The activity of sponsors and Ethics Committees for Research with medicines has increased in recent years. The objective was to design and validate 2 instruments to analyze and evaluate the formal quality of the patient information sheet and the informed consent form of clinical trials with drugs, in accordance with the legislation.
MethodsDesign (Guideline for good clinical practice and European and Spanish regulations); validation (Delphi method and expert consensus: concordance ≥ 80%); reliability (inter-observer method, Kappa index). 40 patient information sheets/informed consent forms were evaluated.
ResultsVery good concordance was obtained in both checklists (k ≥ 0.81, p b 0.001). The final versions consisted of checklist-patient information sheet: 5 sections, 16 items and 46 sub-items; and checklist-informed consent form: 11 items.
ConclusionThe instruments developed are valid, reliable and facilitate the analysis, evaluation, and decision-making on the patient information sheets/informed consent forms of clinical trials with drugs.
La actividad de los promotores y Comités de Ética de la Investigación con medicamentos (CEIm) ha aumentado en los últimos años. El objetivo fue diseñar y validar dos instrumentos para analizar y evaluar la calidad formal de la hoja de información al participante (HIP) y el formulario de consentimiento informado (CI) de ensayos clínicos (EC) con medicamentos, acorde con la legislación.
MétodosDiseño (Buenas Prácticas Clínicas y normativas europea y española); validación (método Delphi y consenso de expertos: concordancia ≥ 80%); fiabilidad (método inter-observadores, índice Kappa). 40 HIP/CI evaluados.
ResultadosSe obtuvo muy buena concordancia en ambos instrumentos (k ≥ 0,81, p < 0,001). Las versiones definitivas estaban formadas por: checklist-HIP: 5 secciones, 16 ítems y 46 sub-ítems; checklist-CI: 11 ítems.
ConclusionesLos instrumentos desarrollados son válidos, fiables y facilitan el análisis, evaluación y toma de decisión sobre las HIP/CI de EC con medicamentos.
In the context of clinical trials (CTs), participant information sheets (PIS’s) and informed consent forms (ICFs) are a reflection of the principle of autonomy. Before the informed consent process is initiated, these documents are typically evaluated by a research ethics committee (REC) in order to ensure that the information they contain is appropriate and aligned with all existing ethical and statutory regulations.1
The EMA Guideline for Good Clinical Practice (GCP) establishes a series of norms on the information that should be included in PIS’s to ensure it is as comprehensive as possible.1 Regulation (EU) no. 536/2014 leaves the actual evaluation of PIS’s/ICFs in the hands of national authorities.2 In the case of Spain,3 the first consensus document on the subject was published in 2017 under the name Guía para la correcta elaboración de un modelo de hoja de información al paciente y consentimiento informado (HIP/CI) (Guidelines for correct preparation of patient information sheets and informed consent forms) and integrated as Annex VIIIA into the Instructions for the performance of CTs with medicinal products published by the Spanish Agency of Medicines and Medical Devices (AEMPS).4
Taking into account the need to consider the current legislation and include the information contained in the EMA’s Guideline for GCP when preparing any PIS/ICF, it would be useful to have at one’s disposal a series of validated instruments that facilitate and speed up the analyses and evaluations that must be conducted by CT sponsors and Ethics Committees for investigation with medicinal products (CEIm).5 The present study was consequently aimed at designing and validating two instruments (checklists) intended to analyze and systematically evaluate the formal quality of the PIS’s and ICFs used in CTs with medicinal products, in accordance with the legislation in force.
