The combination of selective serotonin reuptake inhibitors with rivaroxaban may result in a dual interaction (pharmacokinetic and pharmacodynamic) depending on the type of selective serotonin reuptake inhibitor employed (CYP3A4-inhibiting vs. non-CYP3A4 inhibiting). The purpose of this study was to use real world data to determine if the type of selective serotonin reuptake inhibitor used plays a role in the risk and severity of bleeding in patients receiving rivaroxaban.
MethodThis was a single-center retrospective longitudinal observational study carried out between January 2016 and February 2020 in patients aged 18 years or older treated concurrently with rivaroxaban (prescribed for treatments) and a selective serotonin reuptake inhibitor. Patients were divided into two groups according to the selective serotonin reuptake inhibitor they received, i.e., a CYP3A4 inhibitor (group 1): sertraline, fluoxetine and paroxetine, or a non-CYP3A4 inhibitor (group 2): citalopram and escitalopram. We analyzed the bleeding events and severity, the daily dose of rivaroxaban used and the medication administered concomitantly.
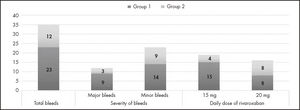
ResultsA total of 146 patients were included (89 in group 1 and 57 in group 2) and 35 bleeding events (24% of patients) were identified, of which 12 were major and 23 were minor. The bleeding rate was higher in group 1 (25.8% vs 21.0%) but there were no differences in major bleeding (10.1% vs 5.3%; p = 0.235) or minor bleeding (13.5% vs 15.8%; p = 0.496). The bleeding rate with a daily rivaroxaban dose of 20 mg was 9% (8/89) in group 1 and 14% (8/57) in group 2 (p = 0.2137), as compared with 16.9% (15/89) in group 1 versus 7% (4/57) in group 2 (p = 0.042) for a daily 15 mg dose.
ConclusionsAlthough the type of selective serotonin reuptake inhibitor used concurrently with rivaroxaban was not found to influence the patients’ bleeding risk, a significant increase in the risk of bleeding was observed based on the dose of rivaroxaban used.
La combinación de rivaroxabán e inhibidores selectivos de la recaptación de serotonina presenta un riesgo de interacción farmacodinámica y farmacocinética que depende del tipo de inhibidor selectivo de la recaptación de serotonina empleado, ya que algunos son inhibidores del citocromo p450, mientras que otros no lo son. El objetivo del presente estudio fue evaluar con datos de vida real si el tipo de inhibidor selectivo de la recaptación de serotonina utilizado influye en la frecuencia y en la gravedad de sangrado en pacientes anticoagulados con rivaroxabán.
MétodoEstudio observacional, longitudinal, retrospectivo y unicéntrico, realizado entre enero de 2016 y febrero de 2020 en pacientes > 18 años que recibían rivaroxabán, en indicaciones autorizadas y financiadas, y que estaban siendo tratados concomitantemente con inhibidores selectivos de la recaptación de serotonina. Se establecieron dos cohortes en función del inhibidor selectivo de la recaptación de serotonina coadministrado: inhibidores del CYP3A4 (grupo 1) —sertralina, fluoxetina y paroxetina—, y no inhibidores del CYP3A4 (grupo 2) —citalopram y escitalopram—. Se analizaron los eventos hemorrágicos, la gravedad del sangrado, la dosis diaria de rivaroxabán y la medicación concomitante que pudiese influir en el riesgo de sangrado.
ResultadosSe incluyeron 146 pacientes (89 en el grupo 1 y 57 en el grupo 2) y se identificaron un total de 35 eventos hemorrágicos (24% de los pacientes), de los que 12 fueron eventos mayores y 23 menores. La frecuencia de sangrado fue ligeramente mayor en el grupo 1 que en el 2 (25,8% versus 21%), pero no se encontraron diferencias significativas entre ambos grupos, ni tampoco en la frecuencia de sangrados mayores (10,1% versus 5,3%; p = 0,235) o menores (13,5% versus 15,8%; p = 0,496). La frecuencia de eventos hemorrágicos con la dosis de 20 mg fue del 9% (8/89) en el grupo 1 y del 14% (8/57) en el grupo 2 (p = 0,2137), mientras que con una dosis de 15 mg la frecuencia de eventos fue del 16,9% (15/89) en el grupo 1 y del 7% (4/57) en el grupo 2 (p = 0,042).
ConclusionesNo se han hallado diferencias significativas en el riesgo de sangrado según el tipo de inhibidor selectivo de la recaptación de serotonina que se administre de forma concomitante al rivaroxabán. Sí se han observado diferencias significativas en función de la dosis de rivaroxabán utilizada.
