The aim of this study was to assess the safety of the most frequently used biologic disease-modifying antirheumatic drugs in rheumatoid arthritis patients in clinical practice.
MethodA retrospective longitudinal observational study was performed. Clinical information was obtained from the electronic health records of patients diagnosed and treated for rheumatoid arthritis, who had received at least one biologic disease-modifying antirheumatic drug dispensed between 2001 and 2013 from a third-level Hospital pharmacy. Adverse reactions during biologic disease-modifying antirheumatic drugs treatments were analysed, as well as the reasons for treatment discontinuation. A disproportionality analysis (odds ratio with 95% confidence interval) was performed to compare adverse drug reactions related to different system organ classes, the period between the drug start date and the reaction start date (latency period), and previous knowledge of the adverse reactions.
ResultsIn total, 210 patients were included in the analysis (73% women, median age 47 years), with 399 prescriptions for biologic disease-modifying antirheumatic drugs and 1,515 adverse reactions potentially related to them. The increased frequency of adverse reactions for each system organ class related to each biologic disease-modifying antirheumatic drug was as follows: general disorders and administration site disturbances with infliximab (2.3 [1.3-4.0]), infections (1.6 [1.3-2.1]) and immune system reactions with etanercept (4.2 [1.2-14.6]), hepatobiliary disorders with adalimumab (2.1 [1.2-3.6]), ophthalmic adverse reactions (1.9 [1.2-3.1]) and cardiac disorders (2.9 [1.0-8.4]) with rituximab, and blood and lymphatic system disorders with tocilizumab (2.9 [1.8-4.7]) and abatacept (3.0 [1.6-5.8)]. The mean latency period was 5 to 33 months. Most adverse reactions were related to adalimumab (93.6%; P < 0.01), whereas the fewest adverse reactions were related to tocilizumab (55.2%; P < 0.01). Most treatment withdrawals related to adverse reactions were identified during the first year of biologic disease-modifying antirheumatic drugs treatment.
ConclusionsTumour necrosis factor a inhibitors were associated with general disorders and administration site disturbances, infections and immune system reactions, and hepatobiliary abormalities, whereas nontumour necrosis factor a inhibitors were associated with cardiac disorders as well as blood and lymphatic system disorders. Treatment withdrawals mainly occurred during the first year of treatment. Most of the adverse reactions have been previously described.
Analizar la seguridad del tratamiento con fármacos biológicos modificadores de la enfermedad prescritos con mayor frecuencia en pacientes con artritis reumatoide en la práctica clínica habitual.
MétodoEstudio observacional retrospectivo, a partir de la historia clínica digitalizada de pacientes con artritis reumatoide de un hospital de tercer nivel, sobre la seguridad de los fármacos biológicos modificadores de la enfermedad, entre los años 2001 y 2013. Además de analizar las reacciones adversas que motivaron la retirada del tratamiento, se hizo un análisis de desproporcionalidad comparando los órganos y sistemas implicados en las reacciones adversas asociadas a los diferentes fármacos biológicos modificadores de la enfermedad calculando la odds ratio con un intervalo de confianza del 95% [odds ratio (IC95%)], del periodo de latencia entre el inicio del tratamiento y el diagnóstico de los efectos adversos, y de su conocimiento previo.
ResultadosSe analizaron las historias clínicas de 210 pacientes (73% mujeres; mediana de edad: 47 años), que incluían 399 líneas de tratamiento con algún fármaco biológico modificado de la enfermedad y 1.545 reacciones adversas potencialmente relacionadas con ellos. Se identificó un incremento significativo de reacciones adversas en los siguientes órganos y sistemas afectados: trastornos generales y del lugar de administración [2,3 (1,3-4,0)] para infliximab; infecciones [1,6 (1,3-2,1)] y trastornos del sistema inmunológico [4,2 (1,2-14,6)] para etanercept; trastornos hepatobiliares [2,1 (1,2-3,6)] para adalimumab; trastornos oculares [1,9 (1,2-3,1)] y cardiacos [2,9 (1,0-8,4)] para rituximab; trastornos de la sangre y del sistema linfático [2,9 (1,8-4,7)] para tocilizumab y abatacept [3,0 (1,6-5,8)]. La latencia media osciló entre 5 y 33 meses. La mayor y menor proporción de reacciones adversas conocidas correspondieron a adalimumab (93,6%; p < 0,01) y tocilizumab (55,2%; p < 0,01), respectivamente. Más de la mitad de las retiradas de fármacos biológicos modificadores de la enfermedad asociadas a reacciones adversas se produjeron en el primer año de tratamiento.
