To develop a risk stratification tool for pharmaceutical care in patients with cardiovascular disease who require a comprehensive and personalized pharmaceutical approach.
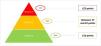
MethodThe risk stratification model was collaboratively developed by hospital pharmacists specialized in managing cardiovascular risk patients, all members of the Spanish Society of Hospital Pharmacy. Through three workshops and a pilot study, relevant variables were identified, grouped into four dimensions, and assigned relative weights. In the pilot study, data from patients in the participating centers were collected and analyzed to determine priority levels and assess the contribution of each variable. The Kaiser Permanente pyramidal model was adopted, classifying patients into three priority levels: priority 1 (intensive pharmaceutical care, 90th percentile), priority 2 (60–90th percentiles), and priority 3 (below the 60th percentile). Cut-off points were established based on this stratification, and each center recorded variables in an Excel sheet to calculate mean weighted scores per priority level and total risk scores.
ResultsParticipants completed a questionnaire consisting of 20 variables grouped into four dimensions: demographic, socio-health and functional status, clinical and healthcare utilization, and treatment-related factors. Based on the tool application in a pretest study, the following cut-off points were established: 23 or more points for priority 1, 17–22 points for priority 2, and fewer than 16 points for priority 3. Over 80% of the total score was attributed to the dimensions of “clinical and healthcare utilization” and “treatment-related factors”. Consequently, interventions based on the pharmaceutical care model were recommended for cardiovascular risk patients, tailored to their prioritization level.
ConclusionThis stratification tool enables the identification of cardiovascular patients who require a higher level of pharmaceutical care, facilitating the adjustment of healthcare capacity. Validation of the model in a representative population is necessary to establish its broader applicability.
desarrollar una herramienta de estratificación de riesgo para la atención farmacéutica de pacientes con enfermedad cardiovascular que requieran un abordaje farmacéutico integral y personalizado.
Métodoel modelo de estratificación de riesgo se desarrolló de forma colaborativa por farmacéuticos hospitalarios especializados en la atención de pacientes con riesgo cardiovascular, miembros de la Sociedad Española de Farmacia Hospitalaria. Mediante 3 talleres y un estudio piloto se definieron las variables relevantes, que se agruparon en 4 dimensiones y se les asignaron pesos relativos. En el estudio piloto se recogieron y analizaron los datos de los pacientes de los centros participantes para determinar los niveles de prioridad y evaluar la contribución de cada variable. Se siguió el modelo piramidal de Kaiser Permanente, clasificando a los pacientes en 3 niveles: prioridad 1 (atención farmacéutica intensiva, percentil 90), prioridad 2 (percentiles 60–90) y prioridad 3 (por debajo del percentil 60). Los puntos de corte se establecieron en función de esta estratificación y cada centro registró las variables en una hoja de Excel para calcular las puntuaciones medias de peso por nivel de prioridad y la puntuación total de riesgo.
Resultadoslos centros participantes completaron un cuestionario compuesto por 20 variables agrupadas en 4 dimensiones: demográfica; sociosanitaria y estado funcional; clínica y utilización de servicios sanitarios; y relacionada con el tratamiento. A partir de un estudio preprueba se definieron los siguientes puntos de corte: 23 o más puntos para la prioridad 1, de 17 a 22 puntos para la prioridad 2 y menos de 16 para la prioridad 3. Se observó que más del 80% de la puntuación total se fundamentó en las dimensiones de «utilización de servicios clínicos y sanitarios» y «relacionada con el tratamiento». En consecuencia, se recomendaron intervenciones basadas en el modelo de atención farmacéutica para los pacientes con riesgo cardiovascular, adaptadas a su nivel de priorización.
Conclusiónla herramienta permite identificar a los pacientes con enfermedad cardiovascular que requieren un mayor nivel de atención farmacéutica, facilitando el ajuste eficiente de la capacidad asistencial. Es necesaria su validación en una población representativa para establecer su efectividad y promover su adopción en la práctica clínica.
Cardiovascular diseases (CVDs) remain the leading cause of morbidity and mortality worldwide1. According to the latest available data from the Global Burden of Disease study, there has been a significant increase in the number of patients with CVD and CVD-related deaths in recent years. There is no sign of this trend abating2. This increase has been attributed to several factors, including an ageing population, an increase in risk factors such as hypertension, diabetes, tobacco use, obesity, and sedentary lifestyles, as well as improvements in diagnostic methods, which allow these diseases to be identified at an earlier stage3–5. Demographic projections suggest that the number of new cases of CVD and CVD-related deaths will continue to increase in the coming decades, and will reach alarming levels if prevention and control strategies are not intensified6.
