The consensus between the Spanish Society of Retina and Vitreous, the Spanish Society of Hospital Pharmacy and the Spanish Society of Ophthalmology on the preparation, distribution, and administration of intraocular medications is presented, which expands the SEO-SEFH Consensus on recommendations for use and preparation of ophthalmic preparations achieved and published in 2018 in the journal “Farmacia Hospitalaria”.
Initially, each society appointed at least two members to form the working group that defined the objectives of the consensus. After that, they carried out a bibliographic search of both scientific articles and documentation from regulatory agencies and manufacturers to write a first draft by internal consensus. This draft was reviewed and corrected by members other than the three scientific societies, and was sent to the societies involved for their endorsement. It was verified that this version complied with the recommendations of the AGREE II guide.
This consensus includes the review of the requirements that the medical devices used must meet to avoid complications such as the release of silicone drops or other lubricants, as well as a selection of products and suppliers that meet these characteristics, the validity periods for the most common preparations, and the techniques for their preparation, handling and administration.
Se presenta el consenso entre la Sociedad Española de Retina y Vítreo, la Sociedad Española de Farmacia Hospitalaria y la Sociedad Española de Oftalmología sobre la preparación, distribución y administración de medicamentos intraoculares, que amplía el Consenso SEO-SEFH sobre recomendaciones de utilización y elaboración de preparaciones oftálmicas publicado en 2018 en la revista Farmacia Hospitalaria.
Inicialmente, cada sociedad nombró al menos 2 miembros para formar el grupo de trabajo que definió los objetivos del consenso. Tras ello, se realizó una búsqueda bibliográfica tanto de artículos científicos como de documentación de agencias regulatorias y fabricantes para redactar un primer borrador por consenso interno. Dicho borrador fue revisado y corregido por otros miembros distintos de las 3 sociedades científicas y se envió a las sociedades implicadas para su aval. Se verificó que esta versión cumpliese las recomendaciones de la guía AGREE II.
Este consenso incluye la revisión de los requisitos que debe cumplir el material sanitario empleado en la preparación y administración de medicamentos intraoculares para evitar complicaciones, como la liberación de gotas de silicona u otros lubricantes, así como la selección de los productos y proveedores que cumplen estas características, los periodos de validez para las preparaciones más habituales y las técnicas para su preparación, manipulación y administración.
Intraocular injections (IOIs) have become the most commonly performed procedure in the majority of ophthalmology departments.1 Antiangiogenics are currently the most frequently prescribed treatment, although corticosteroids, antibiotics (both for treatment and prophylaxis), antivirals, antifungals, tissue plasminogen activator (r-tPA), or gases—considered medical devices (MDs)—are also used to treat retinal detachment.2,3
As IOIs can cause serious adverse events, such as infectious and noninfectious (inflammatory) endophthalmitis, cataracts, ocular hypertension, vitreous haemorrhage, or retinal detachment,2,4 adequate knowledge of the drugs, medical devices used, and the techniques used in their preparation and administration is essential to optimise outcomes and minimise risks.2,5 Currently, only Lucentis6 (ranibizumab), Eylea7 (aflibercept), and Beovu8 (brolucizumab) and sustained-release corticosteroids (Ozurdex and Iluvien) are available in prefilled syringes, eliminating the need for additional handling. All other IOIs should be prepared in pharmacy services, according to quality criteria that ensure their effectiveness, stability, and sterility.5
Recently, the scientific community has focussed attention on the following aspects:
- •
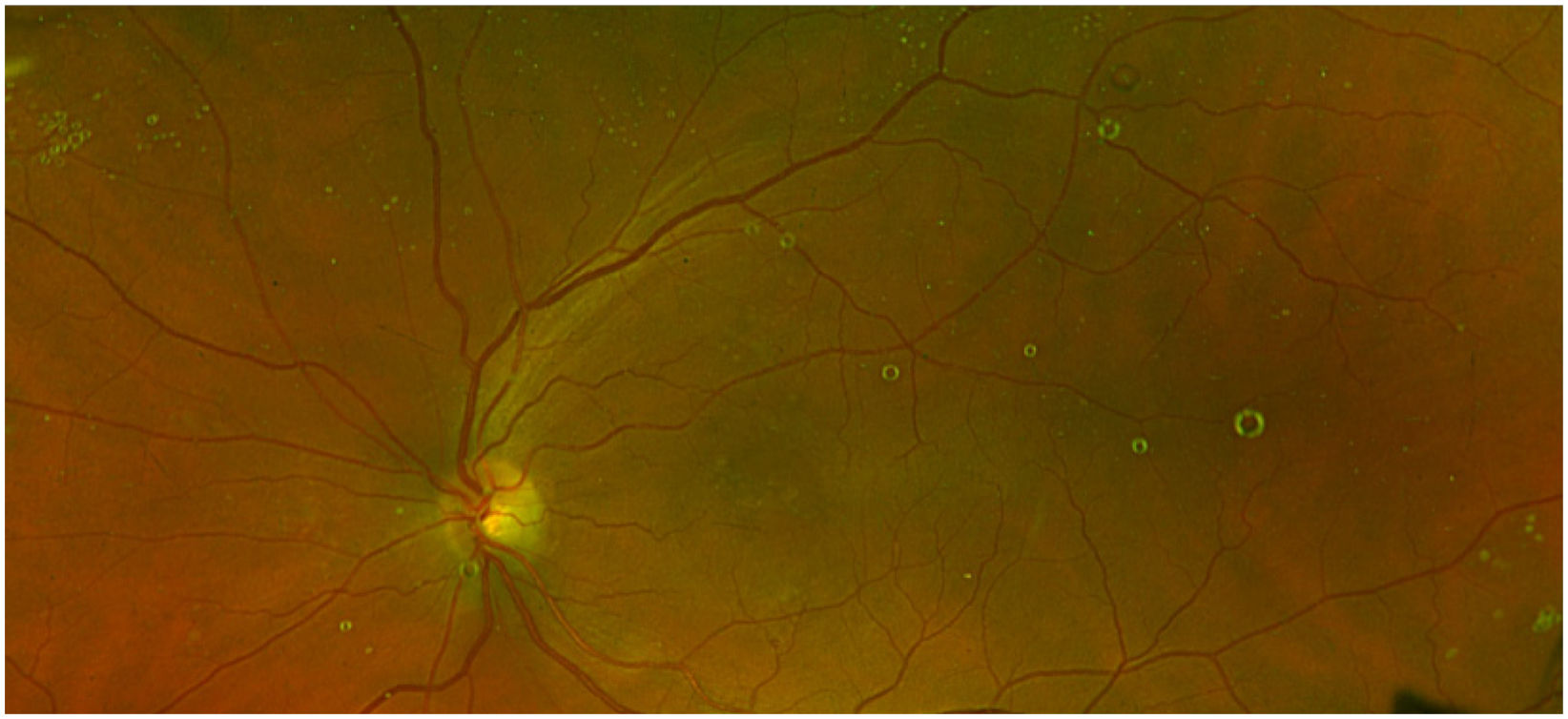
Silicon oil (SO) droplets or other lubricants (Fig. 1) that may be released from syringes and needles.