ConclusionesLos fármacos biológicos modificadores de la enfermedad inhibidores del factor de necrosis tumoral a se asociaron a la presentación de trastornos generales, infecciones y trastornos del sistema inmunológico y a alteraciones hepatobiliares, mientras que los no inhibidores del factor de necrosis tumoral a se relacionaron con un incremento en los trastornos oculares y cardiacos, trastornos de la sangre y del sistema linfático. La interrupción del tratamiento por reacciones adversas sucedió durante el primer año. La mayoría de las reacciones adversas registradas eran conocidas.
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease that symmetrically affects small and medium-sized joints. Its estimated global prevalence is approximately 1% and in the Spanish population it is around 0.5%, according to the EPISER study published in 20021. In the last two decades, advances in understanding the pathophysiological mechanisms of RA, together with the development of molecular engineering techniques, have led to the appearance of new biologic disease-modifying antirheumatic drugs (bDMARDs) that are highly effective in the control of RA2. The bDMARDs currently marketed in Spain include tumour necrosis factor-α (TNF-α) inhibitors (infliximab, etanercept, adalimumab, golimumab, and certolizumab pegol) and non-TNF-α inhibitors (rituximab, abatacept, tocilizumab, anakinra, and sarilumab)3.
Since bDMARDS were first marketed, treatment with them has been closely monitored to identify their long-term effects4. Their marketing has been accompanied by the development of various efficacy and safety registries, which constitute prospective longitudinal cohorts, in the attempt to complete the information provided by clinical trials5. However, the patients included in these registries are heterogeneous and information on some biologic treatments is scarce. In a previous study6, our group analysed the prescribing patterns and clinical outcomes of bDMARDs for RA in Spain. The aim of the present study was to analyse, in routine clinical practice, the safety of the most frequently prescribed bDMARD treatments in patients with RA.
MethodsWe conducted a retrospective observational study using the electronic health records of a 1,039-bed tertiary hospital. We included all patients over 18 years diagnosed with RA, selecting those who received bDMARDs by the Pharmacy Service between 2001 and 2013 and with sufficient follow-up. This date corresponds to the last year prior to the protocolization of biologic prescription in the hospital. We excluded patients who had received bDMARDs as participants in a clinical trial and patients treated with golimumab because the drug was included in the hospital guidelines shortly before the end of the study period. All data were anonymised and coded in accordance with the Personal Data Protection Law. The study protocol was approved by the Research Ethics Committee of the Principality of Asturias (study no. 52/15).