MethodsThe study was divided into three phases: design, validation of contents, and analysis of the instruments’ reliability.6–9
Phase 1: DesignThe two checklists were prepared further to several meetings of a study group formed by four pharmacists and a nurse. The first step was a literature review (EMA’s,1 European,2 and Spanish3 guidelines) which led to preparation of a PIS and an ICF model for CTs with medicinal products. The next step was to select the items and subitems to be included in each checklist, which were worded in simple language and then organized into different sections. The responses proposed were “yes,” “no” and “not applicable” (N/A). Finally, a draft was obtained for each checklist (PIS checklist and ICF checklist). An explanatory document was attached to each checklist, developed in accordance with Annex VIIIA.4
Phase 2: Validation of contentsThe (questionnaire-based) Delphi method was applied by an expert committee8 made up of a multidisciplinary team (two pharmacists, two nurses and two physicians, all of them with experience in evaluating PIS’s/ICFs). An initial e-mail was sent out inviting the committee members to participate in checklist validation process. The e-mail contained information on the purpose of the study as well as a description thereof. Once the members confirmed their participation, they were sent a draft of each of the checklists together with the corresponding Delphi questionnaire.
The questionnaire applied to the draft checklists contained sections allowing respondents to express their agreement/disagreement and remove, add and prioritize items/subitems. A section was also included for “additional comments.”
After administration of the questionnaire, all responses were analyzed, and the percentages of expert agreement were calculated. An item/subitem was considered valid when the percentage of agreement was equal to or higher than 80% Percentages below 30% were indicative that modifications had to be made. All suggestions made were included in each of the draft checklists.
Phase 3: Reliability analysisA total of 40 PISs/ICFs of CTs with medicinal products were used. The expected intraclass correlation coefficient was 0.90, with no items scored below 0.75; statistical power was 90%. An α of 0.05 was used as a cutoff for significance, considering a potential loss of 10%. The checklists were reviewed/approved by Idcsalud a Catalunya, an accredited CEIm between 2016 and 2017) in accordance with the provisions in Royal Decree 1090/2015.3
The reliability of the checklists was confirmed by the inter-observed agreement method6,7 whereby two independent evaluators read each PIS and ICF verifying the appropriateness of the checklists’ items/subitems; a third evaluator was resorted to in case of discrepancy. An item was considered to be valid when it was included in the PIS/ICF, without regard to whether its contents were appropriate or not (formal quality). Strength of agreement between evaluators was determined for each response by means of the overall agreement percentage and the Kappa index (k).9 Interpretation of k was carried out using Landis & Koch’s qualitative scale,9 which includes six levels of strength of agreement: very good (≥0.81), good (0.61-0.80), moderate (0.41-0.60), acceptable (0.21-0.40), low (0.01-0.20), no agreement (<0,00). A value ≥0,60 was considered indicative of reliability [95% confidence interval (CI) and α = 0.05]. Further to this analysis, any changes deemed necessary were introduced in the checklists.
Data analysisData was processed using the SPSS Statistics v. 25.0 software package. A univariate analysis was performed of categorical variables applying absolute and relative frequency tables to the responses obtained from the Delphi questionnaires and to the PIS/ICF checklists during the reliability analysis process.
ResultsPhase 1: DesignA draft version of each checklist (PIS and ICF) was drawn up. The draft PIS checklist comprised 17 items and 51 subitems organized into five sections; the draft ICF checklist contained 15 items. The explanatory documents contained clarifications, examples and recommendations.
Phase 2: ValidationNone of the experts made suggestions concerning 16 items and 33 subitems of the PIS checklist, or 10 items of the ICF checklist as they considered them valid as they were formulated. They did, however, make 23 suggestions regarding other items and subitems of the PIS checklist and five items of the ICF checklist. The versions including the experts’ suggestions were called “interim versions.” The interim version of the PIS checklist contained 16 items and 46 subitems, organized into five sections; the interim version of the ICF checklist comprised 11 items.
Phase 3: Reliability analysisAll items in the PIS checklist displayed “very strong” agreement (k index: ≥0.81, p<0.001), except for item 13, for which agreement was “strong” (k index: 0.61-0.80, p<0.001). All subitems in the PIS checklist (k index: ≥0.81, p<0.001) displayed “very strong” agreement, except for subitems 3a, 4a, 4b, 5a, 5b, 13a and 15d, for which agreement was “strong” (k index: 0.61-0.80, p<0.001). Subitem 15d obtained “moderate” agreement (k index: 0.41-0.60, p<0.001). The PIS checklist obtained “very strong” agreement [k index: 0.931 (95% CI:0.909-0.954), p<0.001].