Atrial fibrillation is the most common kind of sustained arrhythmia in adult patients and it is estimated that over 40 million individuals globally suffered from this condition in 20161. These figures are expected to increase in the future, with forecasts predicting a greater than twofold increase in the number of cases in patients aged 55 or over only in the European Union2. Atrial fibrillation is associated with high morbimortality levels and a high personal and economic burden; it is the most common cause of heart embolism and leads to a four-to-fivefold increase in the risk of stroke3,4. The current evidence-based guidelines recommend administration of anticoagulant treatment in patients with additional risk factors. For example, the CHEST guidelines recommend starting oral anticoagulant treatment in patients with a non-sexrelated CHA2DS2-VASc score higher than or equal to 15 while the joint American Heart Association, the American College of Cardiology and the Heart Rhythm Society guidelines recommend starting treatment when the CHA2DS2-VASc score is 2 in males and 3 in females6. Oral anticoagulant treatment can involve the use of vitamin K antagonists (VKAs) or the so-called directly acting oral anticoagulants (DOACs)6.
Rivaroxaban is a DOAC that does not result in interactions usually seen with VKAs (such as interactions with food) but does interact with other medications, including selective serotonin reuptake inhibitors (SSRIs)7.
Management of depressive disorders commonly consist in the use of a non-pharmacological approach, such as cognitive behavioral therapy, and pharmacological measures with SSRIs considered first-line treatment8. Depression is one of the most common mental diseases in the elderly, with an overall prevalence of 10%9. The American Psychological Association recommends using paroxetine in adult patients over 60 years with persistent depressive disorder10, but warns that according to some authors paroxetine should be contraindicated in these patients due to its anticholinergic side effects (2019 AGS Beers criteria)11 and that many geriatric psychiatrists would prefer SSRIs such as escitalopram or sertraline.
This means that elderly patients might require administration of both types of drugs. Joint use of rivaroxaban and SSRIs could give rise to interactions via both pharmacodynamic and pharmacokinetic mechanisms. On one hand, SSRIs prevent the reuptake of serotonin by platelets and interfere with their antiagregation ability, increasing bleeding risk when administered concomitantly with rivaroxaban. On the other hand, some SSRIs (paroxetine, fluoxetine and sertraline) also inhibit the activity of CYP3A4. Inhibition of this cytochrome, which is involved in rivaroxaban's metabolism, increases the risk of DOAC-induced toxicity12,13. Because of this interaction, physicians recommend that care should be exercised when using rivaroxaban concomitantly with SSRIs14. Yet it is not known whether some SSRIs may present a more favorable profile than others in this context.
The goal of the present study was to use real life data to determine whether the type of SSRI used may influence the frequency and the severity of the bleeding experienced by patients anticoagulated with rivaroxaban, with a view to optimizing the management of patients who require a combination of SSRIs and rivaroxaban.
MethodsThis was a retrospective longitudinal observational cohort study that included patients treated with rivaroxaban and a SSRI between January 2016 and February 2020 in a level 5 general hospital. A review was conducted of patients admitted to the hospital with concomitant rivaroxaban/SSRI treatment and electronic medical records (EMRs) were searched to determine when concomitant treatment with both drugs had begun. All patients aged 18 years or older treated with rivaroxaban for approved indications listed on the drug's SmPC and eligible for reimbursement were included. Cancer patients as well as those with thrombocytopenia diagnosed prior to initiation of rivaroxaban and those with coagulopathies were excluded.
Before the start of the study and the data collection process, an application was submitted to the hospital's Research Ethics Committee for Medicinal Products. The Committee accepted the application (approval nr. 21/026).
Patients were followed from initiation of combined rivaroxaban/SSRI treatment up to a maximum of two years or discontinuation of treatment.
The following variables were analyzed: sex, age, type of SSRI prescribed, occurrence of bleeding, severity of bleeding, number of hemorrhagic events, dose of rivaroxaban administered and concomitant medication that could influence the bleeding risk (non-steroidal anti-inflammatory drugs [NSAIDs], proton pump inhibitors [PPIs], H2 blockers and antiplatelets).
The severe bleeding variable was defined as a bleeding episode resulting in one of the following: death, a space-occupying lesion (SOL), a decrease of hemoglobin levels equal to or greater than 2 g/dL from baseline, the need to transfuse two or more blood units, or permanent disability.
Patients were randomized into two cohorts: group 1 was formed by patients who received SSRIs capable of inhibiting CYP3A4 (sertraline, paroxetine and fluoxetine); and group 2 was formed by patients who received SSRIs that do not inhibit CYP3A4 (citalopram and escitalopram).