Demographic data, the general characteristics of the disease, and treatments used (drug, line of treatment) were collected following the European League Against Rheumatism (EULAR) recommendations for observational studies7. We studied the safety of the bDMARDs analysed by recording all adverse reactions associated with them (excluding golimumab due to the small number of patients under treatment) noted in the clinical history, whether reported by patients or observed by physicians, and analysed the cases in which these adverse reactions were the reason for withdrawing bDMARDs. In line with the Medical Dictionary for Regulatory Activities (MedDRA)8, we coded the recorded adverse reactions according to “Preferred Terms” (level 2, MedDRA Classification) and “System Organ Classes” (level 5, MedDRA Classification). We analysed the following aspects for each of the organs and systems in which there were bDMARD-associated adverse reactions: 1) Disproportionality between notifications with these bDMARDs compared with those of other bDMARDs by calculating the Odds Ratio (OR) with a 95% Confidence Interval (95% CI); and 2) the latency period of the adverse reactions, calculated as the time in months elapsed between starting bDMARD treatment and the date of diagnosis of the adverse reaction. In addition, we studied “previous knowledge” related to each of the adverse reactions recorded. For this purpose, we classified the adverse reactions as follows: a) known: those described in the drug's summary of product characteristics (SPC); b) plausible: not described in the SPC, but reasonable taking into account the mechanism of action of the suspect drug; c) referred to in the medical literature: those that, although not described in the SPC, and cannot be deduced from the drug's mechanism of action, have been previously described in the medical literature; thus, we searched PubMed using the name of the active ingredient and the adverse reaction observed; and d) unknown: those not included in any of the previous categories. In relation to the unknown adverse reactions, we also investigated if there were similar ones recorded in the EudraVigilance database (public version) of the European Medicines Agency9. All suspected adverse reactions were reported to the Spanish pharmacovigilance system.
To record the information extracted from the digitized medical records, we used Microsoft Excel 2010 to create a data matrix in which the study variables were defined. Age was treated as a metric discrete variable and is expressed as the median [quartile 1 - quartile 3; Q1 - Q3]. Other metric variables are expressed as the mean ± standard error of the mean [SEM], and categorical variables are expressed as frequencies and percentages. Statistical analysis was conducted using the IBM SPSS Statistics 24.0 program and the computer application: “Sisa: two by two table analysis”10.
ResultsWe analysed 210 medical records of patients diagnosed with RA, including a total of 399 treatment lines with any bDMARD. The patients had a median age of 47 years (Q1 - Q3: 36.6-55.6) and were mainly women (n = 154; 73%). Mean disease severity was moderate-severe (Disease Activity Score 28 [DAS 28]: mean ± SEM: 5.0 ± 0.2) and the mean time to prescription of the first bDMARD was 5 to 6 years (mean ± SEM: 69.2 ± 5.0 months), having previously received one or two treatment lines with conventional DMARDs (cDMARDs). No treatments with anakinra or certolizumab were identified in patients meeting the inclusion criteria, neither was treatment with sarilumab because it was not included in the Hospital Pharmacotherapeutic Guide during the study period. We observed 1,545 adverse reactions potentially related to bDMARDs.
Overall, adverse reactions associated with bDMARDs led to drug withdrawal in only 20% of the treatment lines. Furthermore, in more than half of the cases, these withdrawals occurred during the first year of treatment (Table 1). The disproportionality analysis of the organs and systems affected by the recorded adverse reactions showed the following increases for each bDMARD analysed: 1) infliximab: general and administration site disorders (perfusion-related reactions); 2) etanercept: infections and infestations (respiratory tract infections) and immune system disorders (allergic reactions); 3) adalimumab: hepatobiliary disorders (increased liver enzymes); 4) rituximab: eye (conjunctivitis) and cardiac (arrhythmia) disorders; 5) tocilizumab: blood and lymphatic system disorders (leukopenia); and 6) abatacept: blood and lymphatic system disorders (anaemia) (Table 2).
Withdrawals of biologic disease-modifying antirheumatic drugs due to adverse reactions
| bDMARD | Patients under treatment, total | Withdrawal associated with adverse-reactions | Treatment lines, total | |||||
|---|---|---|---|---|---|---|---|---|
| First year | Subsequent years | Total | ||||||
| n | n | %a | n | %a | n | %b | n | |
| Infliximab | 75 | 12 | 66.7 | 6 | 33.3 | 18 | 23.7 | 76 |
| Etanercept | 99 | 10 | 76.9 | 3 | 23.1 | 13 | 12.9 | 101 |
| Adalimumab | 109 | 15 | 45.5 | 18 | 54.5 | 33 | 29.7 | 111 |
| Tocilizumab | 54 | 5 | 83.3 | 1 | 16.7 | 6 | 10.9 | 55 |
| Rituximab | 38 | 4 | 57.1 | 3 | 42.9 | 7 | 18.4 | 38 |
| Abatacept | 18 | 1 | 33.3 | 2 | 66.7 | 3 | 16.7 | 18 |
| Total | 47 | 58.8 | 33 | 41.2 | 80 | 20.1 | 399 | |
bDMARD: biologic disease-modifying antirheumatic drugs.