All items in the ICF checklist displayed “very strong” agreement, except for item 8, for which agreement was “moderate” (k index: 0.41-0.60; p<0.001). The ICF checklist obtained “very strong” agreement [k index: 0.969 (95% CI, 0.942-0.996), p<0.001].
Final versionsItems with “moderate” agreement were reworded to make them easier to understand. This rewording process resulted in the final versions of the two checklists:
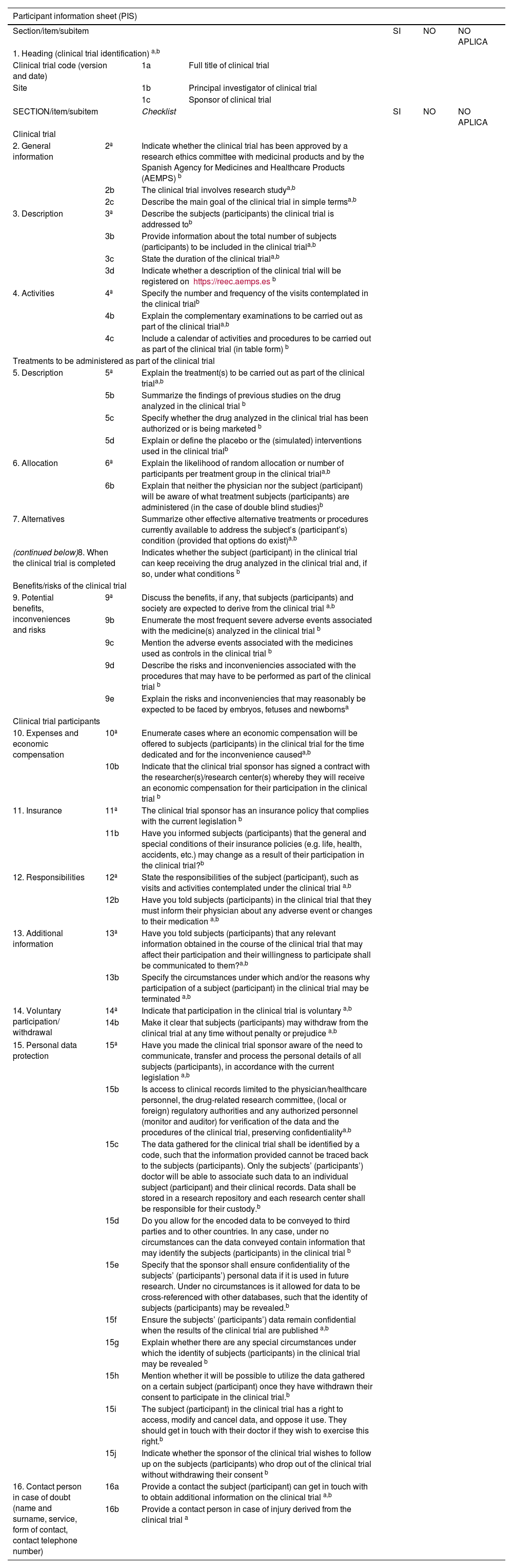
-PIS checklist: The final version was made up of 16 items and 46 subitems, organized into five sections (Table 1).
