (p=0.26), during hospitalization (p=0.91), or at follow-up (p=0.77). There was also no association between death and ADRs (p=0.28) or worse prognosis and ADRs (p>0.05). Higher BADRI scores correlated with more ADRs (p=0.001).
ConclusionsThese data suggest that employing the severity and clinical prognosis scores used in ICUs is not sufficient to direct active pharmacovigilance efforts, which are therefore indicated for critically ill patients.
El manejo de pacientes críticos en Unidades de Cuidados Intensivos (UCI) enfrenta el desafío de la polifarmacia, que puede llevar a Reacciones Adversas a Medicamentos (RAM), particularmente en pacientes ancianos.
ObjetivoDeterminar si las puntuaciones de gravedad y pronóstico utilizadas habitualmente en la UCI están relacionadas con la predicción de RAM en pacientes ancianos ingresados en la UCI de un hospital universitario brasileño.
MétodosEstudio de cohortes en esta UCI, utilizando las puntuaciones APACHE II, SAPS 3, GerontoNet ADR Risk Score y BADRI para evaluar gravedad, riesgo de ADR y pronóstico de los pacientes. Diariamente, se evaluó la gravedad clínica (mediante puntuación SOFA) y las RAM (mediante factores desencadenantes).
ResultadosSe identificaron 1295 factores desencadenantes (mediana 30/paciente, IQR = 28), con 15 sospechas de RAM. No hubo correlación entre la gravedad del paciente y las RAM al ingreso (p = 0,26), durante la hospitalización (p = 0,91) o el seguimiento (p = 0,77). Tampoco hubo asociación entre muerte (p = 0,28) o peor pronóstico y RAM (p > 0,05). Las majores puntuaciones del BADRI se correlacionaron con un mayor número de RAM (p = 0,001).
ConclusionesLos datos sugieren que el uso de puntuaciones clínicas de gravedad y pronóstico utilizadas en las UCI no es suficiente para guiar los esfuerzos activos de farmacovigilancia.
The increasing global elderly population results from medical advances, reduced fertility, and health/sanitation improvements.1 In Brazil, a shift in age structure is evident, with a rising elderly population projected to reach approximately 30% by 2100.2,3 Hospitals and intensive care units (ICUs) face imminent challenges as elderly individuals comprise over half of admissions and experience elevated risks with a significant incidence of adverse drug reactions (ADRs).3
In Brazil, ICUs record 19 ADR events per 1000 patients/day, compared to 10 in other settings.3 Elderly ICU patients are particularly vulnerable due to physiological changes affecting pharmacokinetics/pharmacodynamics and polypharmacy, necessitating monitoring and dose adjustments.2,4 Despite advancements like The GerontoNet ADR Risk Score5 and Brighton Adverse Drug Reactions Risk (BADRI)6 for predicting ADR occurrence in hospitalized elderly individuals, there is still a lack of predictive accuracy in the face of the added complexity of critically ill patients.
This study aims to correlate severity/prognostic scores in ICUs with the prediction of ADRs in the elderly, guiding the selection of effective tools to enhance patient safety, reduce ICU mortality, and healthcare costs.
MethodsThis is a cohort study developed in the ICU for adults of the Clinical Complex Hospital of the Ribeirão Preto Medical School at the University of São Paulo (USP). The research project was approved by the Research Ethics Committee of the Ribeirão Preto Pharmaceutical Sciences School – USP (CAAE 44000715.6.0000.5403).
ParticipantsInclusion criteria: Individuals aged ≥60 years admitted to the ICU for ≥48 h between September 2016 and September 2017, after obtaining informed consent from their legal representative. Data were collected daily until discharge, referral to palliative care, or death (Supplementary Material 1).
Variables of interestSociodemographic variables (age, sex, race/skin color, smoking habit, alcohol consumption), anthropometric measurements [weight (kg), height (m), and calculation of the body mass index (BMI)], and clinical data (prognosis, risk of ADRs, admission diagnosis, renal and hepatic dysfunction, length of stay, reason for follow-up termination) were obtained from secondary sources, i.e., patient records and the institution's electronic database.
