Remdesivir has not shown survival benefit for patients with severe COVID-19. However, subgroup analysis of ACTT-1 Study Group showed an apparent reduction in mortality for patients who required non-high-flow oxygen. Presentation of SOLIDARITY study results were associated by a meta-analysis combining mortality results by subsets from randomized clinical trials. The aim is a methodological assessment of reliability and clinical applicability about findings by subgroups on the effect of remdesivir on mortality in patients with COVID-19.
MethodA validated tool was used to evaluate the findings of subgroup analyses in randomized clinical trials, including meta-analysis attached to SOLIDARITY study. It is structured in preliminary questions to reject subset analyses without relevant minimum conditions, and a specific checklist. The latter considers certain criteria: statistical association, which encompassed p of interaction, prespecification of subgroups, sample size, number of factors analyzed, and overall study result; biological plausibility of observed differences; and consistency between results of similar studies. A score was assigned to each criterion and the tool related global summation to a recommendation on the applicability of subset results in clinical decision making.
ResultsPreliminary questions had positive answers, so checklist was applied. Statistical association obtained “null” assessment (-3 points), including a “doubtful” p of interaction (p = 0.0650) among subgroups and mortality reached no statistical significance for global population. These findings reduced the reliability of subset analysis. Biological plausibility was considered “probable” (+3 points) because antiviral could have a greater effect before the inflammatory process and clinical worsening. Consistency between results of similar studies was evaluated as “possible” (+2 points) analysis for compatibility of ACTT-1 and SOLIDARITY study results. The recommendation about application of subset analysis results according to the risk of patients was “null”.
ConclusionsThis structured interpretation of subgroup analysis suggested too much uncertainty in hypothesis about remdesivir could reduce mortality in patients with severe COVID-19 who required non-high-flow oxygen. It was probably a random finding. Therefore, a randomized clinical trial about effect of remdesivir in mortality in patients with COVID-19 and non-high-flow oxygen is essential.
Remdesivir no ha mostrado beneficio en supervivencia para pacientes con COVID-19 grave. Sin embargo, el análisis por subgrupos del estudio ACTT-1 mostró aparente reducción de mortalidad en pacientes que requerían oxígeno –no de alto flujo–. La difusión de resultados del estudio SOLIDARITY se acompañó de un metaanálisis que combinó resultados de mortalidad por subgrupos de los ensayos clínicos aleatorizados. El objetivo del presente estudio es analizar metodológicamente la fiabilidad y aplicabilidad clínica de los hallazgos por subgrupos sobre el efecto de remdesivir en mortalidad en pacientes con COVID-19.
MétodoSe usó una herramienta validada para valorar los hallazgos de los análisis por subgrupos en ensayos clínicos aleatorizados, incluido el metaanálisis anexo al estudio SOLIDARITY. La herramienta utilizada está estructurada en cuestiones preliminares para descartar análisis por subgrupos sin condiciones mínimas relevantes, y un cuestionario específico. Este último considera determinados criterios: asociación estadística, incluyendo p de interacción, preespecificación de subgrupos, tamaño muestral, número de factores valorados y resultado global del estudio; plausibilidad biológica de las diferencias observadas; y consistencia entre resultados de estudios similares. Se asignó una puntuación a cada criterio y la herramienta relacionó el sumatorio global con una recomendación sobre la aplicabilidad de los resultados de los subgrupos en la toma de decisiones clínicas.
ResultadosLas cuestiones preliminares tuvieron respuestas positivas, aplicándose el cuestionario. La asociación estadística obtuvo valoración “nula” (–3 puntos), con p de interacción dudosa (p = 0,0650) y resultado de mortalidad no significativo en población global, restando fiabilidad al análisis de subgrupos. La plausibilidad biológica fue considerada “probable” (+3 puntos), ya que el antiviral pudiera tener mayor efecto antes del proceso inflamatorio y empeoramiento clínico. La consistencia se valoró “posible” (+2 puntos) por compatibilidad de resultados del estudio ACTT-1 y SOLIDARITY. La recomendación de aplicación del análisis por subgrupos según el riesgo de los pacientes fue “nula”.
