To update and define indicators for improving the quality of care and Pharmaceutical Care for people living with HIV infection in Spain.
MethodThe present project, which updates the previous version of the 2013 document, was developed in four work phases carried out between January and June 2022.
In phase 1, the organization phase, a working group was created, made up of seven hospital pharmacy specialists with extensive experience in pharmaceutical care and from different SFHs in Spain. In addition, another 34 specialists participated in the evaluation of the indicators through two rounds of online evaluation to generate consensus.
For phase 2, initially, a review of the identified reference literature was carried out with the aim of establishing a basis from which to define a proposal for quality criteria and indicators. Then, a preliminary proposal of criteria was made and revisions were established for their adjustment in several telematic work meetings.
In phase 3, consensus was established based on the Delphi-Rand/UCLA consensus methodology.
In addition, all the indicators classified as appropriate and necessary were grouped according to two levels of monitoring recommendation, so as to guide the hospital pharmacy services in the priority of their measurement: key and advanced.
Finally, in phase 4, the final project document was prepared, along with the corresponding descriptive sheets for each indicator in order to facilitate the measurement and evaluation of the indicators by the hospital pharmacy services.
ResultsFollowing the consensus methodology used, a list of items made up of 79 appropriate and necessary indicators was drawn up to establish a follow-up and monitoring of the quality and activity of Pharmaceutical Care for people living with HIV. Of these, 60 were established as key and 19 advanced.
ConclusionsThe indicators defined and updated, since the previous version of 2013, are intended to be a tool for professionals to guide decision-making and facilitate the measurement and assessment of the most relevant aspects of the quality and pharmaceutical care of people living with HIV.
Actualizar y definir los indicadores para la mejora de la calidad asistencial y la Atención Farmacéutica a las personas que viven con infección por VIH en España.
MétodoEl presente proyecto, que actualiza la versión anterior del documento de 2013, se desarrolló en cuatro fases de trabajo realizadas entre enero y junio de 2022.
En la fase 1, de organización, se creó un grupo de trabajo conformado por siete especialistas en Farmacia Hospitalaria con amplia experiencia en atención farmacéutica y procedentes de distintos servicios del territorio nacional. Adicionalmente otros 34 especialistas, participaron en la valoración de los indicadores a través de dos rondas de evaluación online para generación del consenso.
Para la fase 2, inicialmente, se llevó a cabo una revisión bibliográfica con el objetivo de establecer una base a partir de la cual poder definir una propuesta de criterios de calidad e indicadores. A continuación, se realizó una propuesta preliminar de criterios y se establecieron revisiones para su ajuste en varias reuniones de trabajo telemáticas.
En la fase 3 se estableció el consenso basado en la metodología de consenso Delphi-Rand/UCLA.
Adicionalmente todos los indicadores clasificados como adecuados y necesarios fueron agrupados según dos niveles de recomendación de monitorización, de manera que pueda orientar a los servicios en la prioridad de su medición: claves y avanzados.
Por último, en la fase 4 se elaboró el documento final del proyecto, junto con las fichas descriptivas correspondientes para cada indicador con la finalidad de facilitar su medición y evaluación por parte de los servicios de farmacia hospitalaria.
ResultadosSe obtuvo un listado consensuado de ítems conformado por 79 indicadores adecuados y necesarios que permiten establecer un seguimiento y monitorización de la calidad y actividad de la Atención Farmacéutica a personas que viven con VIH. De los mismos, 60 fueron establecidos como clave y 19 avanzados.
ConclusionesLos indicadores definidos y actualizados, desde la versión anterior de 2013, pretenden ser una herramienta para los profesionales para orientar la toma de decisiones y facilitar la medición y valoración de los aspectos más relevantes de la calidad y Atención Farmacéutica de las personas que viven con infección por VIH.
The continuous updating of scientific knowledge and the evolution of care for people living with HIV infection (PLHIV) has led to a redefinition of the roles of healthcare professionals involved in the management of these patients. Thus, in recent years, improvements in care have been proposed and defined for these patients via guidelines and the measurement of care quality indicators1,2 at the national and international level by different scientific societies and institutions involved in this field.