Prognosis was assessed using the Simplified Acute Physiology Score 3 (SAPS 3)7 and Acute Physiology and Chronic Health EvaluationII (APACHE II)8 upon ICU admission. Risk of ADRs was classified using The GerontoNetADRRisk Score5 and BADRI.6 Daily clinical severity assessment was conducted using the Sepsis-relatedOrgan Failure Assessment (SOFA)9 tool, and screening for suspected ADR cases was performed using trigger tools. The IHIGlobal Trigger Tool10 was used to select indicators of adverse events. Additionally, standardized medications, laboratory tests, and the institution's ICU routine were considered in choosing trigger tools for active surveillance (Supplementary Materials 2).
Causality assessment of ADRs was conducted using the Naranjo Algorithm11 (NARANJO), World HealthOrganization-UppsalaMonitoring Center Causality Assessment Scale12 (WHO-UMC), and LiverpoolADRcausality assessment tool13 (LCAT), as referenced below. Suspected ADRs were classified as definite, probable, possible, conditional, or doubtful, with agreement between 2 algorithms. Older adults were categorized as exposed (definite, probable, or possible ADRs) or unexposed, excluding conditional or doubtful cases. For categorical variables, absolute/relative frequencies were reported. Quantitative variables were presented as mean/standard deviation (SD) and median/interquartile range (IQR). Associations between categorical variables were assessed using Fisher's exact test and odds ratio (OR) for ADR risk. Quantitative associations were examined using Pearson's correlation (r). Mean differences were analyzed using the Student's t-test/Mann–Whitney test, with α=0.05. Statistical analyses were performed using SPSS® 17.1.0 for Windows.
ResultsThe study included 41 participants, identifying 1295 trigger tools, [median=30; (IQR 28)]. After chart review, 3.1% (40/1295) detected the occurrence of potential 15 ADRs in 11 patients. Based on the causal attribution, 1 ADR was classified as definite, 3 as probable, and 11 as possible (Supplementary Material 3).
Cardiovascular disorders were the leading admission causes (n=29; 70.7%). During ICU stay, septic shock was diagnosed in 25 patients (61.0%), and 22 (53.7%) showed multi-system physiological compromise. Before admission, 5 (12.2%) had liver disease, and 34 (82.9%) had previously diagnosed acute kidney injury and/or chronic kidney disease. Additionally, 17 patients were hospitalized for ≥14 days (41.5%), and 35 were admitted urgently or <12 h in advance (85.4%).
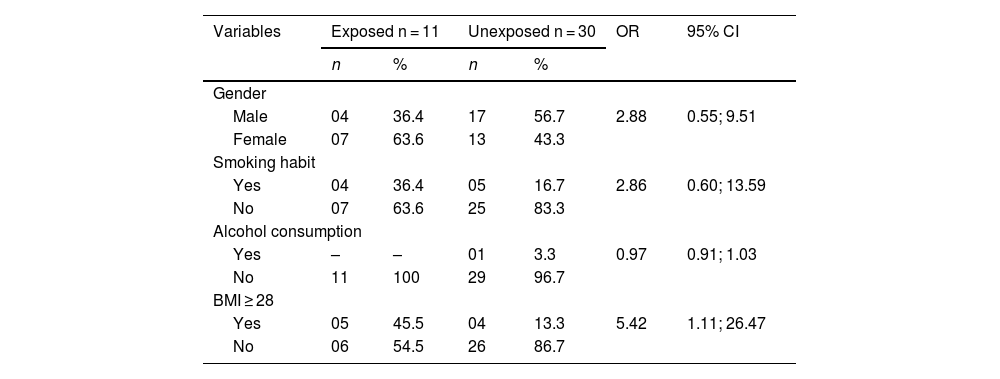
Patients with BMI ≥28 had 5.42 times higher odds of ADRs than those with BMI <28 (95% CI 1.11; 26.47). Sex, smoking habits, and alcohol consumption did not statistically differ between groups (Table 1). The mean age was 66.8 years, with no differences between exposed and unexposed groups (mean difference 1.85; p=0.52).