Classification of adverse reactions associated with biologic disease-modifying antirheumatic drugs
| bDMARD | Classification by organ and system and most frequent reaction | OR (95% CI) |
|---|---|---|
| Infliximab | General and administration site disturbances
| 2.3 (1.3-4.0) |
| Etanercept | Infections and infestations
| 1.6 (1.3-2.1) |
Immune system disturbances
| 4.2 (1.2-14.6) | |
| Adalimumab | Hepatobiliary disturbances
| 2.1 (1.2-3.6) |
| Rituximab | Ocular disorders
| 1.9 (1.2-3.1) |
Cardiac disorders
| 2.9 (1.0-8.4) | |
| Tocilizumab | Blood and lymphatic system disorders
| 2.9 (1.8-4.7) |
| Abatacept | Blood and lymphatic system disorders
| 3.0 (1.6-5.8) |
bDMARD: biologic disease-modifying antirheumatic drugs: CI: confidence interval; OR: odds ratio.
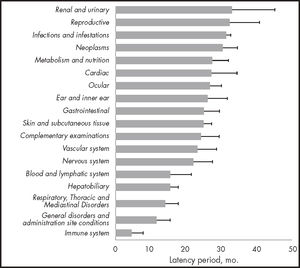
The latency periods of the adverse reactions ranged, on average, from 5 months to 33 months, with immune system disorders and renal and urinary system disorders showing the shortest and longest latency periods, respectively (Figure 1).
Most of the adverse reactions recorded were already known (Table 3). There was significantly more known information (P < 0.01) on adalimumab-associated adverse reactions, which were mainly listed in the SPC (93.6%). However, there was less known information (P < 0.01) on tocilizumab-associated adverse reactions; only 55.2% were listed in the SPC. Although 27% of them were plausible, the potential mechanism involved in their development was in line with knowledge concerning tocilizumab's mechanism of action. Tocilizumab was also the drug with the highest percentage (13%) of unknown adverse reactions recorded in the EudraVigilance database.
Previous knowledge of biologic disease-modifying antirheumatic drugs-associated adverse reactions
| bDMARD | Known | Unknown | Total | P(x2)* | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPC | MJ | BP | Total | EV, yes | EV, no | Total | |||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||
| Infliximab | 414 | 86.2 | 21 | 4.4 | 23 | 4.8 | 458 | 95.4 | 10 | 2.1 | 12 | 2.5 | 22 | 4.6 | 480 | 100 | 0.18 |
| Etanercept | 220 | 73.3 | 17 | 5.7 | 45 | 15.0 | 282 | 94.0 | 15 | 5.0 | 3 | 1.0 | 18 | 6.0 | 300 | 100 | 1.00 |
| Adalimumab | 291 | 93.6 | 2 | 0.6 | 10 | 3.2 | 303 | 97.4 | 6 | 1.9 | 2 | 0.6 | 8 | 2.6 | 311 | 100 | < 0.01 |
| Rituximab | 158 | 80.6 | 6 | 3.1 | 18 | 9.2 | 182 | 92.9 | 9 | 4.6 | 5 | 2.6 | 14 | 7.1 | 196 | 100 | 0.53 |
| Tocilizumab | 100 | 55.2 | 6 | 3.3 | 49 | 27.1 | 155 | 85.6 | 24 | 13.3 | 2 | 1.1 | 26 | 14.4 | 181 | 100 | < 0.01 |
| Abatacept | 49 | 63.6 | 17 | 22.1 | 8 | 10.4 | 74 | 96.1 | 0 | 0.0 | 3 | 3.9 | 3 | 3.9 | 77 | 100 | 0.60 |
| Total | 1,232 | 79.7 | 69 | 4.5 | 153 | 9.9 | 1,454 | 94.1 | 64 | 4.1 | 27 | 1.7 | 91 | 5.9 | 1,545 | 100 | − |
bDMARD: biologic disease-modifying antirheumatic drugs; BP: biologically plausible; EV: EudraVigilance; MJ: medical journals; SPC: summary of product characteristics.
