Design and validate a scale to measure adherence to oral antineoplastic drugs. The availability of a simple, validated tool that can be applied to routine care will make it possible to detect and identify non-adherence in order to establish strategies to improve adherence and optimize the quality of healthcare services.
MethodValidation study of the scale designed to assess adherence to antineoplastic drugs in a sample of outpatients who collect their medication in two Spanish hospitals. Its validity and reliability will be analyzed, based on a previous qualitative methodology study, using classical test theory and Rasch analysis. We will examine its performance, item fit, response structure and person fit to the predictions of the model, as well as dimensionality, item-person reliability, the appropriateness of the level of difficulty of the items to the sample, and the differential performance of the items according to gender.
Diseñar y validar una escala para medir la adherencia a antineoplásicos orales. Disponer de una herramienta sencilla, validada y aplicable a la rutina asistencial permitirá detectar e identificar falta de adherencia para establecer estrategias que permitan mejorarla y optimizar la calidad de los servicios sanitarios.
MétodoEstudio de validación de la escala diseñada para evaluar adherencia a antineoplásicos orales en una muestra de pacientes ambulatorios que recogen su medicación en cuatro hospitales españoles. Se analizará su validez y fiabilidad, elaborada a partir de un estudio previo de metodología cualitativa, mediante teoría clásica de los test y análisis Rasch. Se examinará su funcionamiento, el ajuste de los ítems, la estructura de respuesta y de las personas a las predicciones del modelo, así como la dimensionalidad, la fiabilidad ítem-persona, la adecuación del nivel de dificultad de los ítems a la muestra, y el funcionamiento diferencial de los ítems en función del sexo.
Advances in diagnosis and management often make it possible to treat cancer as a chronic disease, affording patients an acceptable quality of life. The growing availability of oral anti-cancer agents (OAAs) has contributed to improving quality of life, increasing the convenience of treatment, avoiding the complications inherent in the intravenous route, and making patients take responsibility for their treatment1. However, the ultimate success of the oral route depends on the patient or their caregiver and this may result in problems of adherence. Such problems could hamper health outcomes, reducing the treatment's clinical benefit, increasing healthcare costs and negatively impacting quality of life and survival2.
Long-term adherence to OAAs among the general population stands at around 50%. Rates vary according to the chronicity of the disease and to the geographical area considered, being lower in developing countries3. Although cancer patients are generally thought to be highly adherent, the literature contains reports of markedly heterogeneous adherence rates ranging between 46% and 100% depending on the specific drugs considered and the methods used to measure them4. The few studies carried out on the Spanish population report mean rates between 75 and 79%5,6.
Although several methods have been proposed to measure adherence, there is no gold standard for measuring it in cancer patients7,8. Available questionnaires tend to be simple, easy to apply and effective, although some are rather subjective and others overrate adherence. In spite of that, questionnaires are the most commonly used tool to measure adherence and, combined with leftover pill counting, allow a fairly accurate estimation of real adherence rates. Multiple questionnaires have been validated for patients suffering from different chronic conditions9,10. However, few validated questionnaires exist to measure adherence in patients on OAAs11 and none specifically for Spanish patients. The Morisky-Green test is probably the most widely used questionnaire for patients on OAAs, despite not being validated for such individuals and not having been conceived specifically for cancer patients. Recent publications12 have emphasized the need to find a valid and reliable tool to detect lack of adherence in patients on OAAs. Indeed, a series of factors have long been identified that can act either as barriers to or facilitators of adherence in these patients13, mostly related to their pharmacotherapeutic experience, which must be taken into consideration for the design of specific tools to measure adherence to OAAs.
It is therefore of the essence to develop a simple, valid and reliable tool that can be used in clinical practice to assess adherence to OAAs and allow the screening and early identification of patients requiring a closer and more individualized follow-up. Such patients would benefit from early interventions geared towards improving adherence and optimizing health outcomes.
The purpose of this study is to develop and validate a scale aimed at measuring adherence to OAAs in cancer patients, taking into consideration the specific pharmacotherapeutic experience of these patients, in addition to the expertise of the healthcare providers who treat them.