In 2013, “Indicadores para la calidad asistencial y la atención farmacéutica al paciente HIV+”2 (Indicators for the quality of care and pharmaceutical care for HIV+ patients) was published in Farmacia Hospitalaria. This article defined indicators aimed at improvement in these patients from the perspective of hospital pharmacy in order to harmonize and improve their professional activity.
Since then, the pharmaceutical care (PC) of outpatients in general, and PLHIV in particular, has evolved and gained increasing relevance, the final outcome being its consolidation within hospital pharmacy services (HPS). This type of care is routinely included in the quality assurance programs of specialty care centres and is continuously monitored and evaluated.
However, rapid growth in the number of patients treated due to disease chronification, the scarcity of human and material resources, the difficulty of integration within multidisciplinary teams, and the identification of variability in PC at the national level led the Spanish Society of Hospital Pharmacy (SEFH) to establish a joint initiative to address and provide a collaborative response to all these care challenges. Thus, 2015 saw the emergence of the Mapa Estratégico de Atención al Paciente Externo (MAPEX; Strategic Map for Outpatient Care) project, whose strategic pillars are multidisciplinary patient-cantered care, excellence in knowledge, and the evaluation of results3. It is along these lines that, among other initiatives, a model has been developed for continuous improvements in integrating specialist pharmacists in healthcare teams4, stratification tools5, and telepharmacy documents6.
However, as of 2016, one of the most outstanding elements has been the redefinition of the concept itself and the proposed outpatient PC model based on the pillars of Capacity, Motivation, and Opportunity7 (i.e. the CMO Model) adapted to various diseases and which, specifically in the field of PLHIV, has led to improved results regarding its practical application8–10.
Finally, in line with commitment to ongoing quality of care, in 2019, the SEFH also developed the Q-PEX initiative. This is a proposal for excellent PC management in HPSs that establishes a reference framework for quality improvement and is the first quality seal registered by this scientific society11. This standard establishes the general principles of a management system and the technical requirements that HPSs should fulfill in order to promote best PC practice. Therefore, the definition of quality of care and PC indicators for the care of PLHIV should be aligned with the items established in this standard.
Such progress and development entail the need for a specific adaptation to the care of PLHIV.
The main objective of this study was to define and update indicators for improving the quality of care and PC to PLHIV, with the aim of identifying and promoting improvements in health care by outpatient pharmacy units of hospitals (OPUHs) and to provide a reference framework which should be adapted to the situation of each HPS. The secondary objective was to reach a consensus on the suitability and need for the proposed healthcare and pharmaceutical quality indicators.
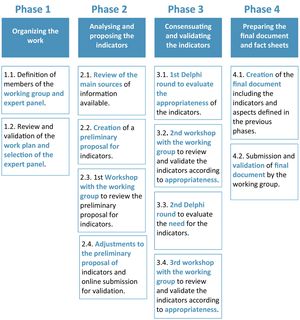
Material and methodThis project was conducted in four phases between January and June 2022 (see Fig. 1).
The first phase involved organizing the work, selecting the experts, and planning (phase 1), followed by analyzing and proposing the indicators (phase 2), consensuating and validating the indicators (phase 3) and, finally, preparing the final document and fact sheets (phase 4).
Phase 1 comprised the creation of a coordinating group consisting of seven HP specialists from different Spanish HPSs with extensive experience in PC to PLHIV.
Initially, this coordinating group reviewed and validated the objectives, scope, expectations, and methodology of the project. The group also participated in the development of the different methodological actions defined (i.e. bibliographic analysis, development of indicator proposals, consensus rounds, review of deliverables, and validation of the final document).
The selected members of the expert panel were 34 HP specialists, all of whom were members of the SEFH's PC-to-PLHIV working group. They were tasked with evaluating the proposed indicators through two rounds of online evaluation with the aim of reaching consensus.
In phase 2, an initial review was conducted of the scientific reference literature relevant to the field of PLHIV that had been disseminated by scientific societies and health institutions, with the aim of establishing a basis from which to define a proposal for quality criteria and indicators by the coordinating group12–14.