- •
Formation of protein aggregates due to the handling and storage of drugs in syringes.
- •
The actual volume of drug injected with each type of needle and syringe, due to the presence of dead spaces where the drug may remain trapped, potentially leading to undertreatment.2,9–28
This document presents a consensus between the Spanish Society of Retina and Vitreous (SERV), the Spanish Society of Hospital Pharmacy (SEFH), and the Spanish Society of Ophthalmology (SEO) on IOI. It serves to extend the 2018 SEO-SEFH consensus document.5
The objectives of this document are as follows:
- •
List the various MDs currently available that can be used to prepare, store, and administer different intraocular treatments.
- •
Specify a set of general recommendations for the preparation of IOIs to standardise their preparation and increase the safety of the procedure.
- •
Specify which drugs, and under what conditions, have sufficient pharmaceutical and clinical evidence to support their storage and use other than that described in the Summary of Product Characteristics (SPCs) to cover the most common therapeutic gaps, and thus facilitate the development of care protocols in healthcare centres.
- •
Specify a set of recommendations to minimise the presence of SOs, other lubricants, and protein aggregates in IOIs to reduce the risk of post-administration complications.
- •
Specify a set of basic recommendations on the process of prescribing and administering IOIs.
In accordance with the collaboration agreements between the three societies, a specific working group was formed, consisting of at least two members from each society. In the case of the SEFH, representatives were sought from the Pharmacotechnics Group and the Medical Devices Group.
In the first stage, the group defined the objectives of the consensus. The second stage involved an information search in Pubmed using the following keywords: drug stability, intravitreal injections, intraocular injections, silicone oil, and drug compounding. Articles published after the 2018 consensus document were reviewed, as well as previous articles deemed relevant due to specific aspects of interest. The SPIs of the drugs were consulted on the Spanish Agency for Medicines and Medical Products (AEMPS) website and the instructions for use of the MDs involved were obtained from the corresponding manufacturers.
Based on this information, a first draft of the consensus was created and circulated to the members of the working group for review and further input, taking into account the expertise of each member. The document was reviewed by at least two other evaluators from each of the societies involved to suggest corrections or new preparations. After appropriate corrections and updating with the latest literature, the document was approved by the SERV, SEO, and SEFH within the framework of the collaboration agreements in place.
ResultsIntraocular medical devicesGiven the increasing use of IOIs, it is essential to identify both the ideal specific characteristics of each MD involved and its broader aspects. It should be borne in mind that in recent years, MDs specific for IOI have been introduced to the market, including some intended to facilitate administration or dosing.
General aspects of MDs to be consideredThe current regulations governing MDs in Europe and Spain are Regulation 2017/74529 and Royal Decree 192/2023,30 respectively, which describe the aspects that MDs must comply with in order to be placed on the market, the requirements for conformity assessment and obtaining the EC marking, as well as aspects relating to post-marketing monitoring and the MD surveillance system.
Therefore, the information that can be requested from the supplier is as follows:
- –
EC Declaration of Conformity
- –
EC-marked MD labelling
- –
Confirmation of communication to the AEMPS marketing register
Another key aspect to ensure is that the MD is intended for IOI, or at least is not contraindicated. For this purpose, the Instructions For Use should be consulted. This document contains the information provided by the manufacturer to inform the user of the intended purpose of a device, its correct use, and the precautions to be taken.
Specific aspects concerning needlesISO 7864:2017 specifies the requirements for sterile, single-use hypodermic needles intended for injecting or aspirating fluids. Hypodermic needle size is described by both the outer diameter and the length of the needle tube in millimetres (e.g. 0.8 × 40 mm needle). Typically, the outer diameter is expressed in gauges (G) and the length in inches, separated by an “x” symbol, taking into account that the larger the G, the smaller the gauge and the thinner the needle. The outer diameter of the needle is identified by a colour code (ISO 6009:2016) on the unit packaging and/or on the needle hub or guard.
Optionally, needle wall thickness can be indicated by the abbreviations RW (Regular Wall), TW (Thin Wall), ETW (Extra Thin Wall), or UTW (Ultra Thin Wall). Some needles with the same external diameter may have small differences in the internal diameter that may affect the force required to deliver fluid; this dimension should also be considered when selecting the needle.
The choice of needle size is essential in IOI, as well as in the process of fractionating drugs to be administered via this route. 30G needles (colour coded yellow) or thinner are usually recommended for administration by this route.
The needle tube is usually lubricated, and the lubricant should be one accepted by the Spanish or European Pharmacopoeia, such as polydimethylsiloxane. The amount used should not exceed 0.25 mg/cm2 of lubricated surface.
The needle tip is usually bevelled and should be sharp and free from jagged edges, burrs, or hook-shaped defects that could damage the tissues. There are also blunt needles for the extraction of substances from vials and ampoules, which should be designed to minimise needle luz blockage and stopper fragmentation when the tip penetrates the vial seal.
These needles may have a filter in the cannula to prevent the passage of particles that may have been entrained when penetrating the vial stoppers or when aspirating liquid from glass ampoules.
Another aspect that should be taken into account is compliance with standards on protection against needlestick injuries, provided that the safety systems do not hinder the administration of the substance, taking into account the route of administration considered. When selecting needles without these safety systems, validated procedures should be specified in the health centres to minimise risks to users and/or patients and to comply with relevant regulations.