ConclusionesEsta interpretación estructurada de análisis por subgrupos sugiere que la hipótesis de que remdesivir podría reducir la mortalidad en pacientes con COVID-19 grave que precisan oxígeno –no de alto flujo– presenta demasiada incertidumbre, y es probable que sea un hallazgo casual. Por tanto, es imprescindible la realización de un ensayo clínico aleatorizado sobre mortalidad en pacientes con oxígeno –no de alto flujo–.
The World Health Organization has recently published the interim results of the SOLIDARITY randomized clinical trial (RTC)1. In this study, remdesivir once again failed to demonstrate a clinical benefit in terms of 28-day mortality reduction in patients diagnosed with severe COVID-19. Nonetheless, on this occasion 28-day mortality was the main endpoint in the study and the number of patients recruited was greater than in previous studies. This null effect on mortality had already been suggested by previous studies with lower statistical power, such as the ACTT-12 and the SIMPLE3 trials, and the paper by Wang et al.4.
In contrast to this, a subgroup analysis of severe COVID-19 patients with non-high-flow (NHF) oxygen support in the ACTT-1 trial suggested a potential mortality reduction in these patients treated with remdesivir2. It seems reasonable to think that an antiretroviral could be effective in controlling the disease in the early stages, before the consequences of an uncontrolled immune response render any kind of viral suppression almost useless. This beneficial effect of remdesivir on the course of the disease, then, seems more likely in patients whose respiratory distress is not yet severe enough to require ventilatory support. However, this effect has not been shown to be life-saving in any of the 7,600 subjects randomized into the four clinical trials performed to date with remdesivir1–4. It should not be forgotten that the main goal of any anti-COVID-19 treatment is to reduce mortality, which is something only dexamethasone has achieved5.
The different RCTs conducted on the use of remdesivir in the treatment of severe COVID-19 have not succeeded in showing any benefit in terms of overall mortality, and have cast many doubts over the benefits that remdesivir could provide to the subgroup of hospitalized patients with NHF oxygen support1–4. It is therefore extremely interesting to investigate the subgroups in the SOLIDARITY trial in search for answers. The published interim results of the SOLIDARITY trial include a meta-analysis that evaluates the outcomes of the subgroup analyses contained in the four RCTs on severe COVID-19 published. The total population of these studies was subdivided into high and low-risk patients (those not receiving ventilatory support), including the subjects with high flow and NHF oxygen support in the SOLIDARITY trial. An analysis of the results of this meta-analysis could provide useful information on the mortality reduction achieved by remdesivir according to the patients’ risk profiles, leading to an increased understanding of the effect of remdesivir on the mortality of patients with NHF oxygen therapy.
It should be noted that application of a subgroup analysis requires the acceptance of a high level of uncertainty6, resulting from the need to make a series of additional measurements and redistribute patients into different groups. An unbalanced distribution of benefit-related factors increases the potential α error, by the possibility of detecting apparent differences that do not really exist. Moreover, dividing the study population into subgroups may make it even less likely to detect the differences between subgroups (increased β error). There is actually no consensus as to what should be the right consideration to subgroup analysis7, which in all cases requires a systematic and methodic evaluation before a clinical decision can be made. Taking into consideration the above, the purpose of this study was to conduct a methodological interpretation of the effect of remdesivir on 28-day mortality in the patients with severe COVID-19 presented in the subgroup meta-analysis of RCTs according to their risk profile.