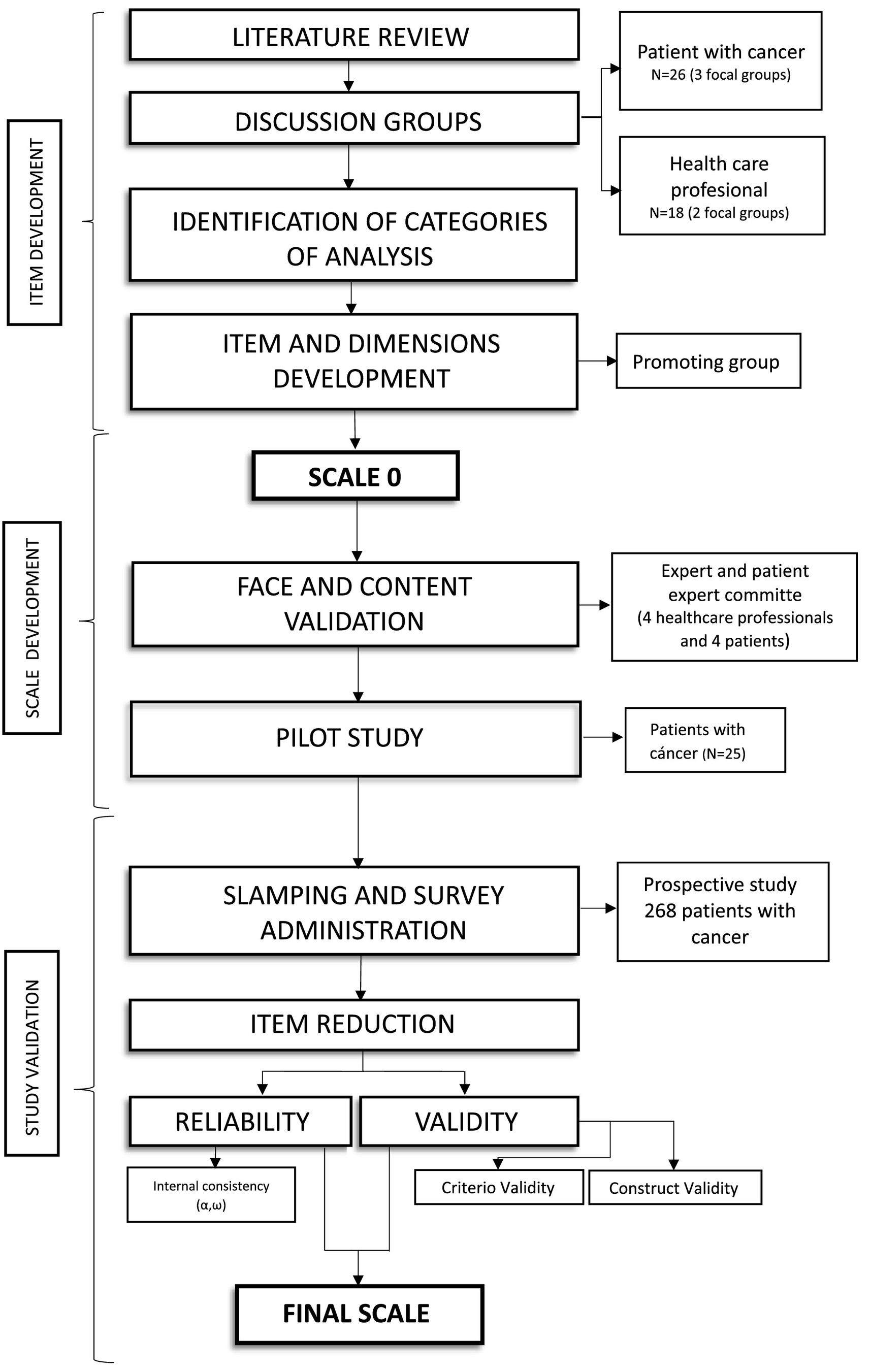
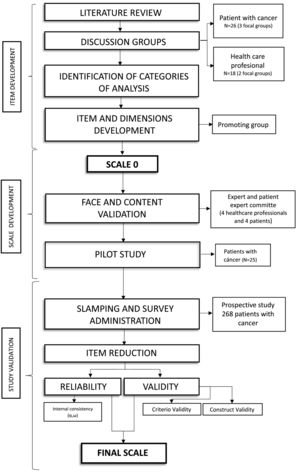
MethodsStudy design and locationThis was a multicenter study aimed at designing and validating a scale to gage adherence to OAAs in cancer outpatients. The proposed scale will be conceived based on an exhaustive literature review and a previous qualitative study by the authors14. Data will be collected prospectively from a sample of cancer patients treated in the outpatient pharmacy units of four hospitals in the Valencia region.
The protocol for this project was structured into three phases: item creation, scale development and validation study (Fig. 1). The work was based on the proposal by Boateng etal.15, Chan's guidelines16 and the COSMIN checklist17.
Phase 1. Item developmentThe scale items will be developed by a task force made up of 6 healthcare professionals: one physician, four pharmacists and one psychologist, who will define the construct and create a reliable and valid questionnaire. The work methodology selected to approve the different versions of the questionnaire is the consensus conference method18. whereby a series of meetings of the task force will be held until an agreement is reached on the definition of the construct, the identification of the various domains and the wording of the items themselves, following different analysis and discussion rounds.