Specific aspects concerning syringesUNE-EN ISO 7886:2018 specifies the requirements to be met, with the exception of insulin syringes (ISO 8537). These MDs are intended for use with hypodermic needles specified in ISO 7864.31
There are two-piece syringes, consisting of a barrel and a piston or plunger, and three-piece syringes, where the plunger and plunger seal are two separate components made of different materials.
For syringes with a capacity of less than 5 mL, the UNE-EN ISO standards require the coupling hub to be located centrally and coaxially with the barrel. These hubs can be of the Luer or Luer-lock type.
To facilitate plunger movement, syringes usually have lubricants (e.g. SOs and fatty acid amides) that comply with the Spanish or European Pharmacopoeia. When the plunger seal is fully inserted, the amount of lubricant applied to the barrel should not reach the Luer connector area. For syringes with lubricated inner surfaces, the amount of lubricant should not exceed 0.25 mg/cm2 of the inner surface in contact with the injection fluid. For syringes containing lubricants in the polymer mix, the amount of lubricant should not exceed 6% (w/w) of the mass of the polymer component. In general, the amount of silicone in a typical 1-mL syringe is between 100 μg and 800 μg, but can be as high as 1000 μg.
The disadvantage of lubricants is that they can be released and transferred to the contents of the syringe, potentially causing compatibility problems with the drug or being administered to the patient. A clear example already mentioned is that of SO droplets found in patients' eyes after IOI.2,9–23 Dounce et al.21 found similar cases with other types of lubricant (oleamide), although their clinical implications are unknown.
Consequently, systems with less lubricant and a reduced likelihood of transfer have been designed, as well as syringes without lubricant. Lubricant-free cycloolefin syringes are associated with reduced particle release into the syringe and reduced protein aggregation.
Another aspect to consider with intravitreal administration is the dead space or residual volume of the syringe.2,14,17,21,27 As very small volumes and high-cost drugs are used, a larger dead space results in more residue being trapped in the syringe, preventing full administration. ISO 7886:201831 specifies that syringes with a nominal capacity of less than 2 mL should have a maximum residual volume of 0.07 mL. However, it may be of interest to have a smaller dead space volume for greater efficiency in the process. It should be noted that if the syringe has a transferrable lubricant, a larger dead space reduces the risk of lubricant reaching the patient, as it remains trapped in this space.
