Adverse drug events are a well-known cause of emergency department admissions. FARM-URG is a project promoted by the REDFASTER working group of the Spanish Society of Hospital Pharmacy. Its aim is to evaluate these adverse events through regular prevalence measurements. The present study shows the results of the first observations. The goal was to determine the prevalence of adverse drug events with respect to the total number of patients treated in emergency departments and carry out a description of the different events identified.
MethodThis is a multicenter cross-sectional study carried out in the emergency room of 13 Spanish hospitals. The identification and registration of patients were obtained from the emergency department patient census at the time of the first prevalence measurement (16 June 2020). The REDCap® platform was used for patient registration.
ResultsThe 2020 FARM-URG registry, which included 13 hospitals, evaluated 772 patients, of whom 57 (7.4%) consulted for adverse drug events. Antithrombotic drugs were responsible for most of these episodes, acenocoumarol being the main drug involved (22.8%). Nine (15.8%) adverse drug events were caused by inappropriate drug prescriptions according to the STOPP-START criteria. Nineteen (33.0%) patients returned to the emergency service within 30 days from discharge.
ConclusionsAdverse drug events are a frequent cause of emergency department visits and are commonly associated with a significant percentage of re-visits. The FARM-URG project has been created with the purpose of obtaining dynamic and updated information on such events.
Los acontecimientos adversos por medicamentos son una causa conocida de asistencia a los servicios de urgencias. El objetivo del proyecto FARM-URG, impulsado por el Grupo de Trabajo REDFASTER de la Sociedad Española de Farmacia Hospitalaria, es evaluar estos acontecimientos adversos mediante cortes periódicos. En este estudio se muestran los resultados del primer registro. Sus objetivos fueron determinar la prevalencia de acontecimientos adversos respecto al total de pacientes atendidos y caracterizarlos.
MétodoEstudio multicéntrico transversal realizado en los servicios de urgencias de hospitales españoles. La identificación y registro de pacientes se obtuvo a partir del censo de pacientes atendidos en urgencias en el momento del corte (16 de junio de 2020). Se revisaron las historias clínicas retrospectivamente y se registraron los datos en la plataforma REDCap®.
ResultadosEn este corte FARM-URG de 2020 participaron 13 hospitales, que evaluaron 772 pacientes, de los cuales 57 (7,4%) habían accessed por un acontecimiento adverso por medicamentos. El grupo de fármacos antitrombóticos fue responsable de la mayor parte de estos episodios, siendo acenocumarol (22,8%) el principal fármaco implicado. Nueve (15,8%) de los acontecimientos adversos fueron causados por fármacos con prescripción inapropiada según los criterios STOPP-START. Diecinueve (33,0%) pacientes volvieron a visitar el servicio de urgencias antes de los 30 días del alta.
ConclusionesLos acontecimientos adversos por medicamentos son un motivo frecuente de visita a los servicios de urgencias y están asociados a un importante porcentaje de visitas posteriores tras el alta. El proyecto FARM-URG nace con el propósito de obtener información periódicamente para la posible implementación de medidas preventivas.
Adverse drug events (ADEs) constitute a major health problem for healthcare systems around the world. It has been estimated that 5–10% of hospital admissions and 10–30% of emergency room visits are due to (mostly preventable) ADEs1–3.
Emergency departments are considered a good observatory for a healthcare system as they operate on a 24/7 basis, are easily accessible, and receive a large number of visits every day. Several studies have been carried out in the last few decades with a view to analyzing the prevalence of ADEs resulting in visits to the emergency room. Studies conducted in Spain have found that between 20% and 30% of all emergency room visits can be attributed to an ADE3–8. This prevalence is similar to that reported for other countries2,9,10. However, the data comes from registers kept at specific hospitals over limited periods of time, which hinders its ability to identify incontrovertible trends regarding drugs leading to ADEs.
Despite the fact that ADEs are an undeniably significant healthcare problem and that many of them are regarded as potentially avoidable, no multicenter registers have been systematized in the emergency departments of our country. In contrast, other countries have successfully created such register systems2, which have given them access to very valuable information for the design of primary prevention strategies in high-risk patient groups. Against this background, the Emergency Pharmaceutical Care Working Group of the Spanish Society of Hospital Pharmacists (SEFH) created the FARM-URG project as a way of furnishing healthcare providers with the information they need on the type and prevalence of ADEs observed in the emergency setting. The project is based on the creation of a regular multicenter register of ADEs resulting in visits to the emergency departments of Spanish hospitals. The present study reports on the results of the first prevalence measurements for the register. The aim is to discuss the incidence of ADEs among patients visiting emergency rooms in Spain and to provide a characterization of such ADEs.
MethodsThis is a multicenter cross-sectional study carried out in the emergency rooms of a series of Spanish hospitals. The studied population comprised patients above 18 years of age presenting to the emergency room of one of the participating hospitals. The measurements reported were made on 16 June 2020 at 12 am. ADEs were defined as any harm caused by the use of a given drug (or the lack thereof)11.