The data was collected in a pseudoanonymized logbook. The information was obtained from each patient's EMR, the hospital computerized prescription systems, and the single prescription module used for ambulatory prescriptions.
Statistical analysis was performed using the SPSS Statistics v.21.0 software package. The Kolmogorov-Smirnov test was applied to determine whether the sample followed a normal distribution. Quantitative variables were analyzed by means of a descriptive analysis using central tendency (means) and dispersion (standard deviation) measurements. Qualitative variables were described by frequency distribution. Student's t test and chi-squared tests (depending on whether the data analyzed was quantitative or qualitative) were used to determine the differences between the cohorts; the p values for the chi-squared test were one-tailed when the bleeding variable was analyzed as the potential interaction could only increase the bleeding risk and not decrease it. Statistical significance was established for a p value < 0.05.
ResultsA total of 146 patients with atrial fibrillation who had received rivaroxaban for the prevention of stroke or systemic embolism were included in the study (Table 1). Eighty-nine subjects comprised the cohort of non-CYP3A4 inhibiting SSRIs (group 1), while the cohort of CYP3A4-inhibiting SSRIs was made up of 57 patients (group 2). Both groups were homogeneous in terms of age and sex. Application of the Kolmogorov-Smirnov test showed that the age variable corresponded to a normal distribution in both groups (group 1: p = 0.364 and group 2: p = 0.292); the mean and standard deviation were 85.7± 8.2 years for group 1 and 83.6 ± 9.7 years for group 2 (p = 0.155). A non-significant value was obtained for homogeneity regarding sex distribution (p = 0.3403).
Demographic characteristics, hemorrhagic events and rivaroxaban dose in the two groups patients were distributed into according to the type of SSRI they received
| Group 1 (n = 89) | Group 2 (n = 57) | |||||||
|---|---|---|---|---|---|---|---|---|
| Fluoxetine | Paroxetine | Sertraline | Total | Citalopram | Escitalopram | Total | Probability | |
| Sex | ||||||||
| Males, n (%) | 0 | 4 (10.8%) | 21 (56.8%) | 25 (67.6%) | 8 (21.6%) | 4 (10.8%) | 12 (32.4%) | p = 0.340 |
| Females, n (%) | 3 (2.7%) | 19 (17.4%) | 42 (38.5%) | 64 (58.7%) | 28 (25.7%) | 17 (15.6%) | 45 (41.3%) | |
| Age (years) | ||||||||
| Mean (SD) | 85.7 (8.18) | 83.6 (9.68) | p = 0.155 | |||||
| Number of hemorrhagic events | ||||||||
| Males, n (%) | 0 | 0 | 4 (100%) | 4 (100%) | 0 | 0 | 0 | p = 0.254 |
| Females, n (%) | 1 (3.2%) | 9 (29%) | 9 (29%) | 19 (61.2%) | 5 (16.2%) | 7 (22.6%) | 12 (38.8%) | |
| Type of bleed | ||||||||
| Major, n (%) | 0 | 2 (16.7%) | 7 (58.3%) | 9 (75%) | 1 (8.3%) | 2 (16.7%) | 3 (25%) | p = 0.236 |
| 0 | 1 (50%) | 1 (50%) | 2 (100%) | 0 | 0 | 0 | |
| 0 | 0 | 0 | 0 | 1 (50%) | 1 (50%) | 2 (100%) | |
| 0 | 0 | 5 (100%) | 5 (100%) | 0 | 0 | 0 | |
| 0 | 1 (33.3%) | 1 (33.3%) | 2 (66.6%) | 0 | 1 (33.3%) | 1 (33.3%) | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Minor, n (%) | 1 (4.7%) | 6 (28.6%) | 5 (23.8%) | 12 (57.1%) | 4 (19.1%) | 5 (23.8%) | 9 (42.9%) | p = 0.496 |
| Rivaroxaban dose | ||||||||
| 15 mg, n (%) | 0 | 7 (36.9%) | 8 (42.1%) | 15 (79%) | 2 (10.5%) | 2 (10.5%) | 4 (21%) | p = 0.042* |
| 20 mg, n (%) | 1 (6.3%) | 2 (12.5%) | 5 (31.2%) | 8 (50%) | 3 (18.8%) | 5 (31.2%) | 8 (50%) | p = 0.214 |
DHb: hemoglobin; SOL: space-occupying lesion; SD: standard deviation.
