To determine and compare the physicochemical and microbiological stability of two 25 lU/mL insulin eye drop formulations made with normal saline and a balanced salt solution, respectively, stored for 120 days under various conditions.
MethodEye drops were compounded in triplicate with 100 IU/mL Actrapid® insulin and either normal saline or a balanced salt solution as vehicles, and they were stored alternatively at room temperature (25 °C), in a refrigerator (2–8 °C) or in a freezer (-20 °C) for 120 days. Insulin concentrations were determined by ultra-high resolution liquid chromatography, and osmolality and pH values were measured at days 0, 3, 7, 15, 30, 60, 90 and 120. Likewise, samples were extracted for microbiological studies on days 0, 30, 60, 90 and 120.
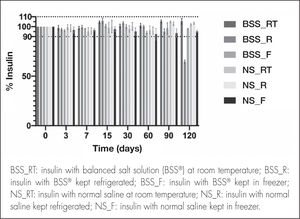
ResultsThe formulation made with normal saline maintained insulin concentrations above 90% of the baseline level after 120 days across all temperature conditions. ln the case of the balanced salt solution-based eye drops, insulin concentration when stored at room temperature or in the freezer remained stable after 120 days, although insulin concentration when stored in the refrigerator fell below 90% on day 90 of the study. Osmolality and pH values remained constant in both formulations and across all storage conditions. No microbiological growth was observed in any of the samples.
Conclusions25 IU/mL insulin eye drops made with normal saline remain stable for 120 days whether they are stored at room temperature, in a refrigerator or in a freezer, provided that they are protected from light. When made with a balanced salt solution, they remain stable for 120 days at room temperature and in a freezer, their shelf life being reduced to 90 days in the case of storage in a refrigerator.
Determinar y comparar la estabilidad físico-química y microbiológica de dos colirios de insulina 25 UI/ml elaborados con suero fisiológico o balanced salt solution bajo diferentes condiciones de conservación durante 120 días.
MétodoLos colirios se elaboraron por triplicado con insulina Actrapid® 100 Ul/ml y balanced salt solution o suero fisiológico como vehículo, y fueron conservados a temperatura ambiente (25 °C), en nevera (2–8 °C) o congelador (–20 °C) durante 120 días. Se determinó la concentración de insulina mediante cromatografía liquida de ultra alta resolución, la osmolalidad y el pH a días 0, 3, 7, 15, 30, 60, 90 y 120. Asimismo, se extrajeron muestras para estudios microbiológicos en los días 0, 15, 30, 60, 90 y 120.
ResultadosLa formulación elaborada con suero fisiológico mantuvo la concentración de insulina por encima del 90% con respecto a la inicial tras 120 días de estudio en todas las condiciones de temperatura. En el caso del colirio elaborado con balanced salt solution, la concentración se mantuvo estable en ambiente y congelador tras 120 días, aunque en nevera descendió por debajo del 90% a día 90 de estudio. Los valores de osmolalidad y pH se mantuvieron constantes en ambas formulaciones y condiciones de conservación. No se observó crecimiento microbiológico en ninguna de las muestras retiradas.
ConclusionesEl colirio de insulina 25 UI/ml elaborado con suero fisiológico es estable 120 días, conservado tanto a temperatura ambiente como en nevera o congelador, protegido de la luz. Con balanced salt solution permanece estable 120 días a temperatura ambiente y congelador, reduciéndose el periodo de validez a 90 días en el caso de la conservación en nevera.
Corneal ulcers, which arise as a result of a rupture or a defect in the corneal epithelium, can cause an underlying inflammation and often give rise to necrosis of the corneal stroma.
The condition is a common cause of ocular morbidity at a world level1. Associated symptoms include irritation, foreign body sensation, conjunctival edema, hyperemia and blurred vision2. Early diagnosis and early initiation of appropriate treatment are essential as lack of treatment may lead to vision loss due to corneal opacity, as well as persistent epithelial defects3.