Sociodemographic and anthropometric characteristics corresponding to the exposed and unexposed groups.
| Variables | Exposed n = 11 | Unexposed n = 30 | OR | 95% CI | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Gender | ||||||
| Male | 04 | 36.4 | 17 | 56.7 | 2.88 | 0.55; 9.51 |
| Female | 07 | 63.6 | 13 | 43.3 | ||
| Smoking habit | ||||||
| Yes | 04 | 36.4 | 05 | 16.7 | 2.86 | 0.60; 13.59 |
| No | 07 | 63.6 | 25 | 83.3 | ||
| Alcohol consumption | ||||||
| Yes | – | – | 01 | 3.3 | 0.97 | 0.91; 1.03 |
| No | 11 | 100 | 29 | 96.7 | ||
| BMI ≥ 28 | ||||||
| Yes | 05 | 45.5 | 04 | 13.3 | 5.42 | 1.11; 26.47 |
| No | 06 | 54.5 | 26 | 86.7 | ||
BMI = body mass index.
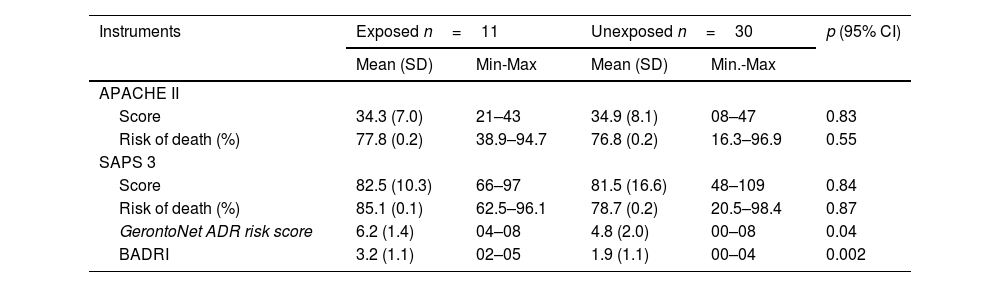
Patients had mean scores of 34.7 points in the APACHE II score and 81.8 in the SAPS 3 score, with average mortality risk of 77.0% and 80.4%, respectively. The mean risk score for ADRs by the GerontoNetADRRisk Score was 5.2, and although higher in the ADR-exposed group, there was no statistically significant relationship (p>0.05). The mean BADRI score was 2.2, and higher scores were associated with increased ADR odds (Table 2). The mean SOFA score at admission was 11.5, and at the end of daily monitoring was 9.7. There was no difference in mean SOFA score prognosis and the occurrence of ADRs at admission, during hospitalization, or at the study's conclusion. Additionally, there was no evidence of an association between hepatic dysfunction (OR=0.65; p=0.59), renal dysfunction (OR=1.54; p=0.59), hemodialysis (OR=1.8; p=0.49), and ADR occurrence.
Clinical prognosis at admission to the intensive care unit for the risk of death and development of ADRs at admission to intensive care unit.
| Instruments | Exposed n=11 | Unexposed n=30 | p (95% CI) | ||
|---|---|---|---|---|---|
| Mean (SD) | Min-Max | Mean (SD) | Min.-Max | ||
| APACHE II | |||||
| Score | 34.3 (7.0) | 21–43 | 34.9 (8.1) | 08–47 | 0.83 |
| Risk of death (%) | 77.8 (0.2) | 38.9–94.7 | 76.8 (0.2) | 16.3–96.9 | 0.55 |
| SAPS 3 | |||||
| Score | 82.5 (10.3) | 66–97 | 81.5 (16.6) | 48–109 | 0.84 |
| Risk of death (%) | 85.1 (0.1) | 62.5–96.1 | 78.7 (0.2) | 20.5–98.4 | 0.87 |
| GerontoNet ADR risk score | 6.2 (1.4) | 04–08 | 4.8 (2.0) | 00–08 | 0.04 |
| BADRI | 3.2 (1.1) | 02–05 | 1.9 (1.1) | 00–04 | 0.002 |
SD = standard deviation. Min. = minimum. Max. = maximum. APACHE II = Acute Physiology and Chronic Health Evaluation II. SAPS 3 = Simplified Acute Physiology Score 3. BADRI = Brighton Adverse Drug Reactions Risk.
The average ICU stay was 15.1 days, longer for patients with ADRs during hospitalization (mean difference 5.21, p<.001). The study concluded with the transfer of 24 patients to the ward, 14 deaths, and 3 referrals to palliative care. There was no evidence of increased ADR occurrence among patients who died compared to those transferred to the ward or referred to palliative care (OR=2.85; p=.28).