In 2023, 9.8% of the Spanish population had some form of CVD, with 52.6% of cases occurring in women and 47.4% in men. These diseases are the second leading cause of death in Spain, accounting for 26.5% of all deaths, behind tumours (26.6%) and ahead of respiratory diseases (10.8%)7.
In response to the need to adopt new perspectives in CVD care, the Spanish National Health System implemented the Cardiovascular Health Strategy8, which aims to minimise the impact of these diseases and to prevent their onset by taking a comprehensive, patient-centred approach to cardiovascular health. This approach encompasses all influencing factors, including social determinants, health education, training of healthcare personnel, improvements in early diagnosis, and the importance of prevention and rehabilitation.
Likewise, the “Strategy for Addressing Chronicity in the National Health System” has established a key strategic approach to reorient healthcare delivery9, aiming to shift the system's focus from disease-centred to people-centred, while considering the needs of both the population as a whole and each individual.
To achieve this, stratification and prediction models have been recommended as the basic tools for managing chronicity. The “Population Stratification Project in the Spanish NHS” has been developed within this framework to promote this change in approach and ensure more personalised, patient-centred care10. Adopting this stratification approach makes it possible to accurately identify individuals within the population who are at the highest risk. This facilitates the design of more effective interventions to improve chronic disease management, as well as the implementation of preventive measures and health promotion.
This approach is consistent with the Capacity-Motivation-Opportunity (CMO) model developed by the Spanish Society of Hospital Pharmacy (SEFH) and is promoted through the SEFH “Strategic Map for Outpatient Care” (MAPEX) project. It aims to improve care and health outcomes for patients attending hospital pharmacy (HP) outpatient clinics and has already been adapted to different patient profiles11,12. At the project's second consensus conference in 2023, 5 strategic lines were prioritised for the period 2024 to 2027. One of these lines was to develop and implement new adaptations of the CMO-MAPEX methodology for different types of patients. This involved collecting and analysing data to enable their parameterisation, utilisation, and clinical evaluation with the aim of obtaining information on adherence, quality of life, and health outcomes13.
Although various tools for stratifying pharmaceutical care (PC) have been developed for patient groups treated by HPs, none to date have focused on CVD14–16.
The aim of this study was to develop a stratification tool for PC for patients with CVD, and to establish interventions tailored to each established priority level.
MethodThe study was developed through a methodological process that combined expert consensus, a critical review of previous models, and empirical validation via a pilot study. The project ran from February 2024 to December 2024, covering all the stages required to define and implement the model.
The first stage comprised organisation and context analysis. To this end, a working group comprising 12 hospital pharmacists specialising in cardiovascular patient care was formed. Most of the pharmacists were members of the SEFH Cardio Group and had either accreditation or experience in advanced PC in the cardiovascular field, working either part-time or full-time in this area. The group conducted an exhaustive review of the literature and previous models in order to identify the specific needs of the cardiovascular population. Given this background, it was deemed relevant to include quantitative and qualitative variables that could be primarily extracted from the electronic medical record and supplemented by structured interviews with the patients in the HF.
In stage 2, the stratification tool itself was defined and designed. Accordingly, during online workshops, the expert group defined the variables to be incorporated into the tool based on previous SEFH models and specific bibliographic searches. They grouped these variables into four dimensions: demographic; socio-health and functional; clinical and service use; and treatment-related.
It was agreed that a score would be assigned to each variable on a scale of 1 to 4, with higher values indicating greater importance for achieving health outcomes. These weights were assigned based on expert assessment and the existing bibliographic support for each item, thus ensuring that the clinical relevance of each variable was given the appropriate weighting.
In stage 3, the pre-test study was conducted and the thresholds between priority levels were defined. To achieve this, a pilot study was conducted with CVD patients recruited from 6 representative hospitals. The pilot study aimed to apply the tool in clinical practice in order to determine the classification thresholds for 3 risk levels (priorities 1, 2, and 3) according to the Kaiser Permanente pyramid care model. Data were collected systematically using a standardised Excel tool, drawing on information available in the electronic medical records of each centre, supplemented by data obtained from structured patient interviews. The weighted mean scores were calculated for the total model and each dimension. A sequential exclusion analysis was then performed to assess the individual impact of each variable on risk classification. The findings of the pilot study enabled the precise definition of the thresholds for the 3 levels, which were adjusted to the clinical reality of the cardiovascular environment.
In the final stage, the final adjustments were made, and the actions were defined and agreed upon, along with the frequency of stratification and restratification for each established priority level.
ResultsThe risk stratification tool comprises a total of 20 variables grouped into 4 dimensions: 4 demographic, 2 socio-health, 8 clinical, and 6 pharmacotherapeutic. Table 1 shows the variables included and their specific weights. A key feature of the tool is that at least two-thirds of the data are already available prior to the patient's face-to-face consultation. This design was chosen to significantly optimise the pre-stratification process and intervention planning within the usual workflow, and to make implementation easier.