A preliminary proposal of criteria was then made and revisions were established for their adjustment in several online workshops. The first activity was the definition of the quality criteria for care and PC to PLHIV based on the analysis of the reference documents identified and aligned with the Q-PEX standard. The proposed criteria were then grouped into dimensions. Additional levels were defined for those with a broader scope.
The proposal was sent online to all members of the coordinating group for review. This was followed by the first online workshop to adjust the preliminary indicators proposed and incorporate possible modifications based on the feedback.
The consensus methodology was established in phase 3. To this end, the indicators defined in the preliminary proposal were determined and validated through the analysis of two rounds of evaluation of an online questionnaire based on the Delphi-Rand/UCLA consensus methodology15.
In the first round, the panelists and members of the coordinating group assessed their appropriateness and, in the second round, the need for the indicator. In both cases, the indicator was scored on an ordinal scale ranging from 1 to 9 points, without taking into consideration any technical difficulties that could be involved in obtaining it through computer systems. In addition to individually scoring appropriateness and need, each panelist freely and anonymously provided comments, clarified opinions on each of the indicators and/or quality criteria, and added proposals or observations for their improvement.
After both rounds were completed, meetings were held to present the results and assess adjustments to the wording of the indicators following the panelists' considerations.
An indicator was considered appropriate if it had the capacity to identify and measure aspects linked to the quality and activity of PC to PLHIV. The appropriateness of the indicators was determined based on the position of the median of the scores awarded and the level of consensus of these scores. Agreement was considered to exist when at least 85% of the panelists awarded scores within the 3-point region (1–3, 4–6, 7–9) in which the median itself fell. Thus, an indicator was considered appropriate when the median was equal to or greater than 7 and there was consensus among the panelists, inappropriate when the median was equal to or less than 3 and there was consensus among the panelists, and lacking in consensus if it did not meet either of the two previous criteria.
After the initial classification according to appropriateness, all the indicators lacking consensus were reviewed based on the score obtained and the comments received. In this way, the indicators that should remain were identified due to their being considered highly appropriate, and the addition of new indicators was proposed based on the information received. Grounded on this review, the list of indicators considered appropriate for measuring the quality of care and PC to PLHIV was created.
Moreover, an indicator was considered necessary if it had the capacity to guide PC-related decision-making. An indicator can be appropriate if it has the capacity to identify and measure aspects linked to PC quality and activity, but unnecessary if these aspects do not improve OPUH decision-making.
Similar to the process followed for identifying appropriateness, after classifying indicators according to their need, the experts and the coordinating group reviewed the indicators that lacked consensus in terms of need in order to determine those that should be included in the final list.
Furthermore, after evaluation by the coordinating group, all the indicators classified as appropriate and necessary were grouped into two levels of monitoring recommendation in order to guide the HPSs in the priority of their measurement.
Key indicators: those whose quality criteria have a direct and relevant impact on decision-making regarding PC to PLHIV, and are considered essential for providing and guaranteeing optimal PC.
Advanced indicators: those that, without being essential, add value to the PC process and are therefore recommended in any OPUHs that can implement and monitor them.
Key indicators were considered to be all those that were both appropriate and necessary with a median need score of between 7 and 9 with a level of agreement of more than 77.78%. The other indicators were classified as advanced. Subsequently, the working group evaluated the classification of each of the indicators such that they correctly reflected the monitoring recommendation.
Finally, in phase 4, the final document and the corresponding fact sheets for each indicator were prepared by the coordinating group to facilitate the measurement and evaluation of the indicators by the HPSs. Each indicator includes a fact sheet describing the following aspects: justification or quality criteria, dimension, formula, monitoring recommendation, explanation of terms, population, type, periodicity, standard, data sources, and comments.
Finally, the document was sent online to the coordinating group for its final validation and, subsequently, to all SEFH members, scientific societies, and patient associations so that they could provide their opinions on the final draft within a period of 15 days prior to its final approval.