A total of 35 hemorrhagic events (24% of patients) were identified (Figure 1). The bleeding rate was higher in group 1 (25.8%; 23/89) than in group 2 (21%; 12/57), without statistically significant differences between the groups (p = 0.2542) Minor bleeds (n = 23) accounted for 65.7% of all hemorrhagic events. Fourteen of these occurred in group 1 (15.7%) and nine in group 2 (15.8%) (p = 0.4962). Regarding major bleeds, nine (10.1%) occurred in group 1 and three in group 2 (5.3%) (p = 0.2359), the most frequent bleeding episodes in this category were decreases in hemoglobin levels > 2 g/dL (seven events, some of which required transfusions) followed by SOLs (Table 1). Two subjects died as a result of bleeding, both of them in group 1 and both on the 15 mg dose; one of them developed subsequent hemodynamic instability and the other a secondary infection of hematoma, which resulted in sepsis.
Forty-seven patients in group 1 and 32 patients in group 2 received a daily dose of 20 mg of rivaroxaban. The bleeding rate in patients on this dose was 9% (8/89) in group 1 and 14% (8/57) in group 2; the p value was calculated to be 0.2137 by the one-tailed chi squared test. Forty-two patients in group 1 and 25 patients in group 2 received the 15 mg rivaroxaban dose. The bleeding rate with this dose was 16.9% (15/89) in group 1 and 7% (4/57) in group 2; the p value in these patients was 0.04165 as calculated by the one-tailed chi-squared test (Ta ble 1).
The data on the concomitant medication (NSAIDs, PPIs/Anti-H2s and antiplatelets) that could result in the development of bleeds is contained in table 2. No significant differences were observed between the groups regarding the use of these drugs (NSAIDs: p = 0.1060; PPIs/ Anti-H2s: p = 0.7321; antiplatelets: p = 0.8477) although there was a higher proportion of patients on NSAIDs in group 2 than in group 1 (16.8% vs 28.1%). A total of 121 patients (81.2% of total) were receiving either a PPI or an anti-H2, while 31 were receiving an NSAID (21.2% of total) and 17 an antiplatelet (11.6% of total). Some patients were on drugs belonging to two or more groups: NSAID + PPI/Anti-H2 (n = 23; 15.6%); antiplatelet + PPI/Anti-H2 (n = 10; 6.8%) and antiplatelet + NSAID (n = 1; 0.7%). Three patients (2%) were on NSAID + PPI/Anti-H2 + antiplatelet treatment.
Concomitant medication with a potential influence on the risk of bleeding
| Group 1 (n = 89) | Group 2 (n = 57) | |||||||
|---|---|---|---|---|---|---|---|---|
| Fluoxetine | Paroxetine | Sertraline | Total | Citalopram | Escitalopram | Total | Probability | |
| Concomitant medication | ||||||||
| PPIs/Anti-H2s (%) | 2 (1.7%) | 18 (14.9%) | 53 (43.8%) | 73 (60.4%) | 27 (22.3%) | 21 (17.3%) | 48 (39.6%) | p = 0.106 |
| NSAIDs (%) | 0 | 3 (9.7%) | 12 (38.7%) | 15 (48.4%) | 10 (32.3%) | 6 (19.3%) | 16 (51.6%) | p = 0.732 |
| Antiplatelets (%) | 0 | 4 (23.5%) | 6 (35.3%) | 10 (58.8%) | 3 (17.7%) | 4 (23.5%) | 7 (41.2%) | p = 0.847 |
Anti-H2: class 2 antihistamine; NSAID: nonsteroidal anti-inflammatory drug; PPI: proton pump inhibitor.
This is one of the first studies seeking to determine the potential dual interaction between CYP3A4-inhibiting SSRIs and rivaroxaban in patients with non-valvular atrial fibrillation. The data provided by previous studies on the combination of DOACs with SSRIs is rather contradictory. A recent study in Korean subjects evaluated the bleeding risk in patients with atrial fibrillation receiving concomitant treatment with an SSRI or an NSAID and found higher risk in patients receiving the combination recommending the use of PPIs to prevent upper digestive tract hemorrhage15. In contrast other studies did not observe an increased risk of bleeding in patients receiving a combination of a DOAC with an SSRI16,17.
Previous studies centered on interactions of a pharmacodynamic kind. A recent United Kingdom population study evaluated the risk of major bleeding resulting from the combination of DOACs with several drugs typically involved in pharmacodynamic and pharmacokinetic interactions (CYP3A4 and P glycoprotein-inhibitors) and observed that pharmacokinetic interactions with DOACs did not lead to an increased risk of major bleeding as compared with pharmacodynamic interactions, which were associated with a higher risk of major bleeding18.