Demographic variables for measuring the overall risk of patients with cardiovascular disease.
| Demographic variables | |
|---|---|
| Age | |
| <18 y | a |
| 50–69 y | 2 |
| ≥70 y | 3 |
| Pregnancy | |
| Pregnant patient | Priority 1 |
| Weight/Nutritional status | |
| Overweight (BMI 25–30kg/m2) | 1 |
| Obesity (BMI >30kg/m2) | 2 |
| Severe obesity (BMI >40kg/m2) | 3 |
| Malnutrition (BMI <18.4kg/m2) | 1 |
| Normal weight (BMI 18.5–24.9kg/m2) | 0 |
| Gender | |
Women with specific CV diseases, including:
| 1 |
| |
| |
| Maximum score for demographic variables | 7 |
| Clinical variables | |
| Underlying CV disease | |
| Patients with 1 of the following underlying CV diseases, associated with higher risk, and requiring closer monitoring: Arrhythmia: atrial fibrillation | 1 |
| Pulmonary hypertension | |
| Heart failure | |
| Secondary prevention in atherosclerotic CV disease (chronic ischaemic heart disease, peripheral arterial disease, cerebrovascular disease) | |
| Acute coronary syndrome | |
| Heart transplant | |
| CV comorbidity | |
| Patients with at least 1 of the following CV diseases, in addition to their underlying CV disease: Cardiac amyloidosis | 2 |
| Ischemic heart disease/CAD/previous myocardial infarction | |
| Valvular heart disease | |
| Peripheral artery disease | |
| Atrial fibrillation | |
| Pulmonary hypertension | |
| Stroke | |
| Heart failure | |
| Patients with a combination of heart failure and ischaemic heart disease | 3 |
| Patients with a combination of heart failure and atrial fibrillation | 3 |
| Non-CV comorbidity | |
| Patients with at least 1 of the following non-CV diseases: Anaemia | 1 |
| Obstructive sleep apnoea | |
| Inflammatory diseases (lymphocyte count below the normal range) | |
| COPD | |
| HIV | |
| Active cancer | |
| Chronic kidney disease (eGFR <60mL/min/1.73m2) | |
| Type 1 or type 2 diabetes mellitus | |
| Dyslipidaemiab | |
| Patients whose LDL levels are above their targets: | 2 |
| LDL-C<1.0μmol/L (<40mg/dL) in patients with CV disease who experience a second vascular event within 2years | |
| LDL-C<1.4μmol/L (<55mg//dL) in secondary prevention of patients at very high risk; in people with FH and very high risk | |
| LDL-C<1.8μmol/L (<70mg//dL), in patients at high risk | |
| Severity of the condition | |
| Patient with left ventricular ejection fraction <40% | 1 |
| Patient with venous thromboembolism | 1 |
| Hypertension | |
| Patient with SBP ≥140mmHg/DBP ≥90 mmHgc or undergoing treatment with antihypertensive drugs indicated for hypertension (if these values are unknown) | 2 |
| Admissions/emergency room visits in the last year due to illness | |
| Patient who has had 2 or more hospitalisations in the previous 12months and has visited the emergency room at least 3 times over the past year, provided that this use of healthcare services is related to poor disease or treatment control | 2 |
| Patient within the first year following a coronary event | |
| Patient with SBP ≥140mmHg/DBP ≥90mmHg or undergoing treatment with antihypertensive drugs indicated for hypertension (if these values are unknown) | 2 |
| Maximum score for clinical variables | 20 |
| Pharmacotherapeutic variablesd | |
| Changes in regular medication regimen | |
| Significant changes in the medication regimen, to be assessed by the healthcare professional, since the last pharmaceutical care visit | 1 |
| Complexity of the pharmacological regimen | |
| The patient takes at least 1 medication with complex administration guidelines (e.g., dosage, pharmaceutical form) | 1 |
| High-risk medications | |
| Patient taking any medication included in the Spanish ISMP list of high-risk medications | 4 |
| Pharmacotherapeutic goals | |
| Patient whose pharmacotherapeutic goals have not been achieved (including comorbidities). The answer will be ‘no’ if the patient has not met the established pharmacotherapeutic goals or has just started treatment and has no previous pharmacotherapeutic goals | 2 |
| The answer will be ‘yes’ if the patient has achieved the established pharmacotherapeutic goals | |
| Polypharmacy | |
| Patient taking between 7 and 10 active ingredients concurrently and chronically | 1 |
| Patient taking more than 10 active ingredients concurrently and chronically | 2 |
| Suspected lack of adherence or nonadherencee | |
| Adherent patient | 0 |
| Patient at risk of nonadherence and suboptimal persistence | 1 |
| Nonadherent patient | 2 |
| Maximum score for pharmacotherapeutic variables | 12 |
| Socio-health variables | |
| Physical activity | |
| Patients with a sedentary lifestyle | 1 |
| Patients with intense physical activity (e.g., elite athletes)f | 1 |
| Tobacco use | |
| Patient who quit smoking within the last 5years | 1 |
| Active smoker | 2 |
| Maximum score for socio-health variables | 3 |
IHD, ischaemic heart disease; CV, cardiovascular; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; BMI, body mass index; ISMP, Institute for Safe Medication Practices; LDL, low-density lipoprotein; HIV, human immunodeficiency virus; SBP, systolic blood pressure; DBP, diastolic blood pressure; FH, familial hypercholesterolaemia.