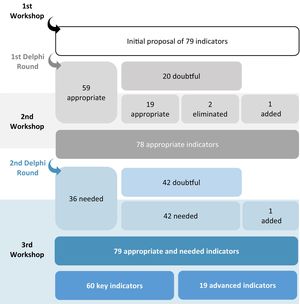
ResultsDuring the first round, 39 completed questionnaires were received out of the 41 sent online. Of the 79 indicators evaluated, 59 were considered appropriate and 20 inappropriate (Fig. 2). During the second workshop, the panelists and the coordinating group evaluated the indicators lacking appropriateness: they identified 19 that should be considered appropriate and eliminated two. Furthermore, during the workshop, another indicator was added based on comments received from the panelists.
In the second round, 33 completed questionnaires were received out of the 41 sent online: of the 78 indicators, 36 were classified as necessary and 42 as lacking appropriateness. Similar to the process followed in the second workshop, the third workshop identified indicators that, although considered doubtful, should be retained because of their ability to guide PC decision-making. The 42 indicators that, according to the working group, lacked appropriateness in terms of need were reclassified as necessary and another indicator was added based on the comments received.
Moreover, during the third workshop, the 79 appropriate and necessary indicators were classified according to the level of monitoring recommendation: 60 indicators were considered key and 19 advanced.
The indicators were finally grouped into five dimensions based on the previously defined quality criteria.
-Leadership and coordination
Four indicators were included whose purpose was to identify aspects related to the leadership and organization of OPUHs in relation to the different levels of health care for PLHIV. Of the four indicators, three were considered key.
-Resource management
Nineteen indicators were included related to the following aspects: human resources (3), infrastructure (2), work tools (11), and communication (3). Their purpose is to enable OPUHs to evaluate the use of all available resources to provide proper PC to PLHIV. Of these, 16 indicators were established as key.
-Pharmaceutical care
The PC indicators are intended to ensure that OPUHs have a record of the patients and the activities conducted in relation to PLHIV. Forty-nine PC-related indicators were identified, distributed into the following dimensions: selection and stratification (6), optimization of medical prescription (5), pharmacotherapeutic follow-up (26), individual perspective (6), dispensing (2), telepharmacy (3), and training (1). Of the total number of indicators, 36 were considered key.
-Research
Four indicators were included that were related to participation in research projects, clinical trials, and publications/communications at congresses. Of the total number of indicators, two were considered key.
-Continuous improvement
Three indicators were included under this heading, aimed at capturing any complaints received, the action procedure in the event of receiving them, as well as the availability of a plan for improvement opportunities. All were considered key.
Table 1 summarizes all the indicators according to the dimensions considered. Table 2 shows all the indicators proposed by dimension LA TABLA 2 debe incorporar la numeración de los indicadores, tal como aparecen en la última reivisión del manuscrito; de otra forma (como se puede ver en el pdf) los indicadores clave, los no clave y los epígrafes se confunden. Además, deben numerarse los epígrafes y subepígrafes.
Proposed indicators by dimension.
| Leadership and coordination |
|---|
| [K] Availability of a person who assumes leadership and commitment regarding adherence to pharmaceutical care |
| [K] Availability of a plan for coordination with other levels of health care |
| Availability of a plan for collaboration with associations of people living with HIV, NGOs, and/or care centres focused on their care and education |
| [K] Participation of specialist pharmacists in multidisciplinary working groups/committees for the protocolization of ART and clinical follow-up |
| Resource management |
| Human Resources |
| [K] Pharmacists available to conduct the pharmaceutical care process |
| [K] Individual continuing education related to the care of people living with HIV |
| [K] Availability of a training plan for resident pharmacists that includes rotation in Infectious Diseases Services |
| Infrastructure |
| [K] Availability of a care hours timetable adapted to needs |
| [K] Availability of pharmaceutical care consultation |
| Working tools |
| [K] Availability of an appointment scheduling system integrated in the Hospital Information System |
| [K] Availability of computer-assisted prescribing for hospital medication |
| [K] Availability of a system to identify individuals at high risk of failing to reach pharmacotherapeutic objectives |
| [K] Availability of a teleconsultation system for telepharmacy programs |
| [K] Availability of a system for the remote dispensing and informed delivery of medication for telepharmacy programs |
| [K] Availability of a barcode, radiofrequency, or automated dispensing system |
| Availability of a traceability system for dispensed batches |
| Availability of a pre-set reminder system for pharmacists |
