To describe the authorisations and funding resolutions for new onco-haematological drugs in Spain between 2017 and 2020, as well as the results of their main trials.
MethodsObservational, cross-sectional, descriptive study conducted between October and December 2022. Onco-haematology drugs approved by the European Medicines Agency between 2017 and 2020 were included, according to EFPIA patients W.A.I.T Indicator 2021 Survey. Authorisation information was obtained from the main study of the European Public Assessment Report. Data were collected on medicines, their authorisation and main study, benefit shown, cost, and status and time to reimbursement.
ResultsForty-one new drugs authorised for 49 indications were identified. More than half (58.5%) were targeted therapies, and 61.2% were for the treatment of solid tumours (61.2%). Most had palliative intent (71.4%) and were indicated in relapsed or refractory disease (55.1%). Of the clinical trials, 57.1% were phase III and 63.3% were randomised. The primary endpoint was overall survival in 16.3%, increasing to 25.8% among randomised clinical trials. Regarding licensed drugs based on response rate, the median response rate was 56.4% [IQI 40–66.3]. In those authorised on the basis of surrogate time-to-event endpoints, the median hazard ratio was 0.54 [IQI 0.38–0.57], and among those using overall survival was 0.71 [IQI 0.59–0.77]. Globally, 22.4% had shown benefit in overall survival, with a median gain of 4 months [IQI 3.6–16.7]. One-third (33.3%) of the indications evaluable according to the European Society for Medical Oncology Magnitude of Clinical Benefit Scale showed substantial clinical benefit. Of the indications, 75.5% were funded, half (48.6%; 36.7% of the total) with restrictions. The median time to funding was 19.5 months [IQI 11.4–29.3].
ConclusionsMost main clinical trials of new onco-haematology drugs approved in Spain used surrogate primary endpoint and, at the time of authorisation, few had shown to prolong overall survival. More than a third were uncontrolled clinical trials.
Describir las autorizaciones y resoluciones de financiación de nuevos fármacos onco-hematológicos en España entre 2017 y 2020, así como los resultados de sus ensayos pivotales.
Material y métodosEstudio observacional, descriptivo y de corte transversal llevado a cabo entre octubre y diciembre de 2022. Se incluyeron los medicamentos onco-hematológicos aprobados por la Agencia Europea de Medicamentos entre 2017 y 2020, de acuerdo con EFPIA patients W.A.I.T Indicator 2021 Survey. La información de las autorizaciones fue obtenida del apartado estudio principal del Informe de Evaluación Público Europeo (EPAR, por sus siglas en inglés). Se recogieron datos de los fármacos, su autorización y ensayo clínico pivotal, beneficio mostrado, coste, y situación y tiempo hasta la financiación.
ResultadosSe identificaron 41 nuevos fármacos autorizados para 49 indicaciones. Más de la mitad (58,5%) eran terapias dirigidas, y el 61,2% para el tratamiento de tumores sólidos (61,2%). La mayoría tenían intención paliativa (71,4%) y estaban indicadas en recaída o enfermedad refractaria (55,1%). El 57,1% de los ensayos clínicos eran fase III y el 63,3% aleatorizados. La variable principal fue la supervivencia global en el 16,3%, aumentando al 25,8% entre los aleatorizados. La mediana de tasa de respuesta fue de 56,4% [IQI 40–66,3] para los fármacos autorizados en base a esta variable de eficacia. La mediana de Hazard Ratio en los autorizados en base a variables subrogadas de tiempo hasta evento fue de 0,54 [IQI 0,38-0,57], y entre los que utilizaron supervivencia global fue 0,71 [IQI 0,59-0,77]. El 22,4% de las indicaciones habían mostrado beneficio en supervivencia global, con una mediana de ganancia de 4 meses [IQI 3,6-16,7]. Un tercio (33,3%) de las indicaciones evaluables según European Society for Medical Oncology Magnitude of Clinical Benefit Scale mostraban beneficio clínico sustancial. El 75,5% de las indicaciones estaban financiadas, la mitad (48,6%; 36,7% del total) con restricciones. La mediana de tiempo hasta la financiación fue de 19,5 meses [IQI 11,4-29,3].
ConclusionesLa mayoría de los ensayos clínicos pivotales de nuevos fármacos onco-hematológicos autorizados en España emplearon variables principales subrogadas y, en el momento de la autorización, pocos habían demostrado prolongar la supervivencia. Más de un tercio eran ensayos clínicos no controlados.