MethodsThe well-organized and systematic interpretation of the subgroup meta-analysis of the SOLIDARITY trial was possible thanks to the use of a validated tool (checklist) to determine the applicability of the subgroup analysis8. The structure of the checklist presented two parts: a first part with preliminary questions aimed at ruling out the subgroup analyses that did not meet a series of minimum requirements, and a second part that consisted of a questionnaire. A negative answer to any of the preliminary questions discarded the applicability of the subgroup analysis without the need of confirmation by the checklist. The checklist considered a series of criteria for interpreting the subgroup analysis: statistical association, which included the interaction p value [p(i)], which indicates the probability that differences between subgroups may be at random, subgroup pre-specification, sample size, number of factors analyzed and overall result of the study; biological plausibility of the differences between subgroups; and consistency between the results of similar studies. When an article did not provide a p(i) value, it was estimated using a subgroup calculator9. Answers to the questions related to statistical association, biological plausibility, and consistency were assigned one of the following scores: probable (+3 points), possible (+2 points), doubtful (0 points) and null (–3 points). The overall cumulative value was associated with a recommendation about the applicability of the findings for a subgroup to the adoption of clinical decisions. A null statistical association or consistency score resulted in a direct exclusion of any findings. Higher scores were related to a greater reliability of the findings of a specific subgroup analysis: the probable score (9-7 points) meant that the findings of the subgroup analysis could be applicable until a confirmatory RCT was developed; a possible score (5-6 points) meant that the findings could be applied with caution in cases of low tolerance, difficulty of use or high cost of therapeutic alternatives; the doubtful score (3-4 points) indicated that applicability was rejected, with a few exceptions; and finally, the null score (<3 points) indicated absolute inapplicability of the subgroup findings.
Subsequently, an estimation was made of the benefit that could be obtained by the low-risk subgroup of meta-analysis that accompanies the SOLIDARITY trial1, as that cohort included the subgroup of patients in the ACTT-1 trial with NHF oxygen therapy, for whom a potential reduction in mortality has been suggested2. The magnitude of the benefit was estimated by calculating the number needed to treat (NNT) to prevent one additional event and the relative risk reduction (RRR).
ResultsAll the preliminary questions of the tool were answered in the affirmative, which meant that the questionnaire could be applied. The subgroup of patients without ventilatory support in the meta-analysis accompanying the SOLIDARITY study exhibited a ratio of death rates (RR) of 0.8 [95% CI (0.63-1.01)], while the subgroup of high-risk patients obtained an RR of 1.16 [95% CI (0.85-1.60)]. With regard to statistical association, it was applied to the result of the meta-analysis in patients without ventilatory support or NHF oxygen therapy as opposed to the rest, a p(i) value of 0.0650 was estimated between the subgroups, using RR values and a 95% confidence interval. A p(i) value between 0.05 and 0.1 was considered acceptable for the subgroup analysis as dividing the samples into the different subgroups reduced statistical power8,10. Thus, this p(i) value was assigned a “doubtful” score. The meta-analysis recreated the group of patients with NHF oxygen therapy and, even if the SOLIDARITY study did not include a pre-specification for this group, the subgroup analysis was developed according to the patients’ risk profile given the apparent mortality benefit found by the ACTT-1 trial2. For this reason, a “probable” reliability score was assigned according to the pre-specification. As the sample size was much greater than 100 patients in both subgroups, a “probable” score was assigned to this criterion. About the number of factors analyzed is concerned, the same factors examined in the ACTT-1 trial were considered2, as the latter gave rise to the subgroup analysis that followed the meta-analysis. Moreover, the meta-analysis cannot evaluate the pre-specified factors as it only examines the factors considered in its constituent RCTs. The ACTT-1 trial analyzed 7 factors (< 10) with a “probable” score2. Mortality did not reach statistical significance for the general population of the meta-analysis of the four trials (RR 0.91; 95% CI 0.79-1.05), which was indicative of an overall negative result. Although the result could have been positive in a specific subgroup, this possibility did not materialize as statistical significance was rot reached even in the subgroup of patients without ventilatory support/high-flow oxygen therapy (RR 0.80; 95% CI 0.63-1.01). As a result of this, statistical association was given a “null” score as the assumption underlying this criterion (a negative outcome for the general population but a statistically significant difference for one of the subgroups) did not hold true.