| [K] Availability of a system to detect loss to follow-up due to treatment abandonment |
| [K] Availability of a medication stock management and ordering system |
| [K] Availability of a system for periodic expiration-date control |
| Communication |
| [K] Availability of tools to communicate with the different levels of health care |
| [K] Availability and provision of direct communication channels with wide coverage to people living with HIV |
| Availability of tools for the transmission of information and training to people living with HIV and members of the public |
| Pharmaceutical care |
| Selection and stratification of individuals |
| [K] Application in routine clinical practice of a methodology for selection and stratification based on the SEFH model |
| [K] Availability of a SOP that includes the development of pharmaceutical care based on the SEFH risk stratification model |
| Availability of a software-integrated tool for screening and stratification |
| People living with HIV stratified into priority 1 according to the SEFH stratification model |
| People living with HIV stratified into priority 2 according to the SEFH stratification model |
| Availability of access by the medical team to the stratification results |
| Optimization of medical prescription |
| [K] Prescriptions validated by the pharmacist at the initiatial visit, changes in treatment, and changes in the patient's clinical status |
| [K] Availability of a SOP that includes validation of the pharmacotherapeutic, technical, and administrative aspects of prescriptions |
| [K] Adaptation of preferred ART initiation guidelines to GESIDA guidelines |
| [K] Adaptation of PrEP guidelines to GESIDA guidelines |
| [K] Adaptation of PEP guidelines to the GESIDA guidelines |
| Pharmacotherapeutic follow-up |
| [K] People living with HIV initiating or changing treatment attended by pharmacists with oral and written information |
| [K] People at higher risk according to the stratification model who require and receive pharmacotherapeutic follow-up at least once a quarter |
| [K] People with undetectable viral load (<50 copies/mL) as of week 48 of treatment |
| [K] Naïve persons with undetectable viral load (<50 copies/mL) as of week 48 of treatment |
| [K] People on ART |
| [K] Persons on long-acting ART |
| [K] Persons who have ever received PrEP |
| [K] People with seroconversion who have received PrEP |
| [K] People who have ever received PEP |
| [K] People with a record of concomitant treatment |
| [K] Polymedicated persons |
| Participation in the HIV and AIDS epidemiological surveillance program |
| [K] People with adherence monitoring |
| Persons with adherence monitoring reports in the medical record |
| [K] People with appropriate adherence to ART |
| [K] Loss to follow-up |
| [K] Establishment of individual pharmacotherapeutic targets |
| [K] People with established pharmacotherapeutic targets |
| [K] Individuals with achieved pharmacotherapeutic targets |
| [K] Individuals with adverse drug reactions that have required intervention |
| [K] Individuals with drug–drug interactions requiring intervention |
| [K] Individuals with prescription incidents |
| [K] Pharmaceutical interventions performed |
| [K] Pharmaceutical interventions accepted |
| [K] Persons admitted who have undergone a therapeutic conciliation procedure |
| [K] Persons admitted with therapeutic reconciliation problems requiring intervention |
| The perspective of the individual |
| Completion of standardized PROM questionnaires |
| Completion of standardized quality-of-life questionnaires |
| People living with HIV with undetectable viral load and good health-related quality of life |
| [K] Availability of training for specialist pharmacists on motivational interviewing for routine use |
| Completion of standardized PREM questionnaires |
| Degree of satisfaction on needs and expectations |
| Dispensing |
| [K] Dispensing errors |
| [K] Availability of a SOP that includes aspects related to medication dispensing |
| Telepharmacy |
| [K] Use of the prioritization model for telepharmacy |
| [K] Individuals included in a telepharmacy program for pharmacotherapeutic follow-up |
| [K] Individuals included in a telepharmacy program for informed dispensing and remote drug delivery |
| Training |
| Training programs for people living with HIV |
| Research |
| [K] Participation in research projects |
| People in clinical trials |
| [K] Publications and conference papers |
| High-impact publications and communications in specialized congresses |
| Continuous improvement |
| [K] Availability of a plan including opportunities for improvement and the actions needed to comply with the requirements established for pharmaceutical care |
| [K] Availability of a procedure for action in case of complaints, claims and suggestions |
| [K] Complaints/Claims received |
[K], Key indicators; PEP, Post-exposure prophylaxis; PREMS, Patient-reported experience measures; PrEP, Pre-exposure prophylaxis; PROMS, Patient-reported outcome measures; ART, Active antiretroviral therapy; GESIDA, Grupo de Educación en sida (AIDS Educational Group); SOP, Standard operating procedure.