Cancer treatment has evolved into precision oncology, where targeted therapies, immunotherapy, and pharmacogenetics are defining a new therapeutic landscape with remarkable advances.1 However, some cancer patients do not have the mutations or markers that would allow them to benefit from the new therapies, while for those who do, the benefits are not always significant.2–4
Another challenge is the rising cost of new drugs, which may be justified by the cost of research, production, and personalisation of treatments, but is not related to the quality of the evidence or the magnitude of the benefit provided.5
Simultaneously, there have been changes in the design of clinical trials (CTs) in oncohaematology, including an increased use of surrogate variables or fewer randomised clinical trials (RCTs), which may result in greater uncertainty in knowing their benefit.6,7
There is a desire to shorten the timeframe for the arrival of satisfactory new therapeutic alternatives. In the case of public health systems, where resources are limited, it is particularly important to make funding resolutions that select interventions with relevant clinical benefit, are efficient, and take into account other socio-economic factors within a reasonable time frame.8–10
The Patients W.A.I.T. (Waiting to Access Innovative Therapies) indicator survey has been produced by IQVIA for the EFPIA since 2004. It provides a detailed breakdown of the proportion of new medicines centrally authorised in the previous 4 years that are available and time to availability for 39 European countries. It excludes new indications for previously authorised drugs, except in the case of rare diseases. Availability is defined as inclusion in the list of medicines funded by the public health system, qualified according to each country's reimbursement process. The EFPIA Patients W.A.I.T. Indicator 2021 Survey,11 with data up to 1 January 2022, includes 41 new oncology drug approvals between 2017 and 2020. In Spain, 25 (61.0%) of these drugs were available, slightly above the European average (24; 58.5%). The time to reimbursement was 469 days, which was below the average (545 days). This indicator does not provide information on the quality of evidence or the magnitude of the clinical benefit of new approvals.
The aim of this study is to describe the authorisations and funding resolutions for new oncohaematological drugs in Spain between 2017 and 2020, as referenced in the abovementioned survey, as well as the design and outcome of their pivotal CTs and the costs associated with the new treatments.
Material and methodsAn observational, descriptive, cross-sectional study was conducted between October and December 2022. The study included new oncohaematology drugs approved by the European Medicines Agency (EMA) between 2017 and 2020, as indicated by the EFPIA Patients W.A.I.T Indicator 2021 Survey.
Variables were collected on the drugs, including the active substance, brand name, mechanism of action, molecule type, technological innovation (defined as a new mechanism of action not overlapping with another previously authorised mechanism for that neoplasm), authorisation details (date and type of authorisation, whether full or conditional), indication specifics (neoplasm, therapeutic setting, target, line of treatment), main trial details (phase of trial, comparator, masking, endpoint, and outcome measures including hazard ratio [HR] and difference in months for time-to-event variables), gain in overall survival (OS), adverse events (AEs; overall incidence and grade ≥3), magnitude of clinical benefit according to the ESMO-MCBS,12 cost, availability, date of the therapeutic positioning report (TPR), and date and funding resolution.
Information about the drugs, their authorisation, and indication was obtained from the EMA website and from the summary of product characteristics sheets. The data and results of the main trial were extracted from the main trial section of the European Public Assessment Report (EPAR), not including subsequent updates of the CTs. If additional data were needed, the TPR and the original publication of the relevant trial were used. The magnitude of clinical benefit according to the ESMO-MCBS was obtained from the published scorecards if they matched the data presented in the main EPAR study. In the event of matching with subsequent updates, the score was assigned by the authors according to the instructions for completion. A score of 4 or 5 (palliative scenario) or A or B (curative scenario) was categorised as indicating substantial clinical benefit. Costs were calculated using the reported laboratory selling prices available in the Nomenclátor and Botplus databases. The TPRs were searched and consulted on the Spanish Agency for Medicines and Health Products (Agencia Española de Medicamentos y Productos Sanitarios [AEMPS]) website. Funding status was extracted using the Search Engine for Information on the Funding Status of Medicines (Spanish acronym: BIFIMED).
For indications for which separate CTs for different populations were conducted, the results of both CTs were collected.13 For authorisations limited to a subgroup of patients, efficacy data were collected for that subgroup. For CTs with an investigator's choice control arm, the most frequently selected therapy of the control arm was recorded. For trials involving multiple doses and indications, only the authorised ones were selected. OS benefit was considered to have been demonstrated if the pre-specified statistical significance had been reached. We also selected cases in which OS had been used for authorisation.14 For more information on this section, see the supplementary material.
Information was collected on all reported AEs and AEs ≥3. If unavailable, information on treatment-related AEs was used. If this information was not reported, data on serious AEs were collected.
This study did not require approval from a pharmaceutical research ethics committee because it used publicly available data.