PIS checklist. Checklist for the information to be included in the participant information sheet used in drug-related clinical trials.
| Participant information sheet (PIS) | ||||||
|---|---|---|---|---|---|---|
| Section/item/subitem | SI | NO | NO APLICA | |||
| 1. Heading (clinical trial identification) a,b | ||||||
| Clinical trial code (version and date) | 1a | Full title of clinical trial | ||||
| Site | 1b | Principal investigator of clinical trial | ||||
| 1c | Sponsor of clinical trial | |||||
| SECTION/item/subitem | Checklist | SI | NO | NO APLICA | ||
| Clinical trial | ||||||
| 2. General information | 2ª | Indicate whether the clinical trial has been approved by a research ethics committee with medicinal products and by the Spanish Agency for Medicines and Healthcare Products (AEMPS) b | ||||
| 2b | The clinical trial involves research studya,b | |||||
| 2c | Describe the main goal of the clinical trial in simple termsa,b | |||||
| 3. Description | 3ª | Describe the subjects (participants) the clinical trial is addressed tob | ||||
| 3b | Provide information about the total number of subjects (participants) to be included in the clinical triala,b | |||||
| 3c | State the duration of the clinical triala,b | |||||
| 3d | Indicate whether a description of the clinical trial will be registered on https://reec.aemps.esb | |||||
| 4. Activities | 4ª | Specify the number and frequency of the visits contemplated in the clinical trialb | ||||
| 4b | Explain the complementary examinations to be carried out as part of the clinical triala,b | |||||
| 4c | Include a calendar of activities and procedures to be carried out as part of the clinical trial (in table form) b | |||||
| Treatments to be administered as part of the clinical trial | ||||||
| 5. Description | 5ª | Explain the treatment(s) to be carried out as part of the clinical triala,b | ||||
| 5b | Summarize the findings of previous studies on the drug analyzed in the clinical trial b | |||||
| 5c | Specify whether the drug analyzed in the clinical trial has been authorized or is being marketed b | |||||
| 5d | Explain or define the placebo or the (simulated) interventions used in the clinical trialb | |||||
| 6. Allocation | 6ª | Explain the likelihood of random allocation or number of participants per treatment group in the clinical triala,b | ||||
| 6b | Explain that neither the physician nor the subject (participant) will be aware of what treatment subjects (participants) are administered (in the case of double blind studies)b | |||||
| 7. Alternatives | Summarize other effective alternative treatments or procedures currently available to address the subject’s (participant’s) condition (provided that options do exist)a,b | |||||
| (continued below)8. When the clinical trial is completed | Indicates whether the subject (participant) in the clinical trial can keep receiving the drug analyzed in the clinical trial and, if so, under what conditions b | |||||
| Benefits/risks of the clinical trial | ||||||
| 9. Potential benefits, inconveniences and risks | 9ª | Discuss the benefits, if any, that subjects (participants) and society are expected to derive from the clinical trial a,b | ||||
| 9b | Enumerate the most frequent severe adverse events associated with the medicine(s) analyzed in the clinical trial b | |||||
| 9c | Mention the adverse events associated with the medicines used as controls in the clinical trial b | |||||
| 9d | Describe the risks and inconveniencies associated with the procedures that may have to be performed as part of the clinical trial b | |||||
| 9e | Explain the risks and inconveniencies that may reasonably be expected to be faced by embryos, fetuses and newbornsa | |||||
| Clinical trial participants | ||||||
| 10. Expenses and economic compensation | 10ª | Enumerate cases where an economic compensation will be offered to subjects (participants) in the clinical trial for the time dedicated and for the inconvenience causeda,b | ||||
| 10b | Indicate that the clinical trial sponsor has signed a contract with the researcher(s)/research center(s) whereby they will receive an economic compensation for their participation in the clinical trial b | |||||
| 11. Insurance | 11ª | The clinical trial sponsor has an insurance policy that complies with the current legislation b | ||||
| 11b | Have you informed subjects (participants) that the general and special conditions of their insurance policies (e.g. life, health, accidents, etc.) may change as a result of their participation in the clinical trial?b | |||||