Persistent epithelial defects are defined as corneal alterations not showing signs of improvement following two weeks of conventional treatment. This absence of epithelialization of the corneal surface may result from multiple causes such as infections, adverse drug reactions, poor epithelial adhesion or trauma4,5. Treatment of this kind of lesion should start with intensive lubrication, the withdrawal of treatments leading to epithelial toxicity, prophylactic antibiotic therapy as well as the use of occlusive bandages and therapeutic contact lenses5–7. In the event of refractoriness, recourse can be made to ophthalmic administration of autologous serum or platelet-rich plasma8.
The last few years have seen an increasing interest in the search of growth factors capable of promoting corneal wound healing, given the presence of receptors for these molecules in the epithelial cells of the cornea9,10. Recent studies have shown the effectiveness of the epidermal growth factor, the nerve growth factor and insulin for treating this kind of lesion due to their epithelial growth promoting properties11.
The use of insulin in corneal ulcers was first proposed by Aynsley in 1945 in a study on the reepithelialization of ulcers refractory to standard treatment12. Topical use of insulin in corneal ulcers is currently reserved mainly to diabetic patients with either postoperative corneal epithelial defects or nonsurgical epithelial defects13,14. In the case of non-diabetic patients, the use of insulin has been described for the treatment of neurotrophic corneal ulcers refractory to conventional treatment and in persistent corneal defects following resection of a neurinoma15,16. As regards the safety of insulin, no side effects or alterations to blood sugar levels, intraocular pressure or the corneal epithelium have been reported as a result of its long-term administration17,18. When no other treatment is available, it is not uncommon to resort to the reformulation of existing medicines to adapt them to an administration route different from the one approved in the drugs’ summary of product caracteristics (SmPC). As there are currently no commercially available insulin-based eye drops in Spain, extemporaneous preparations must be used, based on a fast-acting human insulin solution, intended for subcutaneous or intravenous injection.
Given that no scientific studies have been published to date on the stability of the ophthalmic insulin formulations used to treat persistent epithelial defects, this study can be considered the first of its kind. Its purpose is to determine the stability of two kinds of 25 IU/mL insulin eye drops: one using normal saline (NS) and the other using a balanced salt solution (BSS®) preserved during 120 days in different storage conditions.
MethodsPreparation of the insulin eye dropsA 25 IU/mL insulin-based eye drop preparation was compounded with two different vehicles. The insulin used was Actrapid® [100 IU/mL] (Novo Nordisk®, Bagsværd, Denmark), which contains meta-cresol (m-cresol) as an excipient. The vehicle used was either balanced salt solution BSS® (Alcon Laboratories®, Texas, USA) or NS (Grifols®, Barcelona, Spain).
200 mL of each formulation was compounded by adding 50 mL Actrapid® insulin to 150 mL of BSS® or NS in a 250 mL Vacuflasc®. Three batches of each vehicle were prepared. The solutions were homogenized by shaking them for 30 seconds and later introduced into 5 mL type 1 amber glass vials. The whole process was conducted in aseptic conditions under horizontal laminar flow, the day when the formulations were compounded being considered day 0 of the study.
Conservation conditionsConservation conditions were as follows: room temperature (25 °C), refrigeration (2 °C to 8 °C) or frozen storage (–20 °C). To ensure that temperature remained constant throughout the study, vials stored at room temperature were kept in an ICH L climate chamber (Memmert GmbH + Co®, Schwabach, Germany), which maintains stable temperature (25 °C) and humidity (60%) conditions. Moreover, the fridge and the freezer were equipped with a Siemens® temperature sensor. All vials were protected from light.
Physicochemical characterizationInsulin determination and quantificationDetermination and quantification of insulin were carried out by reverse phase ultra-high performance liquid chromatography (UHPLC) using an ACQUITY UPLC H Class Plus® (Waters) with a photo-diode array (PDA) detector at days 0, 3, 7, 15, 30, 60, 90 and 120. The analytical method used was validated for linearity, accuracy, precision and detection and quantification limits. A linear calibration curve was obtained for both diluents over a concentration range of 0.3–10.0 IU/mL (R2 = 0.999). The detection and quantification limits were 0.15 IU/mL and 0.30 IU/mL, respectively, for both diluents. An analysis was also made to ensure that the method met standard accuracy and precision criteria. Compliance with the analytical validation standards of the European Medicines Agency was also ascertained19.
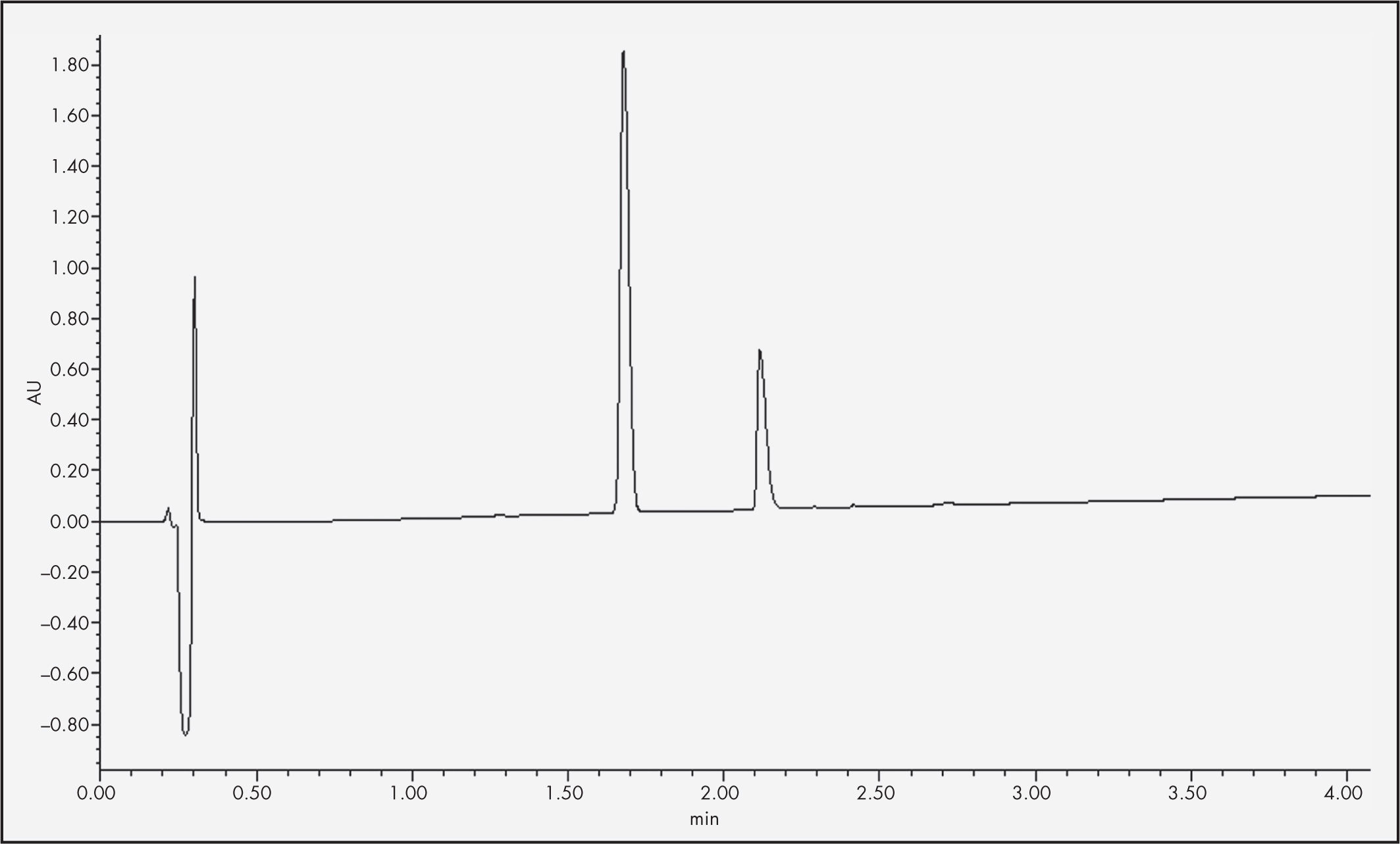
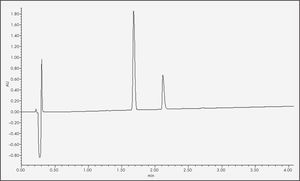
An ACQUITY UHPLC BEH C18 column (2.1 x 50.0 mm, 1.7 µrn) was used at a temperature of 35 °C. The temperature of the sample was 8 °C, the flow rate 0.5 mL/min and the injected volume of the sample 10 µL. The mobile phase comprised the use of 0.1% formic acid (FA) in water (Milli-Q® UHPLC Systems, Merck Millipore®, Madrid, Spain) and 0.1% FA in acetonitrile (ACN) (VWR Chemicals®, Pennsylvania, USA). The chromatographic method used was a gradient elution, starting with 80% 0.1% FA in water and 20% 0.1% FA in ACN and reaching a 30–70% proportion of the respective components at minute 6. Quantification of the insulin required a 1:10 dilution of the samples and their subsequent filtration by 13 mm low protein adsorption Acrodisc® filters (0.2 µm). The data obtained was processed using Empower® 3 software. Under such conditions, insulin retention time stood at 2.1 minutes at a wavelength of 220 nm (Figure 1).
Osmolality and pH determinationOsmolality was determined using a cryoscopic osmometer (Osmo-Special 1, Astori Tecnica®; Poncarale, Italy) where a 150 µL aliquot of each sample was introduced. pH measurements were carried out with a Basic 20 pHmeter (Crison®, Barcelona, Spain). Both variables were determined at days 0, 3, 7, 15, 30, 60, 90 and 120.
Microbiological stabilityThree mL were removed from each formulation at days 0, 15, 30, 60, 90 and 120. The culture media used to evaluate sterility of the formulations were thioglycolate broth (Merck®, Damrstadt, Germany), Columbia blood agar (Merck®, Damrstadt, Germany) and Sabouraud agar (Merck®, Darmstadt, Germany). Samples were incubated in a stove at 37 °C in anaerobic conditions. The thioglycolate was incubated for 10 days, whereas the blood and Sabouraud agars were incubated for 48 hours. Once stove incubation was completed, the plates with Sabouraud agar were incubated again for 13 days in aerobic conditions.
Variation range and statistical analysisThe shelf-life of formulations was established in accordance with the provisions of the Pharmaceutical Codex20.The active pharmaceutical ingredient was considered stable if the compounded formulation retained 90–110% of the initial concentration21–23. As far as osmolality and pH values were concerned, any variation outside the limits accepted for ophthalmic formulations was considered unacceptable, as was the presence of microbial contamination in the analyzed samples21–24.
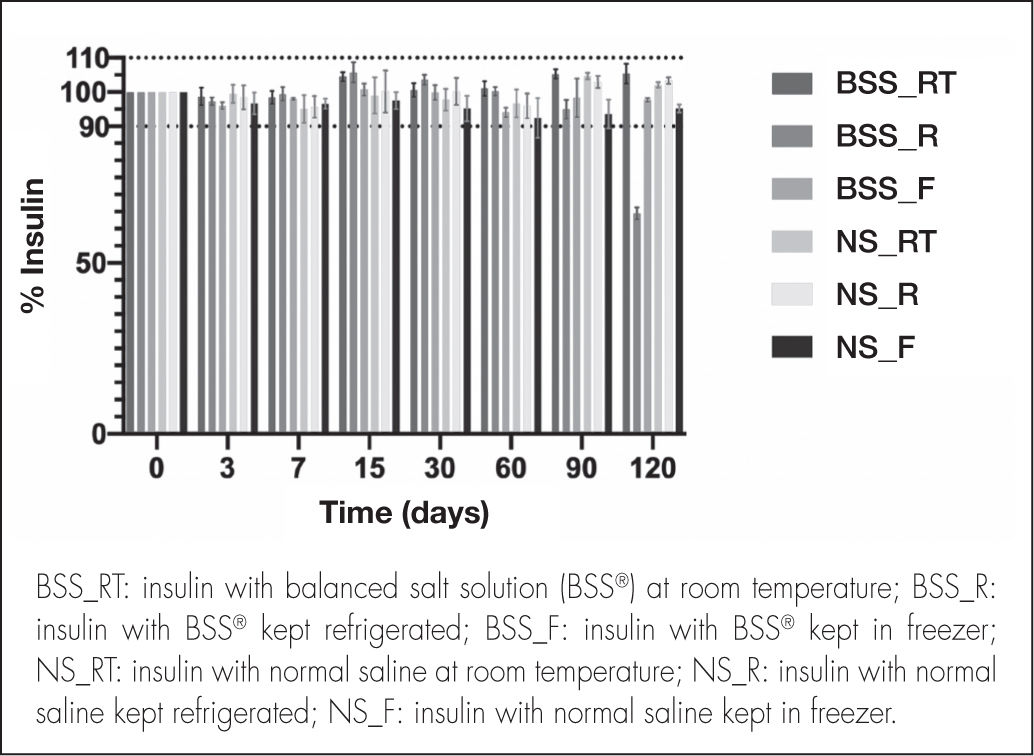
ResultsInsulin quantificationFigure 2 shows the insulin concentration of the NS and BSS® formulations measured at each time point, expressed as percentages of the initial concentration (25 IU/mL).
Although the insulin concentration of the BSS® formulation fell below 90% at 120 days in the refrigerated samples, concentration at 120 days remained within the accepted range in the samples kept at room temperature or in frozen storage. Conversely, the NS formulation showed insulin concentration within the accepted range until the 120th day in all the conservation temperatures analyzed (room temperature, refrigeration and frozen storage), with physicochemical stability being guaranteed for the entire study period.
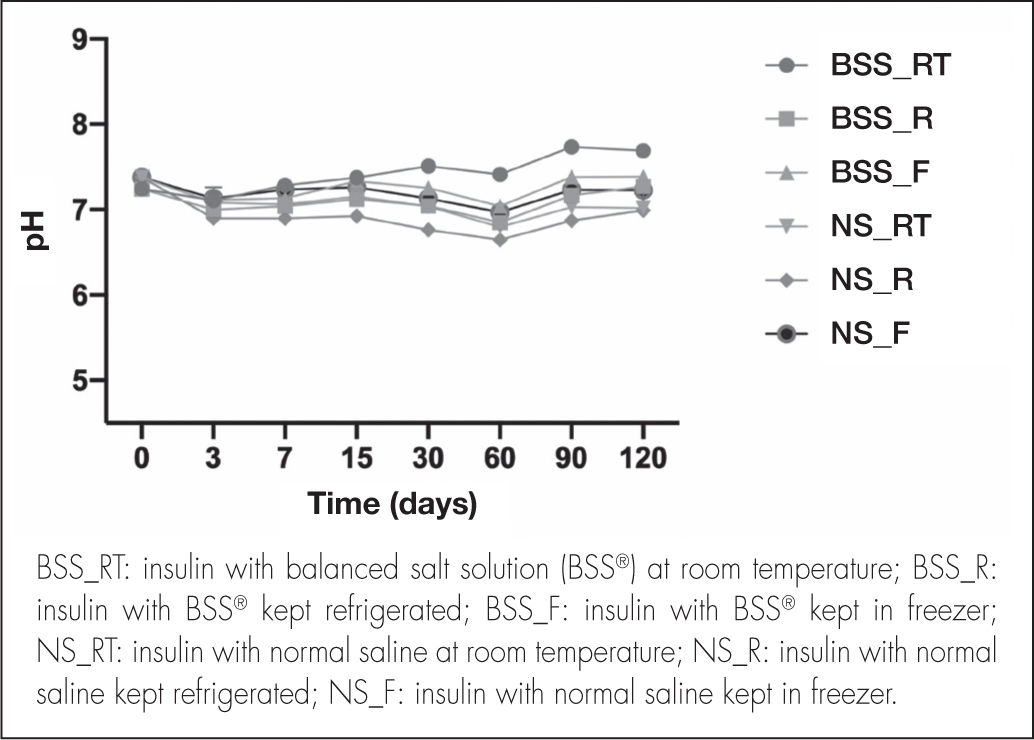
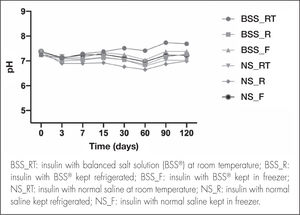
pH and osmolality quantificationpH remained constant, without significant variations, throughout the study (Figure 3). In eye drops elaborated with the BSS® formulation, the mean pH value in the different temperature conditions was 7.21 ± 0.21, whereas the mean pH value obtained for eye drops elaborated with the NS formulation was 7.05 ± 0.20.
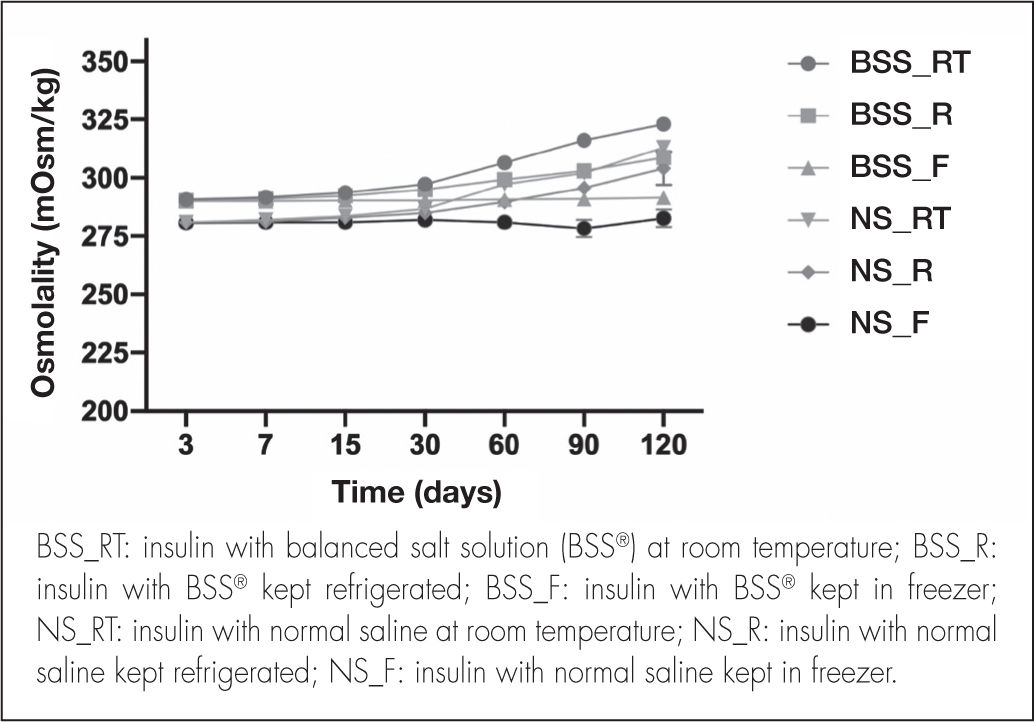
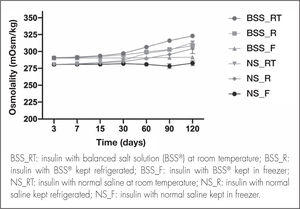
As regards osmolality, no significant variations were detected during the study period (Figure 4). The osmolality of the BSS® formulation was 296.01 ± 9.04 mOsm/kg considering the three storage conditions.
Although the NS formulation showed a similar trend, osmolality values were relatively lower, with a mean value of 288.46 ± 9.02 mOsm/kg.
Microbiological stabilityNo microbiological contamination was observed in any of the samples at the different temperature conditions and time points analyzed. As a result, it can be ascertained that no contaminations occurred during the formulation and conservation process.
DiscussionThe conventional therapeutical arsenal available to address persistent corneal epithelial defects sometimes falls short of the patients’ needs. For that reason, practitioners must resort to therapeutic alternatives such as insulin, endowed with properties capable of promoting epithelial growth. The absence of commercially available insulin-based eye drops makes it necessary to compound them as extemporaneous preparations. To date, several studies have shown the efficacy of this treatment25 although data on physicochemical or microbiological stability remain scarce.
As regards the insulin concentration in the preparation, the literature reports highly variable concentrations, ranging from 1 IU/mL to 50 IU/mL15,25. A decision was made to select the 25 IU/mL concentration for this analysis, based on Fai et al.13. Given that insulin does not feature as an active ingredient in the Spanish register of manufacturers, importers, and distributors of active substances (RUESA)26, we used a fast-acting human insulin solution designed to be injected subcutaneously or intravenously. The requirements to be met by injectable insulin preparations are laid out in in the different national registers of authorized medicines21–23. These preparations must contain between 90% and 110 % of the initial insulin concentration, they must be as isotonic as possible and their pH must be comprised between 6.9 and 7.8. Taking all of this into consideration, the isotonicity, sterility and pH standards associated to ophthalmic formulations can now be met by using this injectable preparation as a basis24. Regarding the osmolality of ophthalmic preparations, although the lacrimal fluid presents with values around 300.5 ± 7.2 mOsm/kg, the human eye can accommodate a wide range of osmotic pressures24, with eye drops of wide ranging osmolarities (260–330 mOsm/kg) being commercially available27. With respect to pH, lacrimal fluid pH values range between 7.4 and 7.7. However, the eye is able to relatively quickly neutralize solutions with wide pH ranges (3.5–10.5). Having said this, the greater the difference between the pH of the administered solution and the physiological pH value of the lacrimal fluid, the longer it will take for neutralization to occur24.
In the formulations considered herein, pH and osmolality were within the established ranges and underwent few changes during the study period. Nonetheless, a slight trend was observed for osmolarity to increase, possibly related to the degradation of insulin. This should be the subject of further analysis.
Another important aspect to consider is the presence of certain excipients. Specifically, 100 IU/mL Actrapid® contains m-cresol as an excipient. M-cresol has been associated with hypersensitivity and irritation reactions, which could cause ocular problems27. This excipient acts as a stabilizer of the insulin molecule in its hexameric form, preventing its aggregation28,29. Although it has been reported that, at a 5% concentration, m-cresol may cause severe ocular toxicity in animals, no ocular damage has been observed with 1% concentrations30. M-cresol is typically used as an antimicrobial preservative at very low concentrations (0.15–0.30%). Specifically, the m-cresol concentration in 100 IU/mL Actrapid® stands at 0.3%, which means that no safety issues are to be expected when this kind of insulin is used to elaborate this compounded formulation28.
This study is not without limitations. Firstly, the stability analysis it limited to the 25 IU/mL concentration, which is only one of the different concentrations reported in the literature. Secondly, the maximum room temperature evaluated was 25 °C, which can easily be exceeded in certain geographical areas. Finally, the degradation mechanism according to which insulin with BSS® is less stable when refrigerated than at room temperature should be subject to further analysis.
The results of the present study demonstrate for the first time the stability of two 25 IU/mL insulin eye drops formulated with NS and BSS®. Both kinds of eye drops remain stable for 120 days across all conservation conditions, with the exception of the one prepared with BSS® when stored in a fridge, which remains stable for 90 days.
FundingNo funding.
AcknowledgmentsAna Castro Balado, Anxo Fernández-Ferreiro and Cristina Mondelo-García are grateful to the Carlos III Health Institute for financing their personnel contracts: CM21/00114, JR18/00014 and JR20/00026.
Conflict of interestNo conflict of interest
Contribution to the scientific literatureThis is the first study to evaluate the physicochemical and microbiological stability of an insulin-based extemporaneous ophthalmic preparation. It therefore constitutes an important turning point, paving the way for this preparation to be compounded by pharmacy departments, ensuring its stability. It would be desirable for this product to be used to treat persistent epithelial defects in routine clinical practice.
Early Access date (10/25/2022).