All data were independently collected by Hilario Martínez-Barros, Jorge Pedreira-Bouzas, and Álvaro Pousada-Fonseca, who pooled the data and discussed differences. If no agreement was reached, it was resolved by Ana Clopés-Estela.
Statistical analysisThe results obtained for the main variables used, their confidence intervals, and the clinical benefit according to the ESMO-MCBS are expressed as medians and interquartile ranges. Non-inferiority CTs were excluded from this analysis.
The difference in AEs was estimated using the t-test for comparisons of means after testing the assumption of homogeneity of variances with Levene's test.
Treatment costs were expressed as monthly costs, using as a reference the dose needed to treat a person of 1.70 m, 70 kg, and 1.81 m2 body surface area for 1 statistical month (30.4375 days) in the first year of treatment. For drugs authorised for use in addition to a basic treatment (add-on), only the price of the new drug was estimated. These calculations included only medicines marketed in Spain.
The time elapsed from the date of marketing authorisation to the publication of the TPR (equated in this analysis with the date of the funding resolution, whether positive or negative) as well as the time from the publication of the TPR to the positive funding resolution (date of registration for reimbursement) were calculated. In the exploratory analysis, these times were compared according to different variables, with a cut-off date of December 31, 2022. For this purpose, survival curves were estimated using the Kaplan–Meier method and COX regression.
All analyses were performed using STATA 17 software. A 2-tailed p-value of <.05 was used as a cut-off for statistical significance.
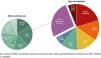
ResultsForty-one authorised drugs were identified for 49 indications: 11 (22.5%) were conditionally authorised, 24 (58.5%) were targeted therapies, 4 (9.8%) were immune checkpoint inhibitors, 4 (9.8%) were antibody-drug conjugates, 3 (7.3%) were chimeric antigen receptor T-cell therapy (CAR-T), 3 (7.3%) were chemotherapeutics, 2 (4.9%) were hormonal therapies, and 1 (2.4%) was an oxidative molecule. Thirty indications (61.2%) were authorised for the treatment of solid tumours (Fig. 1).
Nineteen (38.8%) new indications were categorised as technological novelties. Treatment intent was palliative in 35 cases (71.4%) and 27 (55.1%) were authorised for relapsed or refractory (R/R) conditions after any previous line of treatment.
Of the 30 indications for the treatment of solid tumours, 24 (80%; 49.0% of the total) corresponded to metastatic stages, 3 (10%; 6.1%) to locally advanced stages, 2 (6.7%; 4.1%) to (neo)adjuvant treatments, and 1 (3.3%; 2.0%) to photodynamic therapy for localised low-risk disease. Sixteen (53.3%; 32.7%) were for R/R conditions. Among the indications for haematological malignancies, treatment intention was curative in 11 cases (57.9%; 22.5% of the total) and palliative in 8 cases (42.1%; 16.3%). Eleven (57.9%; 22.4%) were for R/R conditions.
Twenty-eight (57.1%) pivotal CTs were phase III, 20 (40.8%) were phase II, and 1 (2.0%) was phase I. Thirty-one (63.3%) were RCTs. Most of the CTs were open-label (38; 77.6%), even when the analysis was restricted to RCTs (64.5%). The primary endpoint was surrogated in 41 trials (83.7%), the most common surrogates were as follows: response rate (including objective, overall, or complete response rate) in 20 (40.8%); progression-free survival (PFS) in 15 (30.6%); disease-free survival (DFS) in 2 (4.1%); metastasis-free survival (MFS) in 2 (4.1%); and invasive disease-free survival (iDFS) in 1 (2.0%). One drug (2.0%) was approved based on pharmacokinetic equivalence. OS was the primary endpoint in 8 trials (16.3%), increasing to 25.8% in the RCTs. None of the trials used quality of life as a primary endpoint.
The median response rate for authorised drugs based on this efficacy endpoint was 56.4% (IQR 40–66.3). In CTs with time-to-event surrogate endpoints (PFS, DFS, MFS, and iDFS), the median HR was 0.54 (IQR 0.38–0.57) and the range width (95% CI upper bound–95% CI lower bound) was 0.28 (IQR 0.20–0.34). In the 16 (32.7%) indications where the median was reached, the benefit over the control arm was 7.35 months (IQR 5.15–11.35). In trials with OS as the primary endpoint, the median HR was 0.71 (IQR 0.59–0.77) and the median range width was 0.36 (IQR 0.29–0.42). The gain in the 7 (87.5%) indications for which the median had been reached was 3.7 months (IQR 1.5–4.2). A further 3 (6.1%) CTs had OS benefit data as a secondary endpoint, making a total of 11 (22.4%), with a median HR of 0.67 (0.61–0.77). In the 9 (81.8%) indications where the median OS was reached, (7 primary and 2 secondary endpoints), the median benefit was 4 months (IQR 3.6–16.7). In 5 (55.5%), the benefit was ≥4 months.