In line with the final document, it was agreed that the SEFH would make available to all the HPSs a web observatory for monitoring the proposed indicators. The observatory will be available at the following domain: https://observatorio-mapex.sefh.es/vih.
DiscussionThe present study defines and updates the previous indicators2 for improving the quality of care and PC to PLHIV. As tools, they are intended to guide HP specialists, heads of service, and managers in decision-making and facilitate the measurement and assessment of the most relevant aspects of the quality of care and PC to PLHIV. These aspects include coordination of the care team, resource management, monitoring pharmacotherapeutic objectives, research, and evaluation of the care received.
The update also serves to identify the priority lines of action in this setting, which include the promotion of continuous improvement of care to PLHIV and the implementation of an overall model (i.e. CMO) that facilitates standardized decision-making, the standardized recording of information, data mining, and the evaluation and improvement of results.
These indicators are recommended for use as a reference for monitoring and evaluating quality of care in order to guide management and decision-making and to establish a system of continuous improvement. It is also recommended that each HPS adapts the proposed indicators to its particular situation such that it is accurately represented by the indicator. Their measurement and follow-up would require the participation of the healthcare professionals directly and indirectly involved in the provision of PC, as well as the patients themselves, committees, or the patients' associations.
The HPSs should prioritize the monitoring of key indicators, given that adherence to them is considered essential for providing and guaranteeing optimal quality PC. The monitoring of advanced indicators is recommended for those HPSs that have the resources needed to conduct their follow-up.
We recommend, as a support, the use of the fact sheets describing aspects that can facilitate their interpretation and measurement. It would also be advisable to have a checklist adapted to each OPUH to facilitate data collection and the analysis of indicators at the local level.
The orientative standard or desirable level of the indicators has been established based on the literature and the considerations of the working group, with the aim of establishing a frame of reference that guarantees optimal quality of care and PC. Most of the indicators represent a ratio or percentage in order to enable the comparison and monitoring of the results over time. Thus, it is hoped that this approach will promote the implementation of a web observatory and a mechanism for comparisons between the different HPSs. We suggest that measurements are performed once a year, although this approach can be adapted to the needs of each service. Each HPS should define the timetable for the introduction and final implementation of the indicators according to its current situation, although it is recommended that they should be established over a period of between 1 and 3 years. The indicators related to the availability of tools or procedures—mainly those included in the dimension of resource management, and specifically those linked to work tools, communication, and documentation—should not be restricted to PLHIV, but rather their availability should also be used as indicators of the quality of care for all outpatients. Given the continuous updating of scientific evidence concerning the care of these patients, it is recommended that each centre establishes and makes available a continuous improvement plan and defines the corresponding actions for its revision and updating.
The set of criteria and indicators contemplated should be capable of capturing the current and future situation of PC to PLHIV. Therefore, in the same way that this document updates the results of the previous study, it is expected that in the future these indicators will be updated according to new scientific evidence and the situation of the HPSs.
Based on recording indicator data on the web observatory's platform, future lines of research will be able to conduct cross-sectional studies on the situation of HPSs in Spain, observe their evolution over specific periods of time, and identify factors related to adherence to the different blocks of indicators.
In conclusion, updating the proposed indicators on the basis of new developments in PC care for PLHIV allows HPSs to monitor continuous improvement and strategies aimed at improving quality of care.
Authors' statementAll authors contributed to developing the original idea and designing the study. Ramón Morillo-Verdugo was in charge of writing the manuscript, which was reviewed by all the authors, who approved the final version approved for its publication.