To identify the ADEs to be included in the analysis, the pharmacists working at the different emergency departments retrospectively reviewed the discharge reports of their corresponding emergency room. Visits to the emergency department were considered to result from an ADE when the latter was part of the patients’ primary or secondary diagnosis. If nothing was said in the diagnosis about the ADE, the different comments made in the report as well as the clinical and analytical exams performed and the different drugs administered were reviewed. Presence of an ADE was assumed when two investigators independently came to that conclusion. ADEs resulting from the ingestion of drugs for self-harming purposes were excluded.
Information on the following variables was collected: age; sex; origin; comorbidities; number of drugs prescribed in the outpatient setting according to electronic records or reports from the patient's health center; destination following discharge; drug most directly involved in the ADE; other potentially involved drugs; type of ADE (adverse reaction- or medication-related); type of medication error, if applicable (under- or overdosing, interactions, poor adherence, misadministration, unavailability of the required drug); and compliance with the STOPP/START criteria12. No algorithm was applied to determine the cause/effect relationship of the drug with the ADEs observed or to determine the ADE's preventability. Lack of adherence was considered causative of an ADE if mentioned as such in the patients’ discharge report.
The data was collected using the REDCap® platform. Quantitative variables were described using central tendency and dispersion indices, while qualitative variables were expressed as frequencies and percentages.
The present research project benefited from funding from SEFH's working group grants. The study was approved by the research ethics committees of the participating hospitals (Reference hospital: Santa Cruz and San Pablo Hospital, Reference number: 19/294).
ResultsA total of 13 hospitals from six Spanish regions participated in the first round of measurements in the FARM-URG project. Of the participating hospitals, three (23.5%) had > 1,000 beds, six (46.1%) had between 500 and 1,000 beds and four (30.8%) had less than 500 beds.
At the time of the first measurement, a total of 772 patients were being treated at the emergency rooms, which means that the per-hospital mean was 58 patients (range: 17–134). Of these, 57 (7.4%) had consulted for an ADE. The prevalence of ADEs ranged from 0% to 16.5%. The characteristics of all identified patients are shown in table 1. Mean age (SD) was 72.0 ± 18.5 years, 32 (52.1%) for male patients, and the median number of drugs taken on admission was 10 (interquartile range: 6–12).
Characteristics of the patients (n = 57) presenting to the emergency room for drug-related adverse events
| N (%) | |
|---|---|
| Origin of patients (%) | |
| Home | 51 (89.5) |
| Primary care center | 2 (3.5) |
| Nursing home | 2 (3.5) |
| Other | 2 (3.5) |
| Comorbidities (%) | |
| Chronic renal failure | 6 (10.5) |
| Cirrhosis | 2 (3.5) |
| Chronic obstructive pulmonary disease | 9 (15.8) |
| Diabetes mellitus type II | 17 (29.8) |
| Diabetes mellitus type I | 1 (1.7) |
| Immunosuppression | 6 (10.7) |
| Chronic heart failure | 18 (31.5) |
| Destination following discharge | |
| Home | 31 (54.4) |
| Nursing home | 2 (3.5) |
| Extended/long-term care facility | 1 (1.7) |
| Hospitalization | 22 (38.6) |
| Death | 1 (1.7) |
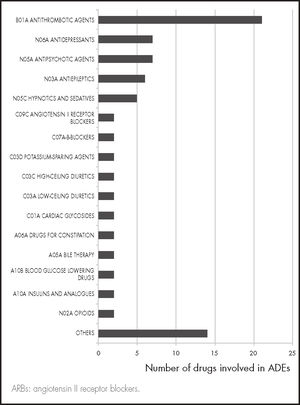
Bleeding episodes were the most common ADE (24.6%), followed by falls (10.5%). Therapeutic groups involved in ADEs are shown in figure 1. Antithrombotic drugs were responsible for 21 (26.3%) of the episodes, among them vitamin K antagonist anticoagulants (n = 13) followed by anti-platelet drugs (n = 4) and direct-acting anticoagulants (n = 2). Benzodiazepines (n = 3) were the main drugs responsible for the 6 falls recorded.
As regards the types of ADEs observed, 15 (26.3%) were the result of overdosing, 8 (14.1%) were due to lack of adherence, 7 (12.3%) were attributable to drug-drug interactions, and 4 (7.1%) were associated with a lack of the required treatment. Twenty-three ADEs (40.3%) were adverse reactions not caused by medication errors. Of the 57 ADEs, 9 (15.8%) were related to the administration of inappropriate drugs according to the STOPP-START criteria12. One patient died while in the emergency room as a result of an ADE. Nineteen (33.0%) patients returned to the emergency room within 30 days from discharge.
DiscussionAt the first prevalence measurement for the FARM-URG register, the 13 participating hospitals recorded a total number of 57 ADEs as the primary or secondary reason for presentation to the emergency room.