According to the ESMO-MCBS, 27 indications were evaluable for clinical benefit, with a median score of 3 (IQR 3–4). Nine (33.3%) provided substantial clinical benefit.
The mean difference in total AEs between the experimental group (97.2%) vs the control arm (94.2%) was 3.0% (95% CI 0.1–6.0). The mean difference in AEs ≥3 between the experimental group (63.6%) vs the control arm (52.2%) was 11.4% (95% CI 0.75–22.1). In 21 of 31 comparator RCTs (67.7%), the experimental drug was associated with more AEs ≥3.
The median monthly cost was €6679.1 (IQR 4972.4–8462.7). The median costs were €7848.4 (IQR 6103.1–20 217.7) for those authorised based on response rate, €4481.1 (IQR 3449.6–7242.0) for time-to-event surrogates, and €7800.0 (6506.0–20 110.5) for OS. Forty-seven indications (95.9%) had a TPR, with a median time to publication of 20.3 months (IQR 13.6–25.8). Thirty-seven indications (75.5%) were funded, 18 (48.6%; 36.7% of total) having restrictions, with a median time to reimbursement of 19.5 months (IQR 11.4–29.3). The exploratory analysis showed a trend toward a shorter time to TPR publication, and thus an earlier funding resolution, for drugs that were ultimately funded by the Spanish health system compared to drugs that were not funded (16.3 months and 28.7 months, respectively; HR 1.94 [95% CI 0.95–3.94]) (Fig. 2). A trend was also observed toward a shorter time to reimbursement for drugs with full EMA approval compared to those with conditional approval (see supplementary material), although this trend did not reach statistical significance (HR 1.95 [95% CI 0.80–4.73]).
DiscussionThis study found that only 16.3% of the pivotal CTs for new drug authorisations in oncohaematology used OS as the primary endpoint. At the time of authorisation, 24.5% had been shown to prolong OS, with a median benefit of 4 months over the comparator. According to the ESMO-MCBS, 33.3% provided a clinically significant benefit. Almost half (42.8%) of the pivotal CTs were phase I or phase II. The exploratory analyses showed an earlier funding resolution for drugs funded by the Spanish health service.
The use of OS as a primary endpoint is becoming less common.7,8 The low percentage observed (16.3% for OS and 25.8% in RCTs) is consistent with the percentages reported in previous studies.3,4,15 A study of EMA authorisations of oncohaematology drugs conducted between 2009 and 2013 found that 26.4% of pivotal RCTs used OS as the primary endpoint.15 Nieto et al.3 analysed authorisations from the AEMPS for the treatment of solid tumours between 2010 and 2022, finding that OS was the most commonly used primary endpoint (39.6%). Gloy et al.4 reviewed oncohaematology drugs authorised by the FDA between 2000 and 2020, finding that OS was the most commonly used primary endpoint in 13.7% of trials, increasing to 28.3% in RCTs. Our results are similar to those of Gloy et al.4 but differ slightly from Davis et al.15 and Nieto et al.3 This difference may be due to the fact that the first 2 studies included only first-time authorisations, whereas the second 2 also included subsequent authorisations. Michaeli et al.16 found that first-time authorisations were based on CTs corresponding to earlier phases of research compared to later phases. In the later phases, we would expect a greater number of RCTs and the use of clinical variables rather than surrogates, which would explain these differences. The percentage of RCTs included in our study and in the study by Gloy et al.4 (63.3% and 48.4%, respectively) was lower than in the studies by Davis et al.15 and Nieto et al.3 (90.3% and 83.3%, respectively).
The low use of OS as a primary endpoint is striking as most indications were palliative in intent and often for R/R conditions. In this context, it could be argued that it is necessary to use tools that allow rapid assessment of the effect of new drugs in order to expedite their incorporation into the therapeutic arsenal.17,18 However, the counter-argument could be raised that this makes it all the more important to determine whether the interventions result in the prolongation or improvement of life, for example, using patient-centred outcome measures.17,19 Furthermore, in order to validate surrogate endpoints, several CTs with drugs with a similar mechanism of action in the same therapeutic setting must have been performed previously, an aspect which calls into question any time savings.18 Given the poor prognosis associated with R/R scenarios, it is likely that there would be sufficient events to demonstrate differences in patient-centred variables. Finally, the benefits observed when surrogate variables are used do not always result in improved OS.17,18 This finding may be due to measurement uncertainty,18,20 informative censoring,18,19,21 or the late negative impact of AEs.17,18
The 4-month OS benefit was superior to that observed by Davis et al.15 (2.7 months) and Gloy et al.4 (2.55 months), and similar to the more recent findings of Nieto et al.3 (4.5 months). The median HR among those with OS benefit was 0.67, which is comparable to the 0.75 reported by Gloy et al.4 According to the ESMO-MCBS, clinical benefit was relevant in 33.3%, which contrasts with the approximately 50% reported by Davis et al.15 and Nieto et al.3 These differences may be attributed to the fact that, in this study, scores were given at the time of authorisation, whereas the reference studies had longer follow-up periods, which may have led to increases in the scores. In addition, Davis et al.15 only performed this analysis in those with OS benefit.