Consider lipoprotein(a) level as supplementary information on CV risk (if this biomarker is available).
If a patient does not have specific pharmacotherapeutic goals, a general review of adherence, disease control, and management should be carried out (e.g., hypertension=patient with controlled blood pressure, diabetes=blood sugar control, etc.).
The pilot study included a total of 212 patients from 6 centres (Table 2). During the study, cut-off points were established for stratifying patients into 3 priority levels, according to the theoretical distribution of the Kaiser Permanente model (60–30–10). The cut-off points were defined as follows: priority 1, at least 23 points (10.4% of the sample); priority 2, 17 to 22 points (29.2%); and priority 3, less than 17 points (60.4%).
Baseline scores of patients included in the pre-test study.
| Positive score/variable | O % | P1% | P2% | P3% | |
|---|---|---|---|---|---|
| Yes | Yes | Yes | Yes | ||
| Demographic variables | Age of patient with CV disease | 86 | 95 | 92 | 81 |
| Pregnant patient | 0 | 0 | 0 | 0 | |
| African American or South Asian patient | 0 | 0 | 2 | 0 | |
| BMI | 56 | 68 | 63 | 50 | |
| Gender: female with specific CV diseases (AF/PH/IHD) | 23 | 50 | 29 | 16 | |
| Clinical variables | Underlying CV disease: AF/PH/IHD/secondary prevention/ACS/transplant | 82 | 91 | 97 | 73 |
| Patient has at least 1 of the following CV diseases, as well as the underlying disease: amyloidosis/IHD/CAD/myocardial infarction/valvular heart disease/PAD/AF/PH/stroke/IHD | 47 | 68 | 68 | 34 | |
| Patient with a combination of heart failure and IHD | 10 | 41 | 15 | 3 | |
| Patient with a combination of heart failure and AF | 11 | 50 | 35 | 5 | |
| Presence of at least 1 non-CV disease: anaemia/apnoea/inflammatory diseases/COPD/HIV | 29 | 55 | 39 | 20 | |
| Patient with LDL levels above target | 49 | 73 | 45 | 46 | |
| Patient with left ventricular ejection fraction <40% | 5 | 23 | 10 | 0 | |
| Patient with venous thromboembolism | 4 | 5 | 10 | 1 | |
| Patient with SBP ≥140mmHg/DBP ≥90mmHg and antihypertensive treatment | 58 | 91 | 74 | 45 | |
| Patient who has had 2 or more hospitalisations in the previous 12months or has attended the emergency department at least 3 times in the past year | 15 | 32 | 23 | 9 | |
| Patient with an acute event or whose last event occurred in the last 12months | 16 | 55 | 11 | 7 | |
| Pharmacotherapeutic variables | Significant changes in medication regimen | 28 | 59 | 42 | 16 |
| Patient taking at least 1 medication with complex administration guidelines | 34 | 68 | 40 | 26 | |
| Patient taking any medication included in the Spanish ISMP list | 49 | 91 | 76 | 29 | |
| Patient with unmet pharmacotherapeutic goals | 45 | 95 | 71 | 38 | |
| Number of active ingredients taken concurrently and on a chronic basis | 72 | 91 | 90 | 70 | |
| Patient with suspected nonadherence or nonadherence | 29 | 41 | 42 | 21 | |
| Socio-health variables | Patient with a sedentary lifestyle or intense physical activity | 49 | 82 | 58 | 40 |
| Current or former smoker | 25 | 36 | 24 | 24 |
IHD, ischaemic heart disease; CV, cardiovascular; COPD, chronic obstructive pulmonary disease; AF, atrial fibrillation; PH, pulmonary hypertension; LDL, low-density lipoprotein; ISMP, Institute for Safe Medication Practices; O, overall; P1, Priority 1; P2, Priority 2; P3, Priority 3; ACS, acute coronary syndrome; HIV, human immunodeficiency virus; BMI, body mass index; CAD, coronary artery disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; PAD, peripheral artery disease.