DiscussionThe analysis of most trigger tools highlighted alternative causes, underscoring the complexity of distinguishing ADRs from other adverse events in the elderly.14,15 This aligns with the inherent complexity of the studied population, highlighting the overlap of clinical conditions and the multifaceted nature of elderly patients in critical situations, where more than half exhibited multi-system physiological compromise.14,16
Although hepatic dysfunction, renal dysfunction, or hemodialysis are recognized risk factors for elderly and critically ill patients,16,17 no evidence of an association with ADR occurrence was found. This lack of association may be related to the pre-existing clinical conditions at ICU admission. The unexpected association between BMI ≥28 and ADRs suggests that overweight or obesity may elevate ADR risk, possibly due to pharmacokinetic changes secondary to obesity.16,17
The results do not indicate that severity at ICU admission, assessed by APACHE II and SAPS 3, or the evolution of organ dysfunction measured by the SOFA score, increase the risk of ADRs in critically ill elderly patients. This contrasts with findings in the literature associating greater severity and morbidity with increased ADR risks,14,15,17 suggesting the need for a larger sample size to validate this finding. However, GerontoNetADRRisk Score and BADRI scores were higher in patients with ADRs, aligning with the literature18 and supporting the potential of these tools to identify high-risk patients for targeted interventions in hospital pharmacovigilance.
The average ICU stay was longer for patients with ADRs, as seen in other studies,17,18 endorsing the practice of active pharmacovigilance as an important care strategy, as it systematizes early detection and management, contributing to reduced patient exposure to iatrogenic events. Age is often a risk factor for ADRs in elderly individuals due to pharmacokinetic and physiological changes in the face of frailty14,17; however, our findings showed no difference in the mean age between exposed and unexposed groups to ADRs. This may be due to the sample size and low age variability observed, as this study did not provide indications of susceptibility differences related to the aging process. Although ADRs do not appear to influence outcomes, as there was no higher occurrence in deaths compared to others (transfer to the ward or palliative care), the sample size may have impacted the result.
Some limitations are presented. The limited number of participants affects generalization to broader populations of critically ill elderly individuals and may have contributed to the lack of statistical significance. Future research should expand the sample, encompassing multiple centers, for validation and representativeness. Additionally, factors such as specific comorbidities and pharmacogenetic characteristics were not considered for a more comprehensive understanding of ADRs in critically ill elderly patients.
The lack of associations with known risk factors and the unexpected association with elevated BMI have implications for pharmacovigilance, as potential risks may be underestimated, compromising the implementation of preventive measures. Despite the study not finding evidence that severity at admission and the evolution of organ dysfunction increase the risk of ADRs, the scores show potential for identifying high-risk patients, warranting their use. The relationship between prolonged ICU stay and ADRs highlights the importance of active pharmacovigilance. These results can guide preventive strategies and inform future research and clinical practices in critically ill elderly patients.
Contribution to the scientific literatureThis study underscores the complexity of identifying ADRs in critically ill elderly patients, emphasizing the need to differentiate alternative causes.
FundingThis study was financed in part by the CAPES—Finance Code 001. National Council for Scientific and Technological Development (CNPq, Brazil).
Ethical considerationsThe research project was approved by the Research Ethics Committee of the Faculty of Pharmaceutical Sciences of Ribeirão Preto at the University of São Paulo (CAAE 44000715.6.0000.5403).
Responsibility and transfer of rights:Todos los autores aceptamos la responsabilidad definida por el Comité Internacional de Editores de Revistas Médicas (disponible en http://www.icmje.org/).
In the event of publication, the authors exclusively transfer the rights of reproduction, distribution, translation and public communication (by any means or sound, audiovisual or electronic support) of our work to Farmacia Hospitalaria and by extension to SEFH. To do this, a letter of transfer of rights will be signed at the time of sending the work through the online manuscript management system.
CRediT authorship contribution statementFabiana Angelo Marques Carizio: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – review & editing. Isabella do Vale de Souza: Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Thalita Zago Oliveira: Formal analysis, Writing – original draft, Writing – review & editing. Luana Sueli Silva: Writing – original draft. Natalia Chaguri Alves Rodrigues: Writing – original draft. Maria Olívia Barbosa Zanetti: Writing – original draft, Writing – review & editing. Fabiana Rossi Varallo: Writing – review & editing, Resources. Leonardo Régis Leira-Pereira: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing.