Heart failure is a prevalent syndrome with high mortality rates, representing a significant economic burden in terms of healthcare. The lack of systematic information about the treatment and adherence of patients with heart failure limits the understanding of these aspects and potentially the improvement of clinical outcomes.
ObjectiveTo describe the clinical characteristics, therapeutic management, adherence, persistence, and clinical results, as well as the association between these variables, in a cohort of patients with heart failure in Andalusia.
DesignThis study will be an observational, population-based, retrospective cohort study. Data of patients discharged from an Andalusian hospital with a diagnosis of heart failure between 2014 and 2023 will be extracted from the Andalusian population health database.
AnalysisThe statistical analysis will incorporate the following strategies: (1) Descriptive analysis of the characteristics of the population cohort, adherence measures, and clinical outcomes. (2) Bivariate analyses to study the association of covariates with adherence, persistence, and clinical results. (3) Multivariate logistic regression and Cox regression analysis including relevant covariates. (4) To evaluate changes over time, multivariate Poisson regression models will be used.
By conducting this comprehensive study, we aim to gain valuable insights into the clinical characteristics, treatment management, and adherence of heart failure patients in Andalusia, as well as to identify factors that may influence clinical outcomes. These findings could be critical both for the development of optimised strategies that improve medical care and quality of life of patients and for mitigating the health burden of HF in the region.
La insuficiencia cardíaca es un síndrome prevalente y con alta mortalidad, que representa una carga económica significativa en términos de atención sanitaria. Hasta el momento, no se disponen de estudios con una metodología robusta que aporten información sobre los patrones de tratamiento, la adherencia y persistencia de los pacientes reales con insuficiencia cardíaca.
ObjetivoDescribir las características clínicas, manejo terapéutico, adherencia, persistencia y resultados clínicos, así como la asociación entre estas variables, en una cohorte de pacientes con insuficiencia cardíaca en la comunidad autónoma de Andalucía.
DiseñoEstudio observacional, poblacional, retrospectivo y de cohortes. Serán incluidos los pacientes dados de alta en un hospital andaluz con diagnóstico de insuficiencia cardíaca entre 2014 y 2023. Dichos datos serán extraídos de la Base Poblacional de Salud de Andalucía.
AnálisisEl análisis estadístico incorporará las siguientes estrategias: 1) Análisis descriptivo de las características de la cohorte poblacional, las medidas de adherencia y los resultados clínicos. 2) Análisis bivariantes para estudiar la asociación de las covariables con la adherencia, persistencia y resultados clínicos. 3) Análisis de regresión logística multivariante y regresión de Cox incluyendo las covariables relevantes. 4) Modelos regresión de Poisson multivariante para la evaluación de cambios en el tiempo.
La realización de este estudio permitirá obtener información relevante para el manejo de la insuficiencia cardiaca en Andalucía, así como identificar los factores que pueden influenciar en los resultados clínicos. Estos hallazgos podrían ser fundamentales tanto para el desarrollo de estrategias optimizadas que mejoren la atención médica y la calidad de vida de los pacientes, como para la mitigación de la carga sanitaria de la insuficiencia cardíaca en la región.
Heart failure (HF) is a complex syndrome resulting from impaired cardiac function. The origin of this functional impairment may be multifactorial and of diverse aetiology.1,2 It is a highly prevalent syndrome with high mortality3 and is one of the most frequent causes of hospitalisation in Europe.4 There are national studies available on hospital admissions and medical emergency episodes.5–10 Studies conducted in Spain have shown an increase in the number of hospitalisations and readmissions for HF, whereas in-hospital mortality has become stable.5–7
In addition to the choice of pharmacological treatment, adherence to treatment is considered one of the key factors for the clinical benefit of patients.8 However, estimates of non-adherence in HF patients vary from 20% to 50%.9–11 Non-adherence is associated with poorer clinical outcomes.12
Since 2001, the Andalusian Health System has been recording all the information produced during the healthcare process in the Population Health Database (PHDB), making it one of the largest repositories of clinical data in the world.13 The aim of this population-based study will be to describe the clinical characteristics, therapeutic management, adherence, persistence, and clinical outcomes, as well as the association between these variables, in a cohort of patients with HF in the Autonomous Community of Andalusia, using data from the PHDB.
MethodologyDesignObservational, population-based, retrospective, cohort study.
PopulationThe population will consist of all patients hospitalised for the first time with a diagnosis of HF in public hospitals in the Autonomous Community of Andalusia between 2014 and 2023.
Selection criteriaInclusion criteria: patients must meet at least one of the inclusion criteria and none of the exclusion criteria in the year prior to the date of discharge from the index hospitalisation (index date, Table 1).
Operational definitions.
| Parameter | Definition |
|---|---|
| Index hospitalisation | Hospitalisation for which includes the diagnosis of HF for the first time |
| Index date | Date of discharge from index hospitalisation |
| End of follow-up period | When the patient is censored or the end of study date is reached. The follow-up period will be limited to 1 year for management and adherence analyses. |
| Censure | Patients will be censored if they move to another autonomous community during the follow-up period or die during the study. In addition, periods during which the patient was hospitalised or if there are no prescriptions for the medicines of interest will be censored. Sensitivity analyses will be conducted without censoring time periods. |
| Initiation of treatment | Prescription of a drug with no previous prescription of the drug in the previous 90 days and no dispensing of the drug in the year prior to the index date |
| Interruption of treatment | Interruption in prescribing for a period longer than 90 days |
| Dose | The dose received by the patient will be defined as the dose at the prescribed dosage |
| Modification of dosage | Changes in the prescribed dosage |
| Addition (combination treatment) | Prescribed treatments for which there is an overlap in time of ≥30 days |
| Changes of treatment (switch) | Prescription of a medicine within a 30-day window both before or after the date of discontinuation of another HF medicine. Will not count as a treatment interruption.When a switch occurs, it will be assumed that the change is immediate, without counting previous dispensing instances in the adherence calculation. |
| Pharmacological treatment | Within 4 cut-off points, prescriptions up to day +30, +60, +180, and +365 after the index date |
| Beginning of follow-up period for estimation of adherence and persistence | Prescription date within 1 month of index date |
| Primary adherence | No. of first prescriptions not withdrawn⁎100/treatments initiated |
| Secondary adherence | Days covered by medication = days dispensed⁎100/days prescribed |
| Persistence | No. of treatment continuations at days 60–90-180⁎100/treatments initiated.There is no break in treatment dispensing lasting ≥30 days within specific time windows (60, 90, and 180 days).Alternatively, the time in which the patient has been persistent (without a break in dispensing ≥30 days) will be calculated. |
| Previous treatment (presupply) | Medication used for the treatment of HF that was prescribed and dispensed prior to the index date. A 30-day presupply will be allowed and counted in the adherence calculation. |
| Stockpiling of medication | Up to a maximum of 90 days. Extra dispensing instances will be made available in the database for use in adherence estimation. |
| Multiple primary adherence | At least one initial dispensing of all prescribed HF treatments occurred during the follow-up period. |
| Multiple secondary adherence | Adherence will be estimated simultaneously for all HF medications, setting 2 thresholds: 67% and 100% |
| Concurrent persistence | Continuation occurred for all HF medicines, at the same established time thresholds. In addition, 2 thresholds are defined to consider adequate multiple persistence: 67% and 100%. |
| Minimum number of dispensing instances for calculating adherence and persistence | No minimum number of dispensing instances will be included |
| Adherence over time | Secondary adherence will be estimated for each month prescribed/dispensed to examine its evolution over time. |
Abbreviation: HF, heart failure.
Patients with a first hospitalisation having an International Classification of Diseases (ICD) code corresponding to HF (table S1) between 2014 and 2023. Patients with an ICD secondary diagnosis code of HF will also be included if the primary diagnosis code indicates ischaemic heart disease (table S1).
Exclusion criteriaPatients younger than 18 years of age. Patients surviving <30 days after index hospitalisation. Cases with a HF code registered in the PHDB in the year prior to the first hospitalisation (table S1). Patients not covered by the Andalusian Public Health System (APHS).
Operational definitionsTable 1 shows the operational definitions that will be used in this study.
The 9th and 10th editions of the ICD will be used to record the diagnostic and procedural covariates.14,15
VariablesDescriptive variablesDemographic and clinical variables: demographic, behavioural, affiliation, and clinical data: sex; smoker; assigned centre; description of assigned primary care area; date of discharge from the APHS user database (UDB); date of discharge from the UDB; code/description of discharge type; date of death; functional status according to the New York Heart Association Functional Class16; ejection fraction; chronic complex; vulnerability measured on the Barthel17 and Pfeiffer18 scales; multi-pathological, polymedication, and comorbidities (table S2).
Organisational variables: health centre; primary care area; health district and hospital. Specialised outpatient consultations: reason for consultation; diagnoses and procedures associated with the consultation, and care unit.
HF treatment prescription (table S3) and dispensing, and dispensing data for other drugs of interest (table S4): therapeutic group; active ingredient; route of administration; start and end dates; duration; number of units; and time between doses.
Concomitant treatment: number of different active ingredients dispensed to the patient per month.
Clinical analysis variables: date of analysis; NT-proBNP; natraemia; kalaemia; haemoglobin; glomerular sedimentation rate; sideremia; transferrin; ferritin; reticulocytes; transferrin saturation index and creatinine index.
Hospital emergencies: date of emergency episodes; reason for the emergency; diagnoses and procedures associated with the emergency; service attending the emergency; and type of discharge.
Hospital admission: date of hospitalisation; major outpatient surgery indicator; description of the circumstances of admission; type of discharge according to PHDB coding; main diagnosis code; codes for the rest of the diagnoses and procedures performed.
Exposure factorsThe various measures of primary, secondary, and persistent adherence to specific HF medications will be considered as exposure factors. Cohorts of patients will be formed based on the presence of each of these factors.
Clinical outcome variablesMain outcome variables: hospital emergencies, hospital admissions, death. Total all-cause events will be analysed.
Secondary: hospital admissions and HF as primary cause.
Data sourceStudy data will be obtained from the PHDB through a request to the Technical Advisory Subdirectorate for Information of the Andalusian Health System. The request will be evaluated to determine whether the variables requested and the relevance of the potential results of the study to the health system are justified, given the potential impact on data protection under current regulations. The data requested by the study will be sent to the pre-approved study investigators for analysis using non-identifiable codes.
Sample sizeAs this is a population-based study, no sampling will be conducted; all patients in the population who meet the selection criteria will be included. Consequently, no sample size calculation will be performed.
Statistical analysisThe statistical analysis will involve the following steps:
- 1.
Descriptive analysis of the characteristics of the population cohort and adherence measures:
- a)
Clinical characteristics of the patients.
- b)
HF treatment patterns: the study will analyse the evolution of patients' treatment during the follow-up period at the proposed cut-offs (Table 1) for the prespecified drugs (table S3). Patterns of treatment modification, frequency of changes, and timing of changes will be analysed (Table 1).
- c)
Adherence measures: primary adherence, secondary adherence, and persistence to treatment will be estimated according to the specifications (Table 1) for the prespecified drugs (table S3) with the exception of diuretics (furosemide, torasemide, hydrochlorothiazide, and acetazolamide) due to the anticipated low precision in adherence estimation resulting from their on-demand use. In the case of secondary adherence, the analysis will be conducted in duplicate: (1) analysis as a dichotomous variable using a threshold of 80%; (2) continuous monthly adherence.
- d)
Multiple adherence and persistence: secondary adherence and persistence will be estimated simultaneously for all prescribed HF treatments; the methodology used to estimate multiple adherence is described in detail in Fig. S1.
- e)
Clinical outcomes: hospital emergency medical episodes, hospitalisations, and deaths will be investigated. All events occurring after the index date will be taken into account; for the main analyses, patients will be censored once the first event occurs, and secondary analyses of subsequent medical emergencies may be conducted.
- 2.
Association analysis: the study will analyse bivariate associations between the different covariates: patient characteristics (socio-demographic and clinical), medications, healthcare professionals and organisation, treatment patterns, primary adherence, and secondary adherence. Corresponding bivariate analysis of clinical outcomes will also be conducted. Multivariate logistic regression analyses will then be used to assess potential independent associations between the covariates of interest and treatment patterns, primary adherence, and secondary adherence. The predictions from these analyses will also be used to construct propensity scores to be included as explanatory variables in the analyses of clinical outcomes. The association between the various covariates and persistence will be studied similarly using Kaplan–Meier models (bivariate analysis) and Cox proportional hazards models. The predictions from the multivariate model will be used to construct the corresponding propensity score. In the case of adherence patterns or trajectories, ordinal regression models will be used to identify patient characteristics associated with each trajectory.
- 3.
Secondary analyses: see Supplement 5.
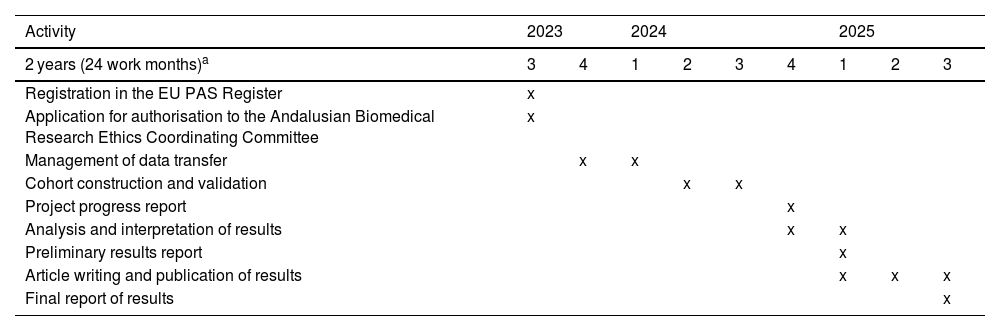
Table 2 presents the planned schedule for the execution of the project, which is expected to last 24 months, starting from the authorisation granted by the Andalusian Biomedical Research Ethics Coordinating Committee (CCEIBA).
Timeline of activities.
| Activity | 2023 | 2024 | 2025 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 years (24 work months)a | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 |
| Registration in the EU PAS Register | x | ||||||||
| Application for authorisation to the Andalusian Biomedical Research Ethics Coordinating Committee | x | ||||||||
| Management of data transfer | x | x | |||||||
| Cohort construction and validation | x | x | |||||||
| Project progress report | x | ||||||||
| Analysis and interpretation of results | x | x | |||||||
| Preliminary results report | x | ||||||||
| Article writing and publication of results | x | x | x | ||||||
| Final report of results | x | ||||||||
Abbreviation: EU PAS, European Union Post-Authorisation Studies Register.
The present study will identify and analyse a cohort of patients with a first HF hospitalisation in an autonomous community of 8.5 million inhabitants.19 A study that uses data from a large cohort of real patients to describe the management of HF treatment, accurately estimate treatment adherence, and identify the factors associated with clinical outcomes and adherence itself could be of great relevance to clinical practice for the following reasons:
Greater representativeness: population-based studies allow a larger and more representative sample of HF patients to be analysed than studies conducted in specific hospital centres and patient registries, which may be subject to selection bias. This will ensure better generalisability of the results and enable the study of HF patients who are typically excluded from pivotal clinical trials due to factors such as the presence of comorbidities, impaired functional capacity, or other issues.
Assessment of treatment in real-world conditions: this type of study provides information on treatment and adherence in routine clinical practice. This is essential to understand how evidence is interpreted and how clinical practice guidelines are applied in our setting. This analysis will facilitate the identification of areas for improvement in treatment, patient follow-up, and the identification of treatment patterns, adherence, and clinical outcomes.
Identification of factors associated with adherence and persistence: population-based studies, such as the one proposed, can help to identify and characterise non-adherent patients and identify factors associated with low adherence and lack of persistence to treatment. This information is relevant for developing intervention strategies aimed at improving medication use in these patients.
Accurate measurement of adherence: the proposed study, due to its robust methodology, can address the methodological limitations of previous studies by providing accurate estimates of parameters such as primary adherence, secondary adherence, and persistence. Furthermore, it will also provide additional insights, such as variations in adherence over time and adherence to the set of drugs used to treat HF, rather than focussing solely on individual drugs. This aspect is key to understanding the combined effect of treatments, validating the importance of adherence in clinical practice, and estimating the desirable level of adherence in patients with HF and its impact on clinical outcomes.
LimitationsIn terms of conducting this study, it is relevant to highlight the following identified limitations: firstly, the data obtained from the PHDB does not include information on the functional class and ejection fraction of the patients. In addition, drug prescriptions do not include information on the reasons for prescribing. Therefore, it will be assumed that prescriptions used in the treatment of HF are applied to this indication after the index date, which corresponds to HF hospitalisation. Secondly, there is a potential risk of reporting bias associated with the lack of recording or differential recording of study variables in the medical record. Thirdly, there is a risk of indication bias because clinical decisions are not made randomly in clinical practice. The choice of treatment will depend on the patient's baseline characteristics, and patients who are more likely to respond to treatment will be selected. To mitigate this problem, propensity scores will be used to adjust models for the propensity to receive treatment.
Ethical responsibilitiesThis study will be conducted in accordance with the current regulatory requirements of Spanish law (Law 14/2007, 3 July), with the approval of the Andalusian Biomedical Research Ethics Coordinating Committee (CCEIBA), code: 1167-N-23. Informed consent will not be obtained, as this is a retrospective study of pseudo-anonymised data, and as such constitutes an exception as authorised by the CCEIBA.
Data will be provided by the authorised health authorities through a pseudo-anonymised database. All study data will be processed in compliance with the current regulations of Organic Law 3/2018, 5 December, on Personal Data Protection and guarantee of digital rights.
FundingThe project is unfunded and there is no remuneration of any kind for the healthcare professionals participating in the study.
CRediT authorship contribution statementHéctor Rodríguez-Ramallo: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Conceptualization. Nerea Báez-Gutiérrez: Writing – review & editing, Validation, Methodology, Conceptualization. Didiana Jaramillo-Ruiz: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Investigation, Conceptualization. Gabriel Sanfélix-Gimeno: Writing – review & editing, Supervision, Methodology, Investigation, Conceptualization. Román Villegas-Portero: Writing – review & editing, Supervision, Methodology, Investigation, Conceptualization. José Luis Jiménez-Murillo: Writing – review & editing, Supervision, Methodology. Carlos Hernández-Quiles: Writing – review & editing, Supervision, Methodology. Bernardo Santos-Ramos: Writing – review & editing, Methodology, Investigation, Conceptualization.
The authors would like to express their sincere thanks to the Chronic-pharma group for their scientific support in the development of the study, and also to the health services research group of the Fundación para la Promoción de la Salud e Investigación Biomédica de la Comunidad Valenciana for their valuable contributions based on their extensive experience in the development of population-based studies.