The biological plausibility of the subgroup analysis was given a “probable” score as there is a possibility that remdesivir may have a greater effect before the full inflammatory process responsible for clinical worsening gets underway, i.e. in patients still at the initial stages of the disease such as those in the low-risk group of the meta-analysis, among them the ones NHF oxygen therapy. Consistency was rated as “possible” as the differential interaction analysis in the ACTT-1 trial2, was not incompatible with the results of the SOLIDARITY trial which pointed in the same direction even if no statistical interaction was found. Although the other two studies in the meta-analysis, Wang et al. and SIMPLE3,4 did not show any consistency, their low statistical power means that they cannot by themselves reject a potential mortality benefit.
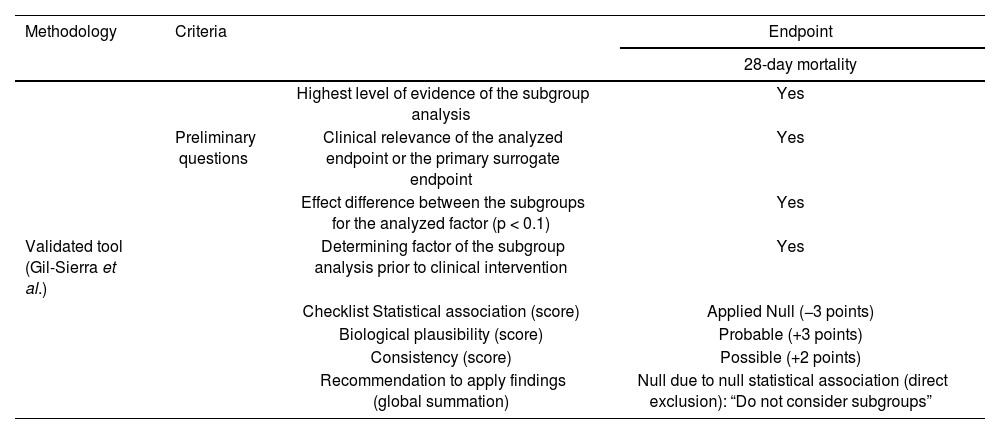
An examination of the findings above in the light of the criteria included in the validated tool8, indicates that recommending application of the subgroup analysis to clinical decision-making according to the patients’ risk profile was “null”, by direct discarding caused by unreliable statistical association. Table 1 summarizes the interpretation of the subgroup analysis based on the patient's clinical status included in the meta-analysis accompanying the SOLIDARITY trial.
Summary of the interpretation about subgroup analysis according to the patients' risk profile as described in the SOLIDARITY trial
| Methodology | Criteria | Endpoint | |
|---|---|---|---|
| 28-day mortality | |||
| Highest level of evidence of the subgroup analysis | Yes | ||
| Preliminary questions | Clinical relevance of the analyzed endpoint or the primary surrogate endpoint | Yes | |
| Effect difference between the subgroups for the analyzed factor (p < 0.1) | Yes | ||
| Validated tool (Gil-Sierra et al.) | Determining factor of the subgroup analysis prior to clinical intervention | Yes | |
| Checklist Statistical association (score) | Applied Null (−3 points) | ||
| Biological plausibility (score) | Probable (+3 points) | ||
| Consistency (score) | Possible (+2 points) | ||
| Recommendation to apply findings (global summation) | Null due to null statistical association (direct exclusion): “Do not consider subgroups” | ||
Should a significant clinical benefit be eventually confirmed for remdesivir, which is something that the meta-analysis has not been able to show, mortality in non-intubated patients/NHF oxygen therapy could decrease from 8.6% to 7.0%. This would entail an absolute risk reduction of 1.6% and hypothetically prevent one additional death in every 62 patients treated with remdesivir (NNT). Overall, in relative terms, it would be possible to prevent one in every five deaths in this patient population (1.6% over 8.6%, an 18.6% RRR).