Based on the results obtained from the previous qualitative research study14, the task force will apply the consensus conference methodology to define the various dimensions of the questionnaire and word the reactive items corresponding to each of them. The number of reactive items will be proportional to the dimensions, ensuring that each dimension contains at least twice as many items as in the definitive scale. The items for each of the dimensions defined will be generated through an iterative process based on deductive methods taken from the literature as well as inductive methods drawn from the patients' experience, the healthcare professionals' prioritization and the contributions of the task group.
Phase 2. Scale developmentFace and content validityAn 8–10 expert-strong panel will be formed. Members will be healthcare professionals, given their knowledge about the disease and their experience in managing and investigating it; and patients, due to their experience with the disease. All of them will be recruited by personal contact.
The expert panel will evaluate the validity of thescale contents with respect to the dimensions and reactive items included, checking for redundant, missing or left-out questions; and face validity (whether questions are easily understood, easy to respond, appropriate and correctly structured). As a result of this qualitative appraisal, changes may have to be introduced in the reactive items of the questionnaire.
Pre-testA pilot test will be run in a group of at least 15 patients to identify potential difficulties in understanding the questions and check the overall performance of the scale. This pre-study will include patients over 18 years of age diagnosed with cancer and treated with L01 or L02 OAAs (according to the ATC classification) who collect their medication from the participating centers and who have been on OAAs for at least one month. Patients with communication difficulties or who refuse to participate in the study will be excluded. The 5-point Likert scale will be used as it the one ensuring the most efficient validation. Respondents will be asked to determine whether questions are coherent and consistent with one another and if they find them easy to understand. The time employed to answer the questionnaire will be measured and the extent to which the items are aligned with their experience will be determined. The investigators will be asked to detect any problems related to the understanding of the questions and to analyze the appropriateness of the scale used to rate the subjects' responses.
Phase 3. Evaluation of psychometric propertiesThe scale will be validated based on a sample of patients treated with OAAs, with variables being recorded in a prospective fashion. Patient selection criteria will be the same as those defined in the previous stage. The sample size will be calculated on the basis of data from other studies, which have found adherence to stand between 75 and 79%. Considering the 78% rate (with a precision of 5% and a 95% confidence interval) obtained by a previous study on Spanish patients5, the sample should be made up of a total of 268 patients, which is in line with the recommendation that a minimum of 175–200 participants be recruited to ensure stable and generalizable results19. When patients who meet the inclusion criteria contact the pharmacy department, they will be consecutively invited to participate in a telephone interview, where different adherence-related sociodemographic and clinical variables will be recorded. They will also be asked questions on literacy and COVID-19 based on their electronic clinical record. The dependent variable, i.e., adherence to OAAs, will be evaluated by means of the newly designed questionnaire, medication collection records, the results of the Morisky-Green, test and leftover pill counts.
Exploratory and confirmatory dimensionality analysisAn exploratory factor analysis will be made using the principal axis extraction method. In addition, a principal component analysis will be performed, together with an oblique rotation method and an optimal implementation of parallel analysis to determine the fit of the items to the model and whether some items should be removed on the basis of the measure of sampling adequacy (MSA) index. MSA values <0.50 suggest that the item in question does not measure the same domain as the other items and should therefore be removed. Factor loads below 0.3 will not be considered either. Bartlett's test will be applied, goodness-of-fit indices and total variance explained will be calculated and an analysis will be made to determine whether the items comprising the questionnaire present with a specific single- or multidimensional structure. A confirmatory factor analysis (CFA) will be used to confirm the theoretical underlying structure, with goodness-of-fit indices being used to determine which of the following CFA models best represents the data set: the Comparative Fix Index (CFI), the adjusted Jöreskog-Sörbom's goodness-of-fit index (AGFI), the goodness of fix index; the standardized root mean squared residual (SRMR) and Jöreskog-Sörbom's goodness of fit index (GFI).
Dimensionality will also be evaluated by means of the Rasch-Andrich rating scale model and the fit of items and persons to the Rasch model will be determined using infit and outfit mean square (MSSQ) fit statistics. If the fit to the Rasch model is good, these values should be close to 1, with values for items in the critical 0.7–1.3 range and below 2 for persons. This would indicate that subject responses are in line with the model's expectations.
Reliability analysisInternal consistency will be measured by means of the usual tests: Cronbach's Alpha coefficient, which makes it possible to determine the items' internal consistency and how they are related with one another, and McDonald's Omega coefficient, which acts as a substitute of Cronbach's in cases where the instrument has a given multidimensional structure. Moreover, Rasch-based person separation reliability will be calculated, which is analogous to Cronbach's alpha coefficient but is based on logits (lineal scores) rather than raw scores. Reliability should be equal to or higher than 0.7 in all cases.