The distribution obtained showed good alignment with the expected pyramid model, allowing resources to be prioritised for patients at the highest risk while ensuring that the remaining patients received efficient care (Fig. 1).
Finally, specific interventions for PC were also established according to stratification level (Tables 3–5).
Definition of pharmaceutical care actions in patients at priority level 3.
| Pharmacotherapeutic follow-up |
| Review and validate cardiovascular treatment to ensure its appropriateness, safety, and effectiveness within the schedule recommended by the guidelines, recording and reporting any adverse reactions observed. |
| Monitor patient adherence to medical prescriptions and establish effective strategies to improve adherence through education and behavioural support, as well as collaborative care and case management, taking into account the characteristics of cardiovascular diseases. |
| Reconcile and review concomitant medication to identify and manage potential interactions, offering therapeutic alternatives if necessary. |
| Patient education, training, and follow-up |
| Encourage patients to become active and informed so that they can share responsibility for treatment outcomes by providing basic information on cardiovascular treatments and managing medication-related problems. |
| Provide information (treatments, cardiovascular diseases, adherence, etc.) and answer questions about the patient's disease. |
| Offer general health education (promoting heart-healthy lifestyle habits, controlling risk factors, correct use of medications, and encouraging adherence to therapeutic goals) via the Internet, pharmacy service websites, the SEFH, and other channels. |
| Promote the use of self-management tools, providing web resources and informative apps for patient education and confirming changes in the patient's real-life habits. |
| Emphasise education on prevention and adherence, highlighting the importance of adhering to treatment and the increased cardiovascular risk for patients due to potential nonadherence. |
| Coordination with the healthcare team |
| Include all information and interventions in the patients' electronic medical records to ensure efficient coordination and integration across levels of care and between autonomous communities. |
| Collaborate in the development and evaluation of policies and guidelines for the safe and effective use of medicines, aligning them with best practices for cardiovascular care, current clinical guidelines for the management of modifiable cardiovascular risk factors, and the needs of the healthcare team. |
| Standardise criteria and messages among different healthcare professionals and across levels of care, promoting effective two-way communication between multidisciplinary teams. |
| Develop specific programmes aimed at achieving pharmacotherapeutic objectives, ensuring the appropriateness of treatments and patient adherence. |
| Collaborate in the development and implementation of a hospital discharge report that includes key information on pharmaceutical care (i.e., treatment plan, detailed follow-up, and therapeutic control objectives) to ensure continuity of care and facilitate the monitoring of the patient's cardiovascular disease. |
| If social inequalities affecting the patient's health are identified, coordinate with other specialities to address the determinants around which these social health inequalities are structured. |
Definition of pharmaceutical care actions in patients at priority level 2.
| Pharmacotherapeutic follow-up |
| Review and validate cardiovascular treatment to ensure its appropriateness, safety, and effectiveness within the schedule recommended by the guidelines, recording and reporting any adverse reactions observed. |
| Monitor patient adherence to medical prescriptions and establish effective strategies to improve adherence through education and behavioural support, as well as collaborative care and case management, taking into account the characteristics of cardiovascular diseases. |
| Reconcile and review concomitant medication to identify and manage potential interactions, offering therapeutic alternatives if necessary. |
| Monitor and make multidisciplinary decisions based on Patient-Reported Outcomes and Patient-Reported Experience Measures used for patient follow-up. |
| Reconcile pharmacological treatment during care transitions (admission, discharge, emergencies) to ensure pharmacotherapeutic adherence, continuity of care, and safety of treatment. |
| Use telecare to maintain additional contact with the patient between visits and to plan the next appointment. |
| Patient education, training, and follow-up |
| Encourage patients to become active and informed so that they can share responsibility for treatment outcomes by providing basic information on cardiovascular treatments and managing medication-related problems. |
| Provide information (treatments, cardiovascular diseases, adherence, etc.) and answer questions about the patient's disease. |
| Offer general health education (promoting heart-healthy lifestyle habits, controlling risk factors, correct use of medications, and encouraging adherence to therapeutic goals) via the Internet, pharmacy service websites, the SEFH, and other channels. |
| Promote the use of self-management tools, providing web resources and informative apps for patient education and confirming changes in the patient's real-life habits. |
| Emphasise education on prevention and adherence, highlighting the importance of adhering to treatment and the increased cardiovascular risk for patients due to potential nonadherence. |
| Offer training and education to family members and carers on how to correctly monitor patients, and encourage them to communicate any new developments in the patient's condition, such as a new illness, a new medication, or a social problem. |
| Coordination with the healthcare team |
| Include all information and interventions in the patients' electronic medical records to ensure efficient coordination and integration across levels of care and between autonomous communities. |
| Collaborate in the development and evaluation of policies and guidelines for the safe and effective use of medicines, aligning them with best practices for cardiovascular care, current clinical guidelines for the control of modifiable cardiovascular risk factors, and the needs of the healthcare team. |
| Standardise criteria and messages between different healthcare professionals and across levels of care, promoting effective two-way communication between multidisciplinary teams. |
| Develop specific programmes aimed at achieving pharmacotherapeutic objectives, ensuring the appropriateness of treatments and patient adherence. |
| Collaborate in the development and implementation of a hospital discharge report that includes key information on pharmaceutical care (e.g., treatment plan, detailed follow-up, and therapeutic control objectives) to ensure continuity of care and facilitate the monitoring of the patient's cardiovascular disease. |
| If social inequalities affecting the patient's health are identified, coordinate with other specialities to address the determinants around which these social health inequalities are structured. |
| Establish joint working procedures with the healthcare team to empower patients and improve their ability to manage their own health conditions. |
Definition of pharmaceutical care actions in patients at priority level 1.