DiscussionConsidering that the final result of the validated tool about applicability of the subgroup analysis was “null” in terms of mortality reduction in hospitalized COVID-19 patients with NHF oxygen therapy receiving remdesivir, the apparent mortality difference between the subgroups could be considered random. This means that mortality should not be assigned –for the time being– a value other than that found in the overall results of the meta-analysis accompanying the SOLIDARITY trial, RR = 0.91 (95% CI 0.79-1.05). Nonetheless, the “null” reliability of this subgroup analysis, based on precarious data, does not exclude the hypothesis of a greater clinical benefit for the subgroup of patients requiring NHF oxygen support.
The meta-analysis accompanying the SOLIDARITY trial presented a significant limitation1: the subgroups studied in the four RCTs analyzed were defined in an inconsistent manner1–4. It was not possible to develop an individualized analysis of the NHF oxygen therapy subgroup, which obtained an apparently significant benefit from remdesivir in the ACTT-1 trial2, so the authors had to be limited to grouping patients with ventilation and patients without it. Patients with high-flow oxygen therapy or non-invasive ventilatory support in the ACTT-1 trial who were observed not to benefit from the treatment were incorporated to the group of patients on ventilatory support, which favored the appearance of differences between the low and high-risk subgroups in the meta-analysis accompanying the SOLIDARITY trial1. Furthermore, the subgroup made up of a combination of patients with and without high-flow oxygen therapy in the SOLIDARITY trial [RR 0.85 (0.66-1.09)] was included in the subgroup receiving no ventilatory support. In addition, the magnitude of the benefit obtained by the low-risk subgroup of the SOLIDARITY trial1 suggests a doubtful mortality benefit in non-intubated patients. If this benefit existed, it could be much smaller than the one allegedly found in the subgroup of patients with NHF oxygen therapy in the ACTT-1 trial2.
The present study is meant to contribute to contextualizing the results of the ACTT-1 trial2, where the subgroup of patients with NHF oxygen therapy obtained p(i) < 0.05. Although the analysis does not seem consistent with previous RCTs, the scenario is biologically plausible3,4. Instead of falling into the typical error associated with subgroup analysis evaluations whereby conclusions are based on whether 95% CIs cross the neutral value or not9,11,12, a systematic interpretation was made of the most relevant results (RCT subgroup meta-analysis) relative to the effect of remdesivir on hospitalized COVID-19 patients according to their risk profile and clinical status.
The current situation caused by the COVID-19 pandemic constitutes an unprecedented challenge for healthcare providers, among them hospital pharmacists13. The lack of effective therapeutic alternatives means that the use of remdesivir may be acceptable in patients with NHF oxygen therapy, even without any certainty that the antiviral can save their lives, and with the possibility that the intervention may just be anticipating the recovery of patients who would recover anyway. The available evidence does not support the conclusion that remdesivir does actually contribute to a reduction in mortality from COVID-19, which is the main goal of treatment, at least in the short term. This should result in the development of well-designed RCTs to investigate alternative treatments, regimens or usages that are really capable of saving patients’ lives. In that regard, it is essential to select 28-day mortality as the main outcome, rather than other variables such as relative recovery, which do not contribute to a better understanding of the disease or to making real solutions available.
Finally, the mortality reduction observed in patients with NHF oxygen therapy is likely to be no more than an random finding. Even if the benefit was real, the results of the meta-analysis under study indicate that it is more modest than suggested by the isolated finding obtained in the ACTT-1 trial. A systematic analysis using the 28-day mortality endpoint discarded the applicability of remdesivir to any of the subgroups studied. Before prematurely embracing the use of remdesivir in patients with NHF oxygen support during the current COVID-19 pandemic, it would be necessary to at least conduct an RCT to study the mortality of these patients so that the administration of remdesivir is warranted by essential clinical evidence.
FundingNo funding.
Conflict of interestsGil-Sierra MD was a member of an advisory board sponsored by Janssen Pharmaceutica and participated in a symposium on oncology drugs. The other authors have no conflict of interest in relation with this study.