The bulk of cases included in the ADE register corresponded to an elderly and polymedicated patient population (82.4% of patients were on five or more drugs as their routine treatment). They were mainly elderly individuals who took a greater number of medications than those described in other studies conducted in our country4–8. The prevalence obtained following this first measurement (close to 10%) was lower than the one reported by the authors of previous studies. This is probably due to the fact that the definition used to define an ADE did not include the possibility that the treatment may be ineffective. Moreover, the retrospective method employed to detect ADEs in those studies may have underestimated the role of poor adherence. Authors who applied a similar methodology to that in this study have found similar prevalence values9,10.
Although antithrombotic drugs, vitamin K antagonist anticoagulants in particular, remain the medicines most frequently responsible for emergency room visits, the importance of direct-acting anticoagulants as causative agents should not be overlooked. Furthermore, falls from a height and the drowsiness associated with drugs acting on the central nervous system have been identified as the second leading cause of ADE-associated emergency room visits. It is a well-known fact that post-fall treatment adjustments in these patients tend to result in fewer subsequent emergency room visits13,14, which makes such patients particularly good candidates for treatment adjustments on discharge. On the other hand, several ADEs seem to be associated with drugs introduced in the last decade, which confirms the importance of keeping an up-to-date and regularly maintained ADE register such as the one proposed herein.
As regards the types of adverse events recorded, ADEs caused by medication errors accounted for nearly 60% of all cases, overdosing being the most common error registered. It should also be mentioned that nearly 16% of ADEs were attributable to inappropriately prescribed medicines according to the STOPP-START criteria12, which points to the need for increased collaboration across different health centers to optimize chronic medication regimens in elderly patients.
Nearly 30% of the patients included in the register returned to the emergency room within 30 from initial discharge, far in excess of the mean observed in the overall patient population in these units. This goes to show the potential impact of implementing strategies focused on secondary prevention when these patients are discharged. The goals of the FARM-URG register for the coming years include conducting an analysis of the number of patients returning to the emergency room because of their pharmacological treatment, and of the treatments associated to a higher number of repeat visits. This should prompt a discussion leading to the design of common prevention strategies.
The limitations of the present study include its observational nature, which limits the possibility to discriminate ADEs associated with lack of adherence from those resulting from administration errors given that no specific patient interviews are contemplated. Nonetheless, although it is a fact that lack of adherence is an important factor in hospital care15, the FARM-URG register seeks to identify drugs associated with ADEs regardless of any patient-specific factors associated to poor adherence to treatment. One of the chief strengths of the study is its multicentric design. The first prevalence measurements were made based on data from 13 hospitals from all over our country. It is to be hoped that more emergency departments will join the register in the future so that the results obtained are more closely representative of this patient population.
In a nutshell, this study shows that ADEs are a common cause of emergency room visits, antithrombotic drugs being the main therapeutic family involved. The first measurement of prevalence was intended as a stimulus for the emergency rooms in other hospitals to join the ADE register so as to boost the amount and the quality of data available on these pervasive adverse events. The data obtained can be used both as a source of information to improve primary prevention of ADEs and to develop specific interventions for this group of patients, with a view to reducing the risk that they may have to return to the emergency room and improving their quality of life.
FundingNo funding.
AcknowledgementsThe following investigators participated in the 2020 FARM-URG register: Ana Ginés Palomares, Nerea Fernández Arberas, Ana Arancón Pardo, Cristina Jiménez Núñez, Javier Álvarez Criado, Macarena García-Trevijano Cabet and Ana Juanes Borrego.
Conflict of interestsNo conflict of interests.
Contribution to the scientific literature
Although adverse drug events are a well-known cause of emergency room visits, no multicenter registers have as yet been implemented in our country to record their prevalence. This paper describes the results of the first prevalence measurement of the FARM-URG project, which represent an effort by a group of pharmacists working in different emergency departments to work collaboratively towards keeping a record of visits to their emergency rooms attributable to adverse drug events. Results show that most drug-related events identified in emergency rooms are caused by antithrombotic drugs and that a significant percentage of such events are caused by inappropriately prescribed medications. The data provided is meant to serve as a source of information to improve primary prevention of adverse drug events and to develop specific interventions for patients suffering these events.
Margarita Prats Riera. Department of Pharmacy, Hospital Can Misses, Ibiza (Baleares). Spain.
Camilla Valls Montal. Department of Pharmacy, Consorci Hospitalari de Vic, Vic (Barcelona). Spain.
María del Mar García Gutiérrez. Department of Pharmacy, Hospital Universitario de Fuenlabrada, Fuenlabrada (Madrid). Spain.
Ana Such Díaz. Department of Pharmacy, Hospital Universitario Infanta Leonor, Madrid. Spain.
Ana de Lorenzo Pinto. Department of Pharmacy, Hospital General Universitario Gregorio Marañón, Madrid. Spain.
Manuel Bonete Sánchez. Department of Pharmacy, Hospital Universitario San Juan de Alicante, Alicante. Spain.
Nuria Gala Ramos. Department of Pharmacy, Hospital Comarcal de Manacor, Manacor (Baleares). Spain.