| Pharmacotherapeutic follow-up |
| Review and validate cardiovascular treatment to ensure its appropriateness, safety, and effectiveness within the schedule recommended by the guidelines, recording and reporting any adverse reactions observed. |
| Monitor patient adherence to medical prescriptions and establish effective strategies to improve adherence through education and behavioural support, as well as collaborative care and case management, taking into account the characteristics of cardiovascular diseases. |
| Reconcile and review concomitant medication to identify and manage potential interactions, offering therapeutic alternatives if necessary. |
| Monitor and make multidisciplinary decisions based on Patient-Reported Outcomes and Patient-Reported Experience Measures used for patient follow-up. |
| Reconcile pharmacological treatment during care transitions (admission, discharge, emergencies) to ensure pharmacotherapeutic adherence, continuity of care, and safety of treatment. |
| Use telecare to maintain additional contact with the patient between visits and to plan the next appointment in coordination with the care team to ensure close and personalised follow-up. |
| Involve the patient in the pharmacotherapeutic plan, share progress on their goals, and agree on actions. |
| Use telemedicine tools to develop structured programmes to detect, prevent, or control specific risk factors (hypertension, dyslipidaemia, diabetes, etc.). |
| Patient education, training, and follow-up |
| Encourage patients to become active and informed so that they can share responsibility for treatment outcomes by providing basic information on cardiovascular treatments and managing medication-related problems. |
| Provide information (treatments, cardiovascular diseases, adherence, etc.) and answer questions about the patient's disease. |
| Offer general health education (promoting heart-healthy lifestyle habits, controlling risk factors, correct use of medications, and encouraging adherence to therapeutic goals) via the Internet, pharmacy service websites, the SEFH, and other channels. |
| Promote the use of self-management tools, providing web resources and informative apps for patient education and confirming changes in the patient's real-life habits. |
| Emphasise education on prevention and adherence, highlighting the importance of adhering to treatment and the increased cardiovascular risk for patients due to potential nonadherence. |
| Offer training and education to family members and carers on how to correctly monitor patients, and encourage them to communicate any new developments in the patient's condition, such as a new illness, a new medication, or a social problem. |
| Develop personalised material for each patient and carer (medication sheet, diary, etc.). |
| Coordination with the healthcare team |
| Include all information and interventions in the patients' electronic medical records to ensure efficient coordination and integration across levels of care and between autonomous communities. |
| Collaborate in the development and evaluation of policies and guidelines for the safe and effective use of medicines, aligning them with best practices for cardiovascular care, current clinical guidelines for the control of modifiable cardiovascular risk factors, and the needs of the healthcare team. |
| Standardise criteria and messages between different healthcare professionals and across levels of care, promoting effective two-way communication between multidisciplinary teams. |
| Develop specific programmes aimed at achieving pharmacotherapeutic objectives, ensuring the appropriateness of treatments and patient adherence. |
| Collaborate in the development and implementation of a hospital discharge report that includes key information on pharmaceutical care (i.e., treatment plan, detailed follow-up, and therapeutic control objectives) to ensure continuity of care and facilitate the monitoring of the patient's cardiovascular disease. |
| If social inequalities affecting the patient's health are identified, coordinate with other specialities to address the determinants around which these social health inequalities are structured. |
| Establish joint working procedures with the healthcare team to empower patients and improve their ability to manage their own health conditions. |
| Coordinate with patient associations to ensure comprehensive care tailored to the patient's needs. |
| Coordinate specialised in-hospital services and recommend the use of personalised dispensing systems in collaboration with community pharmacies. |
| Prepare regular reports (by telephone, electronic medical records, or multidisciplinary sessions) to inform the multidisciplinary team about priority patient cases. |
| Work together and participate in case management teams to discuss and address these patients' specific needs. |
| Develop an action plan across care levels to address adverse reactions to treatment and define rapid communication channels. |
Priority 1: patients at this level require intensive follow-up, including face-to-face visits every 3–6months, a comprehensive review of pharmacotherapeutic objectives, and close coordination with the multidisciplinary team.