| 12. Responsibilities | 12ª | State the responsibilities of the subject (participant), such as visits and activities contemplated under the clinical trial a,b | ||||
| 12b | Have you told subjects (participants) in the clinical trial that they must inform their physician about any adverse event or changes to their medication a,b | |||||
| 13. Additional information | 13ª | Have you told subjects (participants) that any relevant information obtained in the course of the clinical trial that may affect their participation and their willingness to participate shall be communicated to them?a,b | ||||
| 13b | Specify the circumstances under which and/or the reasons why participation of a subject (participant) in the clinical trial may be terminated a,b | |||||
| 14. Voluntary participation/ withdrawal | 14ª | Indicate that participation in the clinical trial is voluntary a,b | ||||
| 14b | Make it clear that subjects (participants) may withdraw from the clinical trial at any time without penalty or prejudice a,b | |||||
| 15. Personal data protection | 15ª | Have you made the clinical trial sponsor aware of the need to communicate, transfer and process the personal details of all subjects (participants), in accordance with the current legislation a,b | ||||
| 15b | Is access to clinical records limited to the physician/healthcare personnel, the drug-related research committee, (local or foreign) regulatory authorities and any authorized personnel (monitor and auditor) for verification of the data and the procedures of the clinical trial, preserving confidentialitya,b | |||||
| 15c | The data gathered for the clinical trial shall be identified by a code, such that the information provided cannot be traced back to the subjects (participants). Only the subjects’ (participants’) doctor will be able to associate such data to an individual subject (participant) and their clinical records. Data shall be stored in a research repository and each research center shall be responsible for their custody.b | |||||
| 15d | Do you allow for the encoded data to be conveyed to third parties and to other countries. In any case, under no circumstances can the data conveyed contain information that may identify the subjects (participants) in the clinical trial b | |||||
| 15e | Specify that the sponsor shall ensure confidentiality of the subjects’ (participants’) personal data if it is used in future research. Under no circumstances is it allowed for data to be cross-referenced with other databases, such that the identity of subjects (participants) may be revealed.b | |||||
| 15f | Ensure the subjects’ (participants’) data remain confidential when the results of the clinical trial are published a,b | |||||
| 15g | Explain whether there are any special circumstances under which the identity of subjects (participants) in the clinical trial may be revealed b | |||||
| 15h | Mention whether it will be possible to utilize the data gathered on a certain subject (participant) once they have withdrawn their consent to participate in the clinical trial.b | |||||
| 15i | The subject (participant) in the clinical trial has a right to access, modify and cancel data, and oppose it use. They should get in touch with their doctor if they wish to exercise this right.b | |||||
| 15j | Indicate whether the sponsor of the clinical trial wishes to follow up on the subjects (participants) who drop out of the clinical trial without withdrawing their consent b | |||||
| 16. Contact person in case of doubt (name and surname, service, form of contact, contact telephone number) | 16a | Provide a contact the subject (participant) can get in touch with to obtain additional information on the clinical trial a,b | ||||
| 16b | Provide a contact person in case of injury derived from the clinical trial a | |||||
Clarifications/remarks:
(a European Medicines Agency (EMA). Guideline for good clinical practice E6 (R2). 2016 [Accessed 09-05-2022]. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-6-r2-guideline-good-clinical-practice-step-5_en.pdf1; b Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Anexo VIIIA. Guía para la correcta elaboración de un modelo de hoja de información al paciente y consentimiento informado (HIP/CI). 2017 [Accessed: 09-05-2022]. Available at: https://www.aemps.gob.es/investigacionClinica/medicamentos/docs/anexo8a-Ins-AEMPS-EC.pdf?x60265;20174).
-ICF checklist: The final version was made up of 11 items (Table 2).
ICF checklist. Checklist of the information to be included in the informed consent form used in clinical trials with medicinal products.