FundingThis project was developed using the SEFH's own funds deriving from collaboration agreements with the following companies: ViiV Healthcare, MSD, Gilead, and Janssen.
Contribution to the scientific literatureImproving the quality of care and pharmaceutical care of PLHIV is a priority objective within current healthcare improvement strategies.
The present study defines and updates previous indicators2. As tools, they are intended to guide HP specialists, heads of service, and managers in decision-making and facilitate the measurement and assessment of the most relevant aspects of the quality of care and PC to PLHIV. These aspects include coordination of the care team, resource management, monitoring pharmacotherapeutic objectives, research, and evaluation of the care received.
We would like to thank the working groups on pharmaceutical care for HIV patients of the Spanish Society of Hospital Pharmacy for their support in the creation, development, and dissemination of the project, as well as Ascendo Consulting for providing methodological support to the project.
The complete document is available at: https://www.sefh.es/mapex/documentacion.php
On behalf of the Working Group on Pharmaceutical Care of HIV patients of the Spanish Society of Hospital Pharmacy.
| Name | Hospital or centre |
|---|---|
| Aitziber Illaro Uranga | Hospital Universitario Marqués de Valdecilla, Santander |
| Alicia Lázaro López | Hospital Universitario de Guadalajara |
| Amparo Moreno Villar | Hospital San Juan de la Cruz, Úbeda (Jaén) |
| Ana Cristina Minguez Cabeza | Hospital Universitario de Araba/Txagorritxu, Vitoria |
| Beatriz Proy Vega | Hospital General La Mancha Centro, Alcazar de San Juan (Ciudad Real) |
| Belén López García | Hospital de Sabadell |
| Carlos Seguí Solanes | Hospital General de Granollers |
| Carolina Aguilar Guisado | Hospital El Escorial |
| Elena Cárdaba García | Hospital Universitario Ramon y Cajal, Madrid |
| Encarna Abad Lecha | Hospital Clínico Universitario de Valladolid |
| Esther Vicente Escrig | Hospital General Universitario de Castellón |
| Gabriel Mercadal Orfila | Hospital Mateu Orfila, Menorca |
| Irene Cañamares Orbis | Hospital Universitario Infanta Leonor, Madrid |
| Jara Gallardo Anciano | Hospital San Pedro, Logroño |
| Javier Casas Arrate | Hospital Universitario de Cruces, Bilbao |
| Javier Sánchez-Rubio Ferrández | Hospital Universitario de Getafe |
| Joaquin Plaza Aniorte | Hospital General Universitario Morales Meseguer, Murcia |
| Jorge del Estal Jiménez | Hospital Universitario Parc Tauli, Sabadell |
| José Alberto Peña Pedrosa | Hospital Clínico San Carlos, Madrid |
| Luis Margusino Framiñan | Hospital Universitario de A Coruña |
| Luis Ortega Valín | Complejo Asistencial Universitario de León |
| Manuel Vélez Díaz-Pallarés | Hospital Universitario Ramón y Cajal, Madrid |
| Maria Angeles Andreu Crespo | Hospital Universitari Germans Trias i Pujol, Badalona |
| Maria Carmen Rosado Maria | Hospital Universitario Central de Asturias, Oviedo |
| María de Miguel Gaztelu | Hospital Universitario de Navarra, Pamplona |
| María García Coronel | Hospital Universitario Reina Sofía, Córdoba |
| Maria Jose Huertas Fernández | Hospital Universitario Puerta del Mar, Cádiz |
| Mercedes Manzano García | Hospital de Mérida |
| Noelia Garrido Peña | Hospital Universitario de Móstoles |
| Oihana Mora Atorrasagasti | Hospital Universitario de Galdakao |
| Patricia Sanmartin Fenollera | Hospital Universitario Fundación Alcorcón |
| Pilar Díaz Ruiz | Hospital Universitario Ntra. Sra. de la Candelaria, Santa Cruz de Tenerife |
| Pilar Taberner Bonastre | Hospital Universitario Arnau de Vilanova, Lérida |
| Vera Áreas del Águila | Hospital General Universitario de Ciudad Real |