On the other hand, certain manoeuvres with IOIs have been described that facilitate lubricant release and thus should be avoided (Table 1).
Recommendations to minimise the release of lubricants in intravitreal syringes and the formation of protein aggregates.
|
|
|
|
|
|
|
- •
Volume adjustment devices: Due to the small volume used in the fractionation and preparation of drugs for intravitreal administration, specific MDs have been developed to enable more precise volume adjustment, reducing substance loss and thus optimising costs. An example is the Microliter Dosing Syringe manufactured by Congruence Medical Solutions (USA).32
- •
Aids for syringe filling, such as the Zero Residual Bubble Adapter, have been developed by SJJ Solutions (Netherlands).33 This allows loading from a vial using a filtered needle and subsequent dosing of the extracted volume into syringes without dead space, thus avoiding the presence of air in the syringe.
- •
Administration aids: These MDs aid in positioning the eye for puncture and administration via this route. Some examples are as follows:
- •
Filters: These are used to prevent particle transfer when drawing a volume of medication from a vial or ampoule. The nature of the drug or substance to be filtered and the filter material must be considered to avoid compatibility problems that may affect the preparation. Disc filters are available with different pore sizes and filters built into needles.
Medical devices for the preparation, fractionation, and administration of IOIs should comply with the following general aspects:
- •
Have a CE mark.
- •
Have recognised intravitreal/intraocular use for its intended purpose, or failing that, that such use is not contraindicated in the instructions for use.
- •
There should be no lubricants that could be transferred to the drug and compromise device stability and/or patient safety. If lubricants are present, they should be used in the smallest quantity possible.43
- •
The composition of the MD should be known to ensure compatibility with the medication used.
- •
Dead space should be minimal to reduce unnecessary loss of the drug being administered.
- •
A Luer-lock hub is recommended for the syringe.
- •
Needles should have an appropriate bevel to prevent particles from being generated during the fractionation process from vials or causing harm to the patient.
- •
30G (yellow) or smaller needles should be used for administration, while 18G blunt needles are recommended for drug extraction for preparation.
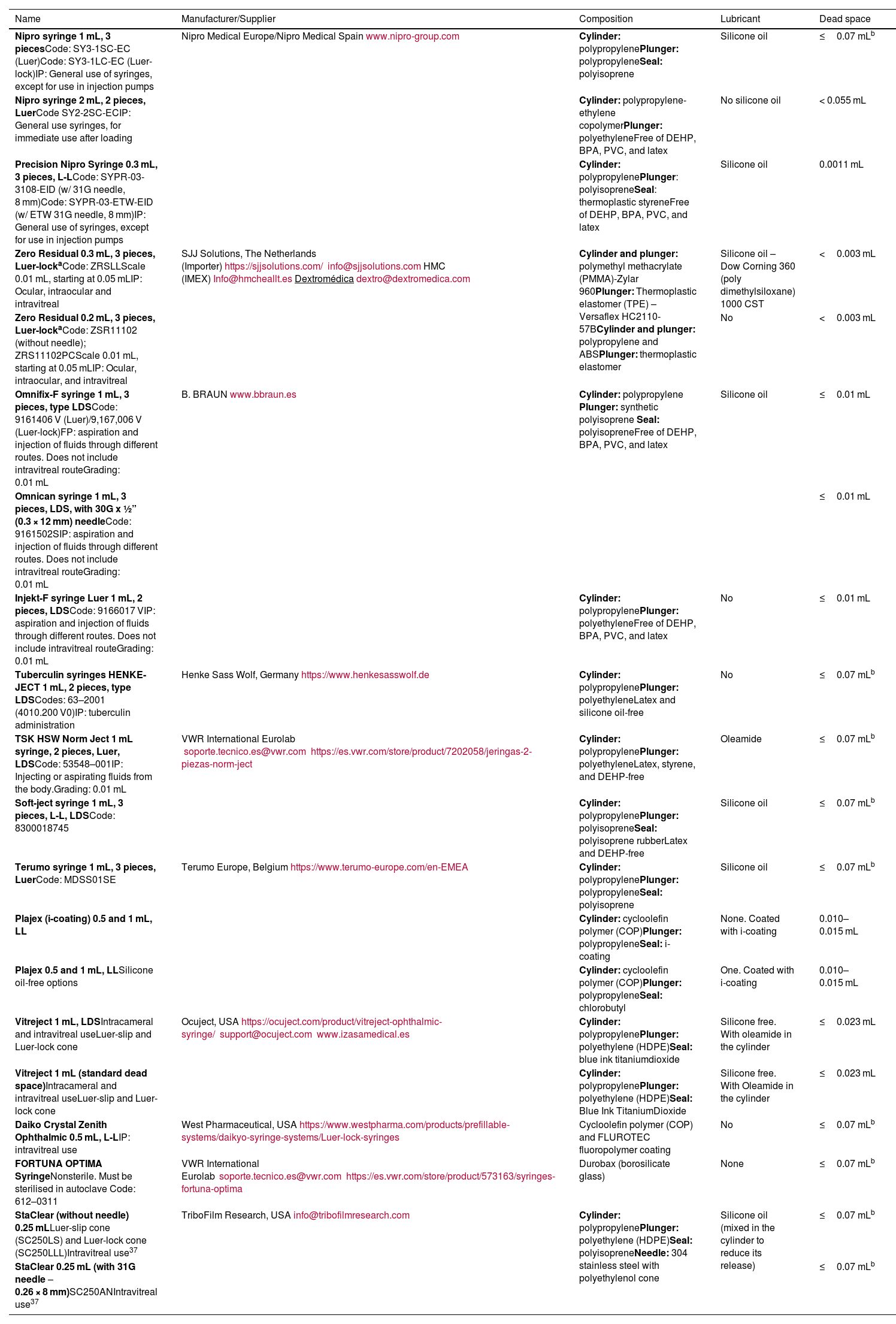
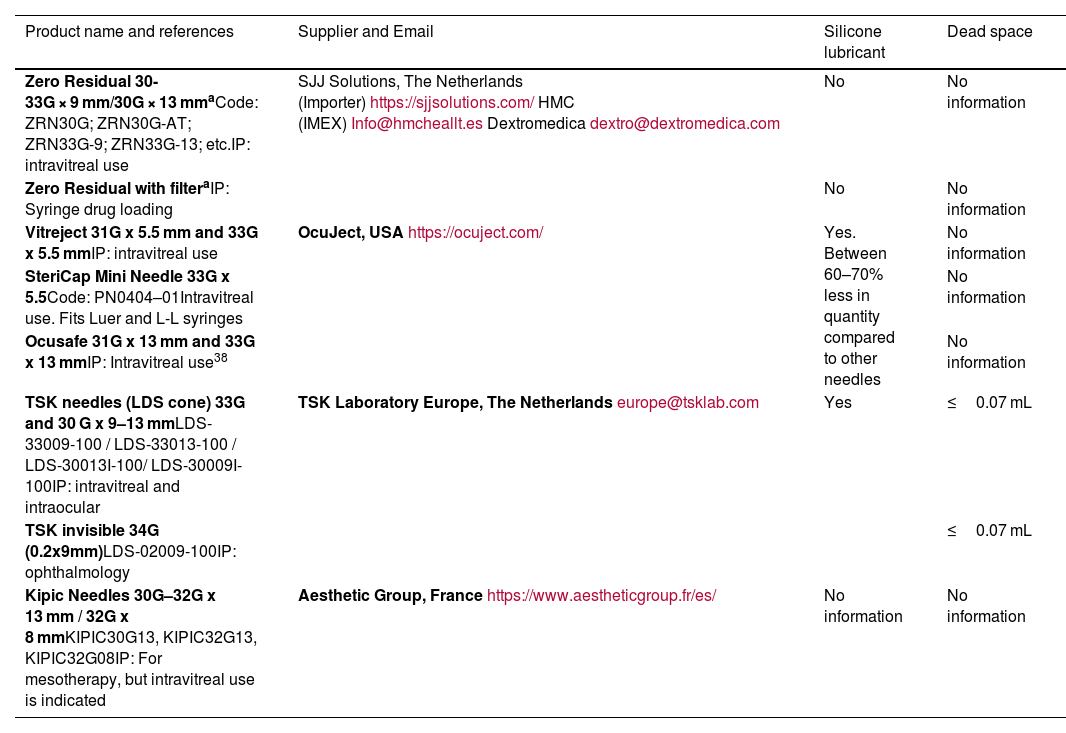
Tables 2 and 3 show syringes and needles that currently meet these characteristics.
Syringes currently available that can be used for the storage and injection of different intraocular drugs.