The demographic characteristics of the study population are consistent with epidemiological data on RA, which show that women are affected 2.5 times as frequently as men and that onset can take place at any age of adult life, although peak frequency is between 40 years and 60 years11. They are also consistent with the demographic characteristics of other previously published patient cohorts, with moderate to severe disease activity at the time of diagnosis12.
Since the first biologic drugs were marketed, the Spanish Agency of Medicines and Medical Devices has published different informative notes on their safety addressed to healthcare professionals, warning of the increased risk of infections, among other adverse effects13.
The safety of bDMARDs and cDMARDS in patients with RA under standard clinical conditions was studied by Abasolo et al.14. They found a treatment discontinuation rate due to adverse reactions of 10% in the population analysed, which was 80% of the total number of patients presenting with adverse reactions. The ANSWER study15 thoroughly investigated the withdrawal of biologic drugs due to their adverse effects. It assessed the maintenance or withdrawal of treatment with seven biologic drugs used in RA under real clinical conditions (750 patients: 1,037 treatments with bDMARDs). The study found that the highest and lowest rates of adverse-effect-related withdrawals were associated with adalimumab and abatacept, respectively, although without reaching statistical significance. These results are in line with the percentages found in the present study: adalimumab and abatacept were associated with the highest (29.7%) and lowest (16.7%) withdrawal rates due to adverse reactions associated with bDMARDs, respectively. However, a meta-analysis included in a Cochrane systematic review16 found that infliximab had the strongest association with treatment withdrawal due to adverse effects.
Of note, the present study found that more than half of the withdrawals due to bDMARD-associated adverse reactions occurred in the first year of treatment with them; tocilizumab was associated with the highest percentage of withdrawals (83.3% of the total treatment lines with this drug), followed by etanercept (76.9% of the total treatment lines).
The typical latency periods described in the studies consulted refer to infections: the greatest risk is during the initial 3 months17, with a gradual reduction as treatment progresses18. Other studies have also reported the latency period of skin reactions, appearing 24 to 48 hours after the start of treatment, or of infusion reactions, appearing after more than 6 months of treatment with adalimumab, etanercept, and infliximab19.
The disproportionality analysis identified a significant increase in general and administration site disturbances related to infliximab. Infusion reactions20 have been widely described in the literature and are the most frequent adverse effect observed. However, the low incidence of general reactions observed in our study could be explained by the prevention protocol adopted in our hospital (i.e. premedication with antihistamines and/or systemic glucocorticoids).
Regarding infections and infestations as well as immune system disorders related to etanercept, it is known that TNF is a relevant component of the immune system response to a variety of infections, so it seems reasonable to consider that its inhibition implies an increase in this risk. Over the course of the development of biologic drugs, treatment with TNF-a inhibitors has been associated with increased susceptibility to infection21,22. In the aforementioned Cochrane review16, the drugs most and least associated with this type of reaction were certolizumab and abatacept, respectively, with no notable differences between the other ones. Three studies used etanercept as a comparator in the incidence of serious infections21,23,24. None of them found significant differences, although a trend was observed toward a weaker association with abatacept in the Italian and British cohorts, which was similar to the findings of the Cochrane review16.