Priority 2: interventions focus on intermediate follow-up, with annual reviews and actions aimed at consolidating therapeutic adherence and adjusting treatments according to clinical needs.
Priority 3: lower-risk patients receive standard follow-up, focusing on health education, prevention, and general monitoring of their clinical situation.
Finally, the defined actions were divided into the following: pharmacotherapeutic follow-up interventions; patient education, training, and follow-up; and coordination with the healthcare team. It was also agreed that these interventions would be cumulative. Thus, priority 1 patients also receive the interventions planned for levels 2 and 3, while priority 2 patients also receive those for priority 3. This approach ensures comprehensive care tailored to the needs of each patient.
DiscussionThis study presents a stratification tool for PC—developed from the perspective of HP—to enable the early and accurate identification of patients at higher risk of poor outcomes and with greater care needs in the CVD setting. Thus, it facilitates personalised pharmacotherapeutic follow-up, allowing interventions to be individualised and reassessment intervals to be set according to the patient's risk profile. By integrating extensive data into the electronic medical record, including information from structured interviews, the model enables a multidimensional assessment that covers demographic, socio-health, clinical, and pharmacotherapeutic variables.
This comprehensive approach optimises resource allocation by focusing interventions on the patients who need them most. It also establishes the foundations for educational interventions that empower patients and improve the control of key factors, such as hypertension, cholesterol levels, and smoking. The tool is thus aligned with the paradigm shift proposed in the national strategies of Spain7,9, which seek to reorient healthcare from a disease-centred model to a patient-centred one.
The tool differs from other traditional and technological approaches that have attempted to address the complexity of cardiovascular risk. For example, tools such as the Medication Risk Complexity Index17 or the LACE index18 focus on one-dimensional aspects—either the complexity of the therapeutic regimen or the prediction of hospital readmissions—without systematically including the clinical, sociodemographic, and behavioural variables that are crucial in the clinical management of patients with CVD.
This difference is particularly relevant in light of the approaches of scientific societies such as the European Society of Cardiology. Its guidelines on cardiovascular disease prevention emphasise the importance of risk stratification to tailor preventive and therapeutic interventions to the individual characteristics of each patient19–21. Similarly, the American College of Cardiology/American Heart Association guideline on the primary prevention of CVD22 highlights the need for comprehensive cardiovascular risk assessment to guide personalised strategies that address both clinical and behavioural factors. These organisations concur that personalising interventions is essential to reducing the incidence of adverse events, thereby validating the implementation of models such as the one outlined in this study.
At the national level in Spain, the SEFH has also promoted initiatives within the MAPEX project—with approaches similar to the one proposed—which have been shown to improve patient health outcomes compared to more traditional approaches23–25.
One of the main advantages of this model is its ability to balance technical sophistication with clinical practicality. In contrast, although emerging models based on artificial intelligence and machine learning achieve high levels of accuracy, they often require complex infrastructures and large volumes of data, limiting their applicability in resource-constrained environments. On the other hand, the approach presented here relies on information already integrated into clinical systems, allowing for immediate and scalable implementation without sacrificing accuracy in risk prediction. One of the model's main strengths is its balance between complexity and operational feasibility, positioning it as a well-founded alternative for transforming clinical practice in the prevention and management of CVD26–28.
Other distinguishing features include the integration of educational interventions and the promotion of interdisciplinary collaboration. Although some models focus solely on number-based risk prediction, the proposed stratification tool encourages coordination between pharmacists, physicians, and other healthcare professionals, facilitating the exchange of information that enriches clinical decision-making. This collaborative approach has been consistently recommended by international organisations, which emphasise that a comprehensive and multidisciplinary care strategy is essential to address patient heterogeneity and improve health outcomes.
Stratification is only one pillar of the CMO methodology that has been proposed and tested in other diseases. Therefore, this process must be complemented by aligning short-, medium-, and long-term pharmacotherapeutic objectives, and by providing longitudinal patient follow-up, thereby enabling real-time or timely responses to their needs. This paradigm, which combines accuracy, operability, and ease of implementation, is emerging as a tool with great potential to guide future PC and cardiovascular prevention strategies, while adapting to the demands of a health system that is increasingly oriented towards personalised care.
This study is limited by its reliance on qualitative data obtained through structured interviews, which could introduce bias in the assessment of adherence and other patient behaviour. Furthermore, the limited sample size of the pilot study means that the results cannot be generalised to more heterogeneous populations. However, standardising the data collection instruments and using pre-existing data from medical records has helped minimise these limitations and promote the tool's reproducibility.