Criterion validityThe criterion validity will be evaluated taking the leftover pill count as a reference as this is one of the most commonly used criteria in routine practice and given the lack of a standard adherence measurement pattern. For chronic conditions, a patient is considered adherent when criterion validity is over 80%. Analyses will also be performed when results are over 90%, 95% and 100% since cancer is a serious condition where no standard cutoff point has been agreed but, rather, each author defines a more or less strict cutoff point depending on their own approach20.
The diagnostic performance of the instrument will be determined based on a calculation of the sensitivity and specificity of all the possible values in the questionnaire. The receiver operating characteristic (ROC) curve will also be represented. The Youden index will be calculated to find a point where both values are optimal. The index, whose values usually range between 0 and 1, makes it possible to establish a cutoff point with greater sensitivity and specificity. The area under the ROC curve will also be calculated to obtain an estimation of the ability of the questionnaire to determine nonadherence to treatment (the larger the area, the greater the diagnostic power).
Known groups construct validationThe validity of the scale construct will be evaluated by comparing two groups created based on the results of Morisky's test, which is the most commonly used tool for this kind of patient (convergent validity). A comparison will also be made of the scores obtained in the questionnaire to be validated with those from a questionnaire that measures literacy (divergent validity). Differences in the prevalence of adherence/nonadherence between both questionnaires will be analyzed (chi-squared test) as well as the differences in the scores from the adherence and literacy instrument (Student's t test).
Finally, the Rasch model will be used to determine goodness of fit of the level of difficulty of the items to the sample. The fit between items and persons is considered good when the mean score for persons is close to 0 logits, which is the value at the center of the scale and corresponds to the mean item value. Sex-related differential item functioning (DIF) is also an important consideration. An item presents with severe DIF if the contrast between groups (DIF size) is >1 and the result of Student's t test is statistically significant at p = 0.05 (with the Bonferroni correction). The analyzes will be made with the SPSS v.28, Winsteps 5.1.4 and Jamovi 1.6.2 software packages.
DiscussionThis study is aimed at designing and validating the first scale specifically intended to evaluate adherence to OAAs in cancer patients in Spain. One of its main strengths will be the process followed to design the questionnaire, which will be based on real-world data, taking into consideration the experiences and perceptions of cancer patients and the opinions of the professionals dedicated to their management. Subsequently, the validation process will allow selection of the most appropriate items to evaluate adherence and study the psychometric properties and the validity and reliability of the final scale.
The purpose is to develop a simple, valid and reliable tool that helps pharmacists evaluate the behavior of cancer patients with respect to their medication, minimizing subjectivity as much as possible.
Dispensation of OAAs by hospital pharmacy departments in Spain provides for a close contact between cancer patients and hospital pharmacists, placing the latter in an ideal position to detect and assess nonadherence and identify barriers and difficulties. Indeed, pharmacists play a central role in the establishment of individualized plans for these patients that go beyond mere dispensation, allowing a comprehensive management of cancer treatment. An improvement of adherence would result in more effective control of the disease, improving the patients' quality of life, reducing the healthcare bill and boosting the quality of the care provided.
The main limitation of the study is the lack of a validated, objective method that can be used as a gold standard to measure adherence. However, the combination of a personalized scales-based interview and leftover pill counts is likely to provide a result that paints a fairly accurate picture of reality. Another limitation has to do with the variability in the collection of data, typical of multicenter studies. To address this problem, the research team will be provided with specific training. Finally, there could be a potential selection bias as the patients who agree to participate in the study could well be the most adherent ones. Although this bias will be difficult to avoid, an attempt will be made to encourage participation and recruit as many patients as possible, minimizing the number of refusals to participate.
Ethical considerationsThe present study was approved by the Research Ethics Committee of the Elda General Hospital on 14 April 2020 (PI2020/12). Subsequently, in March 2021, a series of amendments were approved. The collection of data took longer than expected because of the outbreak of Covid-19. The study has been registered on clinicaltrials.gov (https://clinicaltrials.gov/): NCT04550533.
Authorship statementThe conception of the article and the design of the protocol were developed by Talens-Bolós, López-Pintor, Lumbreras-Lacarra, Aznar-Saliente and Orozco-Beltrán. The overall design was conceived by Talens-Bolós, López-Pintor, Guilabert-Mora and Lumbreras-Lacarra. The literature review and the discussion section were undertaken by all contributing authors. The first author prepared the drafts of the manuscript and the other co-authors reviewed it, made contributions to the text and approved the final version submitted for publication.
FundingNo funding.
Liability and transfer of rightsAll co-authors accept the responsibilities defined by the International Committee of Medical Journal Editors. In the event of publication, the authors exclusively transfer to the Revista and, by extension, to SEFH their rights to reproducing, distributing, translating, and publicly communicating their work by any sound, audiovisual or electronic medium of format. A specific rights transfer letter will be sent once the paper is submitted through SEFH's online manuscript processing system.