At first, the risk of hepatotoxicity with TNF-a inhibitors seemed low, although in 2004 the Food and Drug Administration issued a safety alert of this risk in association with infliximab25. In general, although there are few accounts in the literature of this risk in association with adalimumab, there are published cases that have described toxicity similar to that observed in our study26,27. The SPC lists this reaction as very frequent (> 1/10) based on controlled phase-III clinical trials in patients with RA and arthritis28.
There was a disproportionate number of adverse reactions associated with rituximab vs other DMARDsb: almost 60% of the ocular disorders were conjunctivitis, which could be because rituximab can produce hypogammaglobulinemia29 and thus increase the risk of infection. In any case, conjunctivitis is described in the SPC as a frequent disorder (between > 1/100 and < 1/1030. The SPC also lists visual disturbances and tearing disorders among the other reactions observed, whereas corneal ulcer, subconjunctival haemorrhage, and styes have not been previously described.
Cardiac disorders were observed as early as the time of the clinical trial that identified the indications (mainly in patients with pre-existing heart disease and/or cardiotoxicity). Subsequently, several studies published cases of transient hypotension or hypertension31, arrhythmias, heart failure, and myocardial infarction.
In our study, abatacept was the drug with the fewest recorded reactions. Articles and systematic reviews that have analysed its toxicity profile have mainly focused on its low risk of infections, which is similar to that of other non-NF-a inhibitors32, or, compared to tocilizumab, its lower incidence of admissions for serious infections, such as sepsis, bacteraemia, pneumonia, or diverticulitis33.
Regarding blood and lymphatic system disorders, Espinoza et a/.34 found abatacept-associated neutropenia in 3.8% of patients. This value was much lower than that obtained for tocilizumab (18.6%), but higher than that obtained for infliximab (2.8%). It should be noted that most of the patients who presented with this reaction had previously presented with neutropenia while under other treatments, especially with methotrexate.
The most frequently observed haematologic reaction in our cohort (9/12) was anaemia; however, it is difficult to establish whether the origin of this reaction was due to the treatment or if it was secondary to the disease itself. Tocilizumab had a similar profile to that of abatacept, with significantly more blood and lymphatic system reactions. Its SPC lists leukopenia and neutropenia as “frequent” (≥ 1/100 to < 1/10)35.
In general, there was a good level of knowledge on the safety profile of the drugs. Of note, we observed adverse reactions with tocilizumab that are not included in the SPC or scientific publications. These reactions are in line with those reported to EudraVigilance, and so we recommend that its SPC should be modified to include them.
One of the main limitations of our study is that clinical histories were used as the data source. Issues regarding their degree of completeness could involve information bias and should be taken into account. We conducted the analysis of adverse reactions by treatment line rather then by patient, which could also be limitation when comparing our results with those of previous studies. Another possible source of inaccuracy could be the observer review of the records. However, we believe that the strengths of this study include the large detailed data collection used and the long follow-up period.
TNF-α-inhibiting DMARDs were associated with general disorders, infections and immune system disorders, and hepatobiliary abnormalities, whereas non-TNF-α inhibitors were associated with an increase in ocular and cardiac disorders as well as blood and lymphatic system disorders. Treatment withdrawal due to adverse reactions was infrequent and mainly occurred during the first year of treatment. Most of the adverse reactions recorded were known and the largest percentages of the unknown ones were associated with tocilizumab.
FundingNo funding.
Conflict of interestNo conflicts of interests.
Contribution to the scientific literature
Biologic therapies are increasingly used for different diseases, so it is relevant to conduct a retrospective analysis of their safety in real-life conditions. Thus, within the setting of rheumatoid arthritis, with its characteristic chronicity and polymedicated patients, we analysed the toxic profile of biologic drugs in first-line and successive treatments.
We linked the adverse effects of each drug to specific organs and systems, thereby providing potential information for decision-making in clinical settings. Adverse reactions mainly occurred during the first year of treatment, thus reducing their likelihood during prolonged treatment. Only 5% of these adverse reactions were unknown, and so we conclude that their potential toxicity is well documented through clinical trials and national and international registries.
Early Access date (12/11/2021).