Almost half (42.8%) of the authorised indications were based on phase I or II trials, which is higher than the figure found by Nieto et al. (20.8%),3 which also included subsequent authorisations. In phase I studies, compared to phase II, the response rate is often overestimated21 due to selection bias, small sample sizes, or short follow-up period.22
In terms of indications based on RCTs, most of the RCTs (64.5%) were open-label trials, similar to the results of previous studies.3,4 The open-label design may exaggerate the magnitude of the effect of the intervention, irrespective of the type of variable.19,20
Access to new therapies is not a new issue,10 but it is of renewed interest because of the increasing time between the date of authorisation and funding resolution.11 Recently, 2 documents related to this issue were published by the Advisory Committee for the Funding of the Pharmaceutical Provision of the Spanish Health System.23,24 It is challenging to deliver new drugs that provide value to patients in need within a reasonable timeframe. However, it does not seem correct to speak simply of availability or time to availability; instead, we should focus on appropriate resolution, providing it occurs within suitable timeframes.10 To this end, we need to be aware of the uncertainty associated with new authorisations, as well as their high cost.5 This results in significant differences in public coverage of new interventions, even in high-income countries.9 Moreover, there appears to be no association between higher spending and improved cancer mortality rates.25
This study has limitations. By analysing only drugs included in the W.A.I.T. indicator, the sample size limits the ability to draw conclusions and make formal associations, and excludes successive authorisations of multi-indication drugs. Compared to successive approvals, the former receive more accelerated approvals, focus on diseases of lower prevalence, target more advanced therapeutic lines, are based on earlier phases of research, and show greater benefit in life-years gained.16,26
Other factors that may affect the evaluation of new drugs were not analysed. The use of a suboptimal control arm may exaggerate the benefit of the intervention.21 Between 2013 and 2018, 17% of FDA cancer drug authorisations were based on RCTs with controls considered suboptimal.27 In addition, this study did not assess appropriate crossover,21 which may or may not be desired,28 early termination, which tends to exaggerate the observed benefit,29 or strict eligibility criteria, which may limit applicability.30
Only data used by the EMA at the time of authorisation were analysed. However, updates published in the meantime may show increases in the clinical benefit of interventions. Nevertheless, we believe that this would have little impact on the results observed. After a median of 5.4 years, only 3 of 44 indications that initially showed no OS benefit had demonstrated an increase in OS benefit in subsequent updates or trials.15 Another limitation is that the reported price was used to analyse the cost of new drugs.
In conclusion, most pivotal CTs of new oncohaematology drugs used surrogates as the primary endpoint, and few were shown to prolong survival at the time of authorisation. More than one-third were uncontrolled pivotal CTs. These findings raise questions and should be taken into account in the debate on reimbursement and access to new oncohaematology drugs.
Contribution to the scientific literatureThis study describes the characteristics of the new drugs authorised for use in oncohaematology, the design and results of their pivotal CTs, clinical benefit, and reimbursement.
The results of this study will enable a more informed and contextualised debate about the reimbursement process for new oncohaematology drugs.
Ethical responsibilitiesAll authors accept their responsibilities as defined by the International Committee of Medical Journal Editors (Available at: http://www.icmje.org/).
FundingNone declared.
Presentation at ConferencesPresented in Bilbao at the Congress of the Spanish Society of Hospital Pharmacists, 5–7 October, 2023, and in Bordeaux, 20–22 March, 2024, at the European Association of Hospital Pharmacists Congress.
CRediT authorship contribution statementHilario Martínez-Barros: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Investigation, Data curation, Conceptualization. Álvaro Pousada-Fonseca: Writing – review & editing, Methodology, Formal analysis, Data curation, Conceptualization. Jorge Pedreira-Bouzas: Writing – review & editing, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Ana Clopés-Estela: Writing – review & editing, Supervision, Methodology, Conceptualization.