Although the model has been designed such that approximately two-thirds of the required data are already recorded in the electronic medical record—which, based on our experience, makes completing the data easier—the time required to complete the remaining data was not measured systematically. Future research should focus on developing automated processes that reduce the healthcare burden.
On the other hand, the main strength of the model lies in its multidimensional approach and its ability to integrate objective and subjective variables, enabling a comprehensive assessment of cardiovascular risk. The potential for pre-stratifying patients using information already available in clinical systems optimises the allocation of resources, ensuring that patients with the greatest need receive intensive and personalised interventions. In addition, the ease with which the model can be integrated into clinical practice—without requiring excessively complex technological infrastructures—underscores its operational viability across settings with varying resource levels, which is particularly important in the current context.
To consolidate the validity of the model and broaden its applicability, multicentre prospective studies incorporating a greater diversity of populations and healthcare centres are required. In this context, the integration of technological tools could further optimise how variables are weighted, enabling the detection of risk patterns that might be overlooked by traditional methods. Likewise, developing automated follow-up protocols that facilitate real-time restratification could allow interventions to be adjusted dynamically according to the patient's clinical evolution.
In addition to its operational viability in specialised care settings, the model has high scalability potential in other healthcare settings, particularly primary care, where the early detection and long-term management of cardiovascular risk are crucial.
Another promising line of research could be extending the model to healthcare settings other than specialised care, enabling the implementation of comprehensive, inter-level PC strategies across various therapeutic contexts. Finally, the impact of educational and collaborative interventions on adherence and clinical outcomes should be systematically evaluated, and the reduction in adverse events and savings in healthcare resources quantified, thereby supporting the added value of the stratification methodology adopted.
In conclusion, the proposed stratification tool represents a significant innovation in PC, as it enables personalised monitoring and optimal resource allocation. Its coordinated implementation across hospital pharmacy services would generate a uniform set of comparable indicators which, when integrated into institutional scorecards, would provide added strategic value: identifying gaps in care, guiding resource allocation, and designing common training programmes based on real needs. The multidimensional approach of the model, underpinned by the recommendations of scientific societies, promotes the adoption of educational and collaborative interventions among healthcare professionals. Applied in the context of the CMO methodology, this tool could—after future multicentre validation and automation—contribute to transforming clinical practice into more multidimensional, accurate, and efficient PC that is fully aligned with national health strategies in Spain.
Contribution to the scientific literatureThis study introduces an innovative risk stratification tool for patients with cardiovascular risk in the field of pharmaceutical care. The tool comprises 20 variables grouped into 4 dimensions (demographic, socio-health and functional, clinical and service use, and treatment-related), enabling patients to be classified into 3 priority levels. This classification facilitates the adoption of interventions tailored to the intensity of patient needs, thereby optimising the allocation of healthcare resources.
The results of the pilot study showed that the clinical and service use dimensions accounted for more than 80% of the total score, which underscores the importance of targeting interventions in these critical areas. Specific strategies were established based on the defined cut-off points. Priority 1 patients receive intensive pharmaceutical care, including more frequent follow-ups, detailed reviews of their therapeutic regimen, and close multidisciplinary coordination. Priority 2 and 3 patients are managed with less intensive interventions, adapted to the complexity of their clinical situation and optimising the use of available resources.
This contribution to the literature highlights the importance of having a stratification tool that can objectively identify the risk level of each patient and enable interventions to be tailored to the individual. In addition, the data provide a practical basis for strategic planning and the implementation of healthcare policies aimed at improving the efficiency and quality of pharmaceutical care in the cardiovascular context.
En representación de MAPEX-SEFH y grupo Cardio de la Sociedad Española de Farmacia Hospitalaria (por orden alfabético de apellidos): Cristina Díez Vallejo; Sara Guijarro Herrera; Sara Ibáñez García; Aránzazu Linares Alarcón; José Antonio Martín Conde; María José Mauriz Montero; Nuria Martínez Casanova; Rebeca Pelegrín Cruz.
Declaration of authorshipAll authors contributed to developing the original idea and designing the study. Ramón Morillo-Verdugo was responsible for writing the manuscript. The final version was reviewed and approved for publication by all the authors.
None declared.
1 Miembros en representación de MAPEX-SEFH.
Cristina Díez Vallejo, Sara Guijarro Herrera, Aránzazu Linares Alarcón y Rebeca Pelegrín Cruz.
2 Grupo Cardio de la Sociedad Española de Farmacia Hospitalaria.
Sara Ibáñez García, José Antonio Martín Conde, María José Mauriz Montero y Nuria Martínez Casanova.