| Name | Manufacturer/Supplier | Composition | Lubricant | Dead space |
|---|---|---|---|---|
| Nipro syringe 1 mL, 3 piecesCode: SY3-1SC-EC (Luer)Code: SY3-1LC-EC (Luer-lock)IP: General use of syringes, except for use in injection pumps | Nipro Medical Europe/Nipro Medical Spainwww.nipro-group.com | Cylinder: polypropylenePlunger: polypropyleneSeal: polyisoprene | Silicone oil | ≤0.07 mLb |
| Nipro syringe 2 mL, 2 pieces, LuerCode SY2-2SC-ECIP: General use syringes, for immediate use after loading | Cylinder: polypropylene-ethylene copolymerPlunger: polyethyleneFree of DEHP, BPA, PVC, and latex | No silicone oil | < 0.055 mL | |
| Precision Nipro Syringe 0.3 mL, 3 pieces, L-LCode: SYPR-03-3108-EID (w/ 31G needle, 8 mm)Code: SYPR-03-ETW-EID (w/ ETW 31G needle, 8 mm)IP: General use of syringes, except for use in injection pumps | Cylinder: polypropylenePlunger: polyisopreneSeal: thermoplastic styreneFree of DEHP, BPA, PVC, and latex | Silicone oil | 0.0011 mL | |
| Zero Residual 0.3 mL, 3 pieces, Luer-lockaCode: ZRSLLScale 0.01 mL, starting at 0.05 mLIP: Ocular, intraocular and intravitreal | SJJ Solutions, The Netherlands (Importer)https://sjjsolutions.com/info@sjjsolutions.comHMC (IMEX)Info@hmcheallt.esDextromédicadextro@dextromedica.com | Cylinder and plunger: polymethyl methacrylate (PMMA)-Zylar 960Plunger: Thermoplastic elastomer (TPE) – Versaflex HC2110-57BCylinder and plunger: polypropylene and ABSPlunger: thermoplastic elastomer | Silicone oil – Dow Corning 360 (poly dimethylsiloxane) 1000 CST | <0.003 mL |
| Zero Residual 0.2 mL, 3 pieces, Luer-lockaCode: ZSR11102 (without needle); ZRS11102PCScale 0.01 mL, starting at 0.05 mLIP: Ocular, intraocular, and intravitreal | No | <0.003 mL | ||
| Omnifix-F syringe 1 mL, 3 pieces, type LDSCode: 9161406 V (Luer)/9,167,006 V (Luer-lock)FP: aspiration and injection of fluids through different routes. Does not include intravitreal routeGrading: 0.01 mL | B. BRAUNwww.bbraun.es | Cylinder: polypropylene Plunger: synthetic polyisoprene Seal: polyisopreneFree of DEHP, BPA, PVC, and latex | Silicone oil | ≤0.01 mL |
| Omnican syringe 1 mL, 3 pieces, LDS, with 30G x ½” (0.3 × 12 mm) needleCode: 9161502SIP: aspiration and injection of fluids through different routes. Does not include intravitreal routeGrading: 0.01 mL | ≤0.01 mL | |||
| Injekt-F syringe Luer 1 mL, 2 pieces, LDSCode: 9166017 VIP: aspiration and injection of fluids through different routes. Does not include intravitreal routeGrading: 0.01 mL | Cylinder: polypropylenePlunger: polyethyleneFree of DEHP, BPA, PVC, and latex | No | ≤0.01 mL | |
| Tuberculin syringes HENKE-JECT 1 mL, 2 pieces, type LDSCodes: 63–2001 (4010.200 V0)IP: tuberculin administration | Henke Sass Wolf, Germanyhttps://www.henkesasswolf.de | Cylinder: polypropylenePlunger: polyethyleneLatex and silicone oil-free | No | ≤0.07 mLb |
| TSK HSW Norm Ject 1 mL syringe, 2 pieces, Luer, LDSCode: 53548–001IP: Injecting or aspirating fluids from the body.Grading: 0.01 mL | VWR International Eurolab soporte.tecnico.es@vwr.comhttps://es.vwr.com/store/product/7202058/jeringas-2-piezas-norm-ject | Cylinder: polypropylenePlunger: polyethyleneLatex, styrene, and DEHP-free | Oleamide | ≤0.07 mLb |
| Soft-ject syringe 1 mL, 3 pieces, L-L, LDSCode: 8300018745 | Cylinder: polypropylenePlunger: polyisopreneSeal: polyisoprene rubberLatex and DEHP-free | Silicone oil | ≤0.07 mLb | |
| Terumo syringe 1 mL, 3 pieces, LuerCode: MDSS01SE | Terumo Europe, Belgiumhttps://www.terumo-europe.com/en-EMEA | Cylinder: polypropylenePlunger: polypropyleneSeal: polyisoprene | Silicone oil | ≤0.07 mLb |
| Plajex (i-coating) 0.5 and 1 mL, LL | Cylinder: cycloolefin polymer (COP)Plunger: polypropyleneSeal: i-coating | None. Coated with i-coating | 0.010–0.015 mL | |
| Plajex 0.5 and 1 mL, LLSilicone oil-free options | Cylinder: cycloolefin polymer (COP)Plunger: polypropyleneSeal: chlorobutyl | One. Coated with i-coating | 0.010–0.015 mL | |
| Vitreject 1 mL, LDSIntracameral and intravitreal useLuer-slip and Luer-lock cone | Ocuject, USAhttps://ocuject.com/product/vitreject-ophthalmic-syringe/support@ocuject.comwww.izasamedical.es | Cylinder: polypropylenePlunger: polyethylene (HDPE)Seal: blue ink titaniumdioxide | Silicone free. With oleamide in the cylinder | ≤0.023 mL |
| Vitreject 1 mL (standard dead space)Intracameral and intravitreal useLuer-slip and Luer-lock cone | Cylinder: polypropylenePlunger: polyethylene (HDPE)Seal: Blue Ink TitaniumDioxide | Silicone free. With Oleamide in the cylinder | ≤0.023 mL | |
| Daiko Crystal Zenith Ophthalmic 0.5 mL, L-LIP: intravitreal use | West Pharmaceutical, USAhttps://www.westpharma.com/products/prefillable-systems/daikyo-syringe-systems/Luer-lock-syringes | Cycloolefin polymer (COP) and FLUROTEC fluoropolymer coating | No | ≤0.07 mLb |
| FORTUNA OPTIMA SyringeNonsterile. Must be sterilised in autoclave Code: 612–0311 | VWR International Eurolab soporte.tecnico.es@vwr.comhttps://es.vwr.com/store/product/573163/syringes-fortuna-optima | Durobax (borosilicate glass) | None | ≤0.07 mLb |
| StaClear (without needle) 0.25 mLLuer-slip cone (SC250LS) and Luer-lock cone (SC250LLL)Intravitreal use37 | TriboFilm Research, USAinfo@tribofilmresearch.com | Cylinder: polypropylenePlunger: polyethylene (HDPE)Seal: polyisopreneNeedle: 304 stainless steel with polyethylenol cone | Silicone oil (mixed in the cylinder to reduce its release) | ≤0.07 mLb |
| StaClear 0.25 mL (with 31G needle – 0.26 × 8 mm)SC250ANIntravitreal use37 | ≤0.07 mLb |
IP, intended purpose; LDS, low dead space; L-L, Luer-lock; HDPE, high-density polyethylene; TPE, thermoplastic elastomers; BPA, bisphenol A; DEHP, diethylhexylphthalate; COP, cycloolefin polymer; PVC, polyvinyl chloride.