| Informed consenta by subject (participant) / by a legally designated representative* | ||||
|---|---|---|---|---|
| Item | Checklist | YES | NO | NOT APPLICABLE |
| 1 | Clinical trial title and code (version and date) | |||
| 2 | I have read the information sheet given to me about the clinical trial* …….. declares that, in his/her presence, Mr. / Ms. << participant’s name and surname >> was given comprehensive information and read the clinical trial information sheet that was given to him/her. | |||
| 3 | I / * He/she was able to ask questions and/or received sufficient information about the clinical trial | |||
| 4 | I / * He/she spoke to <>> | |||
| 5 | I understand / * understands that my / * his/her participation in the clinical trial is voluntary | |||
| 6 | I understand / * understands that I / * he/she may withdraw from the clinical trial whenever I / * she/he / wish/wishes to do so, without giving any reason and without my/ * his/her present or future medical treatment being affected | |||
| 7 | I have / * he/she has received a signed and dated copy of the informed consent form for the clinical trial | |||
| 8 | I freely agree/ / * he/she freely agrees to participate in the clinical trial | |||
| 9 | I / * he/she would like to be kept informed about any development that emerges from the research, which could be relevant to my / his/her health. | |||
| 10 | Date, name and signature of the subject/participant in the clinical trial / * legally designated representative | |||
| 11 | Date, name and signature of the researcher responsible for the clinical trial | |||
Clarifications/Remarks:
(a Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Anexo VIIIA. Guía para la correcta elaboración de un modelo de hoja de información al paciente y consentimiento informado (HIP/CI). 2017 [Accessed: 09-05-2022]. Available at: https://www.aemps.gob.es/investigacionClinica/medicamentos/docs/anexo8a-Ins-AEMPS-EC.pdf?x60265;20174)
Possible responses were “yes, “no” and “not applicable.” A “clarifications/remarks” section was also added. Both the checklists and the explanatory documents attached to support application of the checklists are available at http://www.ub.edu/farcli/.
DiscussionThe greatest innovation in this study is that the checklists underwent a rigorous internal validation process,6–9 considering that in the future they could be applied by other drug research ethics committees (external validation).
The checklists prepared as part of this study were intended to put an end to a situation where ethics committees have no validated instruments at their disposal to evaluate the PIS’s/ICFs used in CTs with medicinal products. The checklists may also be useful as facilitators for the design of CTs by CT sponsors and by individuals and agencies wishing to participate in public calls for independent CTs within the framework of national/regional research programs.
From the ethical point of view, both checklists contribute to identifying relevant information that may allow subjects and/or their legal representatives to make the right decisions. The ultimate goal is to reinforce the subjects’ right to autonomy and guide them along the decision-making process.1 The ICF checklist may be used for informed consent forms.1,4
In a recent study,10 the two checklists were used to evaluate the formal quality of 21 PIS’s/ICFs used in CTs conducted by our hospital’s Neurology Service. The results showed the need to improve PIS’s/ICFs. Ruiz de Hoyos et al.11 developed and validated a questionnaire intended to analyze the informed consent process from the participant’s point of view.
The design of checklists and similar instruments is a dynamic process. For that reason, it is indispensable to state the date when each version was completed as well as the corresponding version number, so that the necessary updates can be made when new regulations are introduced. Although this aspect may be considered a limitation tothe use of checklists, these instruments may be used and kept up-to-date in Spain and in other Spanish-speaking countries like Peru,12 Ecuador,13 and Argentina,14 with due regard to the specificities of each country.
Finally, it can be said that the procedure followed for the design and validation of the two checklists, as well as the reliability levels achieved, confirm that both are valid and reliable and can be safely used by professionals dedicated to preparing, analyzing and evaluating PIS’s/ICFs.
Authorship statementAGJV, MAC, MGP, ELM and PM participated in the conception and design of the manuscript as well as in the collection, analysis and interpretation of the data and in the drafting, review and approval of the final version submitted for publication.
FundingNo funding.
Contribution to the literatureThis study provides valid instruments in accordance with current regulations for use by professionals involved in the informed consent process of clinical trials with medicinal products.
These instruments will help to analyse and ensure the formal quality of the Participant Information Sheet and Informed Consent for clinical trials with medicinal products.
The authors would like to thank Idcsalud and the General University Hospital of Catalunya for their support with the validation of the checklists. They are also indebted to the Secretariat for Higher Education, Science, Technology and Innovation (SENESCYT) of Ecuador for granting AGJV a scholarship to read the official Master’s program in Medicines, Health and Healthcare System of the University of Barcelona.