The present study did not show a significant bleeding risk difference between using CYP3A4-inhibiting SSRIs (pharmacodynamic + pharmacokinetic interaction) and non-CYP3A4 inhibiting SSRIs (pharmacodynamic interaction only). It appears that the potential effect of SSRI on cytochrome P450 is not clinically significant in patients treated with rivaroxaban. These results are in line with the information contained in rivaroxaban's SmPC, which specifies that only potent CYP3A4 inhibitors may have a clinically significant effect given the low contribution of said cytochrome to the drug's metabolism14. Nonetheless, significant differences were found when the bleeding risk was analyzed by stratifying patients according to the rivaroxaban dose administered (15 mg vs 20 mg), with a higher bleeding risk being observed in patients who received the 15 mg dose with CYP3A4-inhibiting SSRIs compared with patients who received the 15 mg dose with non-CYP3A4-inhibiting SSRIs. This result may appear counterintuituve, but taking into account that the 15 mg dose is the one administered to patients with atrial fibrillation with impaired renal function, in whom the pharmacokinetic interaction could exert a greater clinical impact as rivaroxaban's half-life may be extended, increasing the bleeding risk.
No statistically significant differences were observed between CYP3A4-inhibiting and non-CYP3A4-inhibiting SSRIs regarding the severity of bleeding, although a higher rate of severe bleeds was observed in patients on CYP3A4-inhibiting SSRIs (10.1%) than in those on non-CY3A4-inhibiting SSRIs (5.2%). The most frequent adverse event was a decrease in hemoglobin levels by more than 2 g/dL without the need for subsequent transfusion.
The presence of concomitant medication that could affect bleeding risk in our population was high but there were no statistical differences in their presence between both groups. The high percentage of patients who received concomitant medication, particularly PPIs/Anti-H2s highlights the importance of prescribing correctly and using tools like deprescription to optimize rational use of drugs and avoid the potential risk inherent in long-term administration and drug-drug interactions19. This is especially important in the case of elderly patients such as those included in our study, who tend to experience a higher risk20.
Exposure to rivaroxaban, and to DOACs in general, may be influenced by other factors including advanced age, low body weight and renal and liver failure21. One of the limitations of this study is the fact that some of these factors, particularly renal failure, were not recorded. Future studies should direct their attention to renal failure, taking into account that DOACs (unlike VKAs) are closely dependent on renal clearance. Other limitations include those intrinsic to retrospective studies. Cases of mild bleeding are often not recorded as they seldom require treatment; neither is all the medication taken by the patient; for the purposes of this study this information was obtained from the patients’ EMR, which does not include over-the-counter medicines, among them certain pain killers. P glycoprotein-inhibiting medications were not considered either. Lastly, the small sample size of the study made it impossible to conduct a subgroup analysis to determine whether other variables could play a role in the bleeding risk.
The interaction between rivaroxaban and SSRIs remains controversial. The literature is contradictory and the current recommendations are based on an exhaustive assessment of the risk/benefit ratio associated with each patient7. Given that treatment with rivaroxaban, unlike VKA therapy, does not have well-established monitoring techniques defined, potential interactions must be considered prior to initiation of treatment. In patients who require combination treatment, it is important to select the most appropriate SSRI considering the patient's situation and characteristics. Non-CYP3A4 inhibiting SSRIs could be more beneficial than CYP3A4 inhibiting SSRIs in patients with renal failure, taking into consideration other factors such as citalopram and escitalopram-induced QT interval prolongation22, although it is true that the prolongation in itself is often minimal on these cases and its clinical relevance negligible23. For that reason, it is necessary to conduct further studies on the subject, particularly considering the increased prevalence of coadministration of SSRIs and rivaroxaban in elderly patients24, which could have a negative impact on patient safety with serious consequences in some cases.
In conclusion, this study did not find the combination of rivaroxaban with SSRIs to entail a higher risk of hemorrhagic events regardless of severity. A higher bleeding risk was observed in patients who received the 15 mg dose concurrently with CYP3A4-inhibiting SSRIs. Given the prevalence of said combination and the clinical importance of the resulting adverse events, additional studies should be conducted to further analyze the evidence presented in this study and improve patient safety.
FundingNo funding.
Conflict of interestsNo conflict of interests.
Contribution to the scientific literature
The present study provides information about the incidence and severity of bleeding in patients treated with a combination of rivaroxaban and different kinds of selective serotonin reuptake inhibitors. The study focuses specifically on the dual interaction observed between rivaroxaban and a subgroup of these antidepressants.
The findings obtained should allow optimization of the therapeutic management of patients requiring treatment with selective serotonin reuptake inhibitors plus rivaroxaban with the aim of reducing their risk of developing hemorrhagic events.
Early Access date (11/29/2021).