Needles currently available for intraocular drug administration.
| Product name and references | Supplier and Email | Silicone lubricant | Dead space |
|---|---|---|---|
| Zero Residual 30-33G × 9 mm/30G × 13 mmaCode: ZRN30G; ZRN30G-AT; ZRN33G-9; ZRN33G-13; etc.IP: intravitreal use | SJJ Solutions, The Netherlands (Importer)https://sjjsolutions.com/HMC (IMEX)Info@hmcheallt.esDextromedicadextro@dextromedica.com | No | No information |
| Zero Residual with filteraIP: Syringe drug loading | No | No information | |
| Vitreject 31G x 5.5 mm and 33G x 5.5 mmIP: intravitreal use | OcuJect, USAhttps://ocuject.com/ | Yes. Between 60–70% less in quantity compared to other needles | No information |
| SteriCap Mini Needle 33G x 5.5Code: PN0404–01Intravitreal use. Fits Luer and L-L syringes | No information | ||
| Ocusafe 31G x 13 mm and 33G x 13 mmIP: Intravitreal use38 | No information | ||
| TSK needles (LDS cone) 33G and 30 G x 9–13 mmLDS-33009-100 / LDS-33013-100 / LDS-30013I-100/ LDS-30009I-100IP: intravitreal and intraocular | TSK Laboratory Europe, The Netherlandseurope@tsklab.com | Yes | ≤0.07 mL |
| TSK invisible 34G (0.2x9mm)LDS-02009-100IP: ophthalmology | ≤0.07 mL | ||
| Kipic Needles 30G–32G x 13 mm / 32G x 8 mmKIPIC30G13, KIPIC32G13, KIPIC32G08IP: For mesotherapy, but intravitreal use is indicated | Aesthetic Group, Francehttps://www.aestheticgroup.fr/es/ | No information | No information |
IP, intended purpose; LDS, low dead space; L-L, Luer-lock.
The current regulations, the SPC of the drugs, the manufacturer's instructions for use of the MDs, and the materials to be used in IOIs will be taken into account when designing work procedures, and the compatibility of all of these aspects will be verified.
Place of preparation- •
In this regard, the regulations vary widely between countries.39 In the USA, there are no specific requirements for the rooms in which they are prepared. Since the publication of Resolution CM/ResAP (2011)140 in Europe and the Guide to Good Practice in the Preparation of Medicines in Hospital Pharmacy Services (GBPP)41 in Spain, intravitreal preparations should be performed under laminar airflow (Class A laminar airflow cabinets [LFC], equivalent to ISO class 4 according to UNE-EN ISO 14644-1). As these preparations are made from sterile products, this cabinet could be in a Class C environment, equivalent to ISO 7. An alternative could be an isolator (internal class A) in a Class D environment.
- •
Although accidents and negligence in the centralised preparation of drugs have been documented, international population studies suggest that the incidence of endophthalmitis secondary to intravitreal administration is less frequent in preparations made in Pharmacy Services than in those prepared in clinical units.42
- •
The preparation of an IOI in a clinical unit, in an unclassified environment, should be considered an exceptional situation and justifiable only when there are no other alternatives for appropriate patient care.
- •
18G blunt needles are recommended for drawing drugs from vials, while 30G or thinner needles are recommended for administration.4 Under no circumstances should the needle used to pierce the rubber stopper of the vial for drug extraction be used for patient administration.
- •
When preparing products requiring sterilisation at the end of the process, double sterilisation filtration with 0.22-μm filters should be performed before filling the final container.41
- •
When starting from sterile material, use 5-μm particle filters to prevent glass particles from ampoules or elastomers from the vials from entering the preparation. For added safety, 0.22-μm filters can be used before filling the final container.
- •
To date, the SPC of anti-VEGF drugs marketed in Spain44–46 for this type of treatment recommend drawing the drug from the vial through a needle with a 5-μm suction filter to prevent glass particles from the vials or elastomers from vials from entering the preparation. As these are provided in the vial packaging, this can be considered a general recommendation for such preparations.
- •
In any case, compatibility between the product to be filtered and the filter components must be verified.
- •
Drugs currently marketed for this route of administration consider the vial to be for single use only.44–46 In these cases, according to article 7 of Royal Decree Law 16/201247 and the GBPP,41 the prescribing medical services and the hospital pharmacy service will jointly decide—through the Pharmacy and Therapeutics Committee or those created for this purpose—on the inclusion of the fractionation and preparation of the IOI in the care protocols.
- •
If vials are to be fractionated to improve their use, a potential technique is as follows5,48:
- (1)
Check all starting materials to ensure they meet the required specifications. Special attention should be paid to drugs that are commercially available in different concentrations.
- (2)
Let the vial rest in a vertical position on the surface of the cabinet for a few seconds to allow all the drugs to run down the walls to the bottom of the vial.
- (3)
Draw the drug into a syringe through a needle with a built-in suction filter that has been previously tested for compatibility.
- (4)
Load the volume of medication agreed with the ophthalmology service into syringes with minimal dead space. The ophthalmology and pharmacy services will work together to determine the size and characteristics of the syringe and needle. These syringes (final containers) will be loaded through the hub of a larger volume syringe previously loaded with the drug. In this case, extreme care should be taken to prevent the microneedle of the syringe with minimal dead space from rubbing any surface of the syringe containing the drug. Extraction can be facilitated by using a pipette holder—previously sterilised and prepared for work under a laminar flow hood—to hold the syringe loaded with medication in a vertical position, allowing extraction through the hub with syringes without dead spaces.
- (1)
- •
Another option is to prepare the final syringes preloaded to a prespecified volume according to the drug, and to load them through their hubs from the larger-volume syringe pre-loaded with the drug.
- •
An alternative technique for fractionating high-cost drugs is to load the drug from the rear of the syringe, after removing the plunger from the syringe. This method requires extreme care when reinserting the plunger and removing air from the syringe to minimise drug loss. It is essential to ensure the absence of bubbles in the final preparation.
- •
If specific commercial devices are used for IOI preparation, the instructions provided by the manufacturer should be followed.24,25,33
- •
The use of a decapper to remove the metal top of the vial prevents the transfer of particles to the vial contents and the needle, while also contributing to better use of the vial by preventing drug wastage on the walls of the rubber stopper.5,48
- •
Throughout the preparation, storage, and distribution process, it is essential to ensure the following:
- •
Avoid the formation of bubbles and the introduction of air into the syringes as this can lead to protein instability. Although the SPCs of some intravitreal drugs recommend shaking or gently tapping the syringe to remove these bubbles, the international literature recommends avoiding these procedures as they would promote the release of particles or droplets from the syringe itself.2,9–23 Any turbidity observed in the drug should be noted, as this would indicate aggregate formation.49
- •
A stable temperature should be maintained and unintentional/accidental freezing and thawing avoided.2,5
- •
- •
Due to the difficulty of having sterile labels available in the hospital environment, prepared syringes should never be labelled directly. They should be packaged inside the LFC in sterile self-sealing bags, with at least one side being transparent, which will be the ones to be labelled.5
- •
Depending on the working methods of each centre, it may be advisable to use a double sterile bag to package each syringe. That is, each bag containing the syringe will be re-packaged in a second sterile bag, which will be the one to be labelled.
- •
Depending on the resources available at each centre—and as most of the drugs have similar names or phonetics (i.e. Look-alike, Sound-like drugs)—the label design will include measures, such as consensus colour codes, tallman-lettering, barcodes, QR codes, to reduce potential distribution or administration errors.
- •
If electronic drug administration is not available, additional labels identifying the batch and expiry date of the preparation can be added to the patient's medical record to improve the traceability of preparations.
- •
In turn, all labelled bags will be packaged in a lightproof bag for conservation and transport.5
- •
The auxiliary support staff in the sterile area should check the volume in the syringe, bag labelling, and so on, to prevent the individuals preparing the syringes from taking their hands away from the sterile areas and/or touching nonsterile material.5
- •
In general, fractionation should be conducted close to the time of administration.
- •
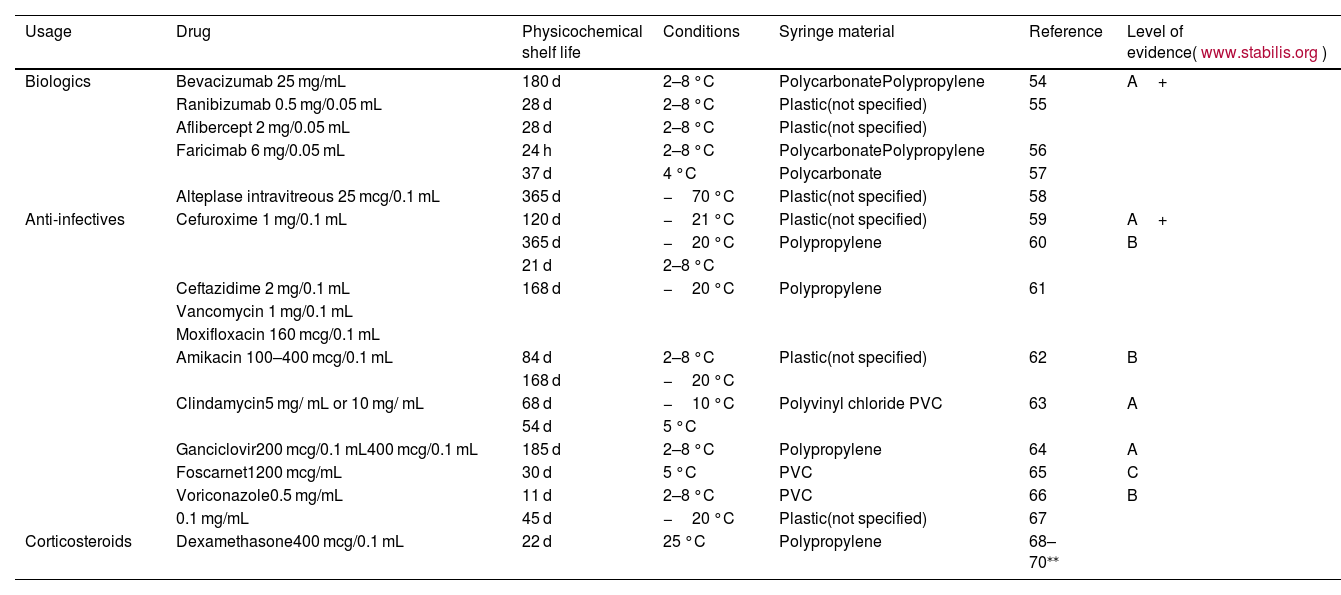
Shelf-life should be determined according to the latest quality evidence and the recommendations from health authorities and scientific societies. Table 4 shows the recommended physicochemical shelf life for the most commonly used intravitreal preparations.
Table 4.Physicochemical shelf life of most used intravitreal preparations. The applicable shelf life is limited by the microbiological controls described in the GBPP, depending on batch size or potential third-party manufacturing.
Usage Drug Physicochemical shelf life Conditions Syringe material Reference Level of evidence(www.stabilis.org) Biologics Bevacizumab 25 mg/mL 180 d 2–8 °C PolycarbonatePolypropylene 54 A+ Ranibizumab 0.5 mg/0.05 mL 28 d 2–8 °C Plastic(not specified) 55 Aflibercept 2 mg/0.05 mL 28 d 2–8 °C Plastic(not specified) Faricimab 6 mg/0.05 mL 24 h 2–8 °C PolycarbonatePolypropylene 56 37 d 4 °C Polycarbonate 57 Alteplase intravitreous 25 mcg/0.1 mL 365 d −70 °C Plastic(not specified) 58 Anti-infectives Cefuroxime 1 mg/0.1 mL 120 d −21 °C Plastic(not specified) 59 A+ 365 d −20 °C Polypropylene 60 B 21 d 2–8 °C Ceftazidime 2 mg/0.1 mL 168 d −20 °C Polypropylene 61 Vancomycin 1 mg/0.1 mL Moxifloxacin 160 mcg/0.1 mL Amikacin 100–400 mcg/0.1 mL 84 d 2–8 °C Plastic(not specified) 62 B 168 d −20 °C Clindamycin5 mg/ mL or 10 mg/ mL 68 d −10 °C Polyvinyl chloride PVC 63 A 54 d 5 °C Ganciclovir200 mcg/0.1 mL400 mcg/0.1 mL 185 d 2–8 °C Polypropylene 64 A Foscarnet1200 mcg/mL 30 d 5 °C PVC 65 C Voriconazole0.5 mg/mL 11 d 2–8 °C PVC 66 B 0.1 mg/mL 45 d −20 °C Plastic(not specified) 67 Corticosteroids Dexamethasone400 mcg/0.1 mL 22 d 25 °C Polypropylene 68–70⁎⁎ - •
In the case of biologics, quantitative studies alone will not be considered valid. Such studies must include techniques that ensure the stability of the three-dimensional structure of the molecule or its biological activity.
- •
In general, biologics should not be frozen.2 This technique can only be used if high-quality literature has shown that the three-dimensional structure of the drug is preserved.5,49
- •
According to the GBPP, medium-risk substances prepared in LFCs in a controlled environment have a microbiological shelf life of 9 days in refrigerators (2–8 °C) provided their physicochemical shelf life is not less than this period and the substances can be stored in refrigerators.41 Longer shelf-lives may be assigned when documented in a high-impact publication and the formulation is the same as that reported in the literature, or when the responsible pharmacist conducts the final sterility test to validate the preparation and routinely performs periodic tests to ratify the assigned shelf life.5,41
- •
All starting materials and packaging materials should be checked prior to use to ensure that they meet all specifications.41
- •
According to the GBPP, batches of more than 25 units must undergo microbiological analysis.41,50
- •
It is unnecessary to perform sterility testing on individualised ad hoc preparations.51 In such cases, the appearance and cleanliness of the final product should be visually checked.41
- •
In any case, validation of the operator's aseptic technique is necessary through simulating preparation using a culture medium in place of the drug.52
Previous studies have shown that the risk of endophthalmitis after intravitreal injection is low, regardless of the site of administration. Thus, there is no specific recommendation as to where injections should be given (e.g. consultation room, treatment room, or operating room), as long as it is comfortable for the patient and the staff involved in the procedure and ensures the use of sterile techniques.4 A recent meta-analysis53 of a total of 276,774 anti-VEGF injections showed that the site (operating room or consultation room) had no significant effect on the risk of endophthalmitis (level of evidence 1A/grade of recommendation A).4
Pre-administration handling- •
Visually inspect before use to ensure that the packaging is correct and meets specifications. Discard any containers that raise doubts about their conservation and quality.
- •
Include the batch number in the patient record, either by using a sticker or scanning the barcode/QR code.
- •
Extract in a clean field. Handle gently to avoid sudden movement of the contents.2
- •
Place the needle in the syringe (if not available). A 13–18 mm long needle3 and 30G or smaller4 is recommended.
- •
Flush to the administration dose, preferably with the needle cap on, to prevent any excess from being dispersed in the room. Do not tap the syringe to remove the air bubble2 and avoid manoeuvres that could facilitate lubricant transfer or the formation of bubbles or aggregates (Table 1).
- •
The injection procedure should follow the clinical practice guidelines of the SERV4 or those of the centre.
- •
This should be performed perpendicularly through the sclera with the tip pointing towards the centre of the globe to avoid damaging the lens, or bevelled injection to avoid reflux.2,4
- •
Special care must be taken not to contaminate the needle by contact.
- •
The injection of the product (0.05–0.1 mL depending on the drug) should be performed gently and steadily to avoid a diffusion effect4 and avoid loss of the more viscous drugs through the syringe-needle junction if the syringe is not Luer-locked. In this case, the needle should be held during injection to avoid dislodging it from the syringe.
- •
Patients should be advised that the more viscous drugs are more visible upon injection as they produce more bubbles. If they notice a bubble, it will persist for 24–48 h.
- •
The needle should be removed gently. A sterile cotton swab or the measuring instrument can be used on the opposite side to prevent drug or aqueous vitreous reflux and subsequent bleeding.4 If a vitreous wick is observed when the needle is removed or the injection site is touched with the cotton swab or haemostat, it should be trimmed at its base with scissors.
- •
Check the patient's light perception after injection.4
- •
Dispose of the syringe, needle, and cap in a waste container in accordance with local regulations, taking particular care with sharps.
It is recommended that these substances are prescribed for outpatients using the computer application available at the hospital. This prescription will also serve to confirm the injection schedule, manage dispensing per patient, and facilitate the traceability and statistics of the drugs used.5
Traceability- •
With the agreement of the ophthalmology service, and in order to help ensure the traceability of the preparations, it is recommended to send a label identifying the batch and expiry date of the preparation, which can be attached to the patient's medical record in case of a paper record or scanned with a bar code or QR code in the case of electronic administration.
None declared.
Declaration of authorshipGiven the working procedure, it is requested that the collective authorship of the 7 members of the drafting committee mentioned on the first page be acknowledged.
CRediT authorship contribution statementGonzaga Garay-Aramburu: Writing – original draft, Validation, Supervision, Formal analysis, Conceptualization. José María Alonso Herreros: Writing – original draft, Validation, Supervision, Formal analysis, Conceptualization. Marta Núñez Izquierdo: Writing – original draft, Validation, Supervision, Formal analysis, Conceptualization. Juan Francisco Márquez Peiró: Writing – original draft, Validation, Formal analysis, Conceptualization. Erika Vázquez Cruchaga: Writing – original draft, Validation, Supervision, Formal analysis, Conceptualization. Fernando González del Valle: Writing – original draft, Validation, Supervision, Formal analysis, Conceptualization. José Ignacio Fernández-Vigo: Writing – original draft, Validation, Supervision, Formal analysis, Conceptualization.
None declared.
We would like to thank Dr. Alfredo García Layana (President of the SERV) and the members of the Board of Directors of the SERV, Dr. José Manuel Benítez del Castillo (President of the SEO) and the members of the Board of Directors of the SEO, and Dr. Cecilia Martínez Fernández Llamazares (President of the SEFH) and the members of the Board of Directors of the SEFH for supporting the project and signing the collaboration agreement between the societies, as well as the members of the Pharmacotechnics Group and the Medical Devices Group of the SEFH for their collaboration throughout the project. We would also like to thank Dr. Silvia Berisa Prado (Servicio de Farmacia Clínica Universidad de Navarra, Pamplona), Dr. Virginia Puebla García (Servicio de Farmacia Hospital Universitario Clínico San Carlos, Madrid), Dr. Anxo Fernández Ferreiro (Servicio de Farmacia Hospitalaria, Unidad de Investigación e Innovación, Hospital Clínico Universitario de Santiago de Compostela), and Dr. Ana María Martín de Rosales (Servicio de Farmacia, Hospital Fundación de Alcorcon) for their critical reading of the document.