To describe the marginal cost and survival of patients treated with tocilizumab in a university hospital under real-life conditions and to evaluate factors that could influence costs and health outcomes will be evaluated.
MethodsObservational, single-center, retrospective study of a cohort of adult patients infected with SARS-COV2 treated with tocilizumab. The 1 year restricted mean survival time was analyzed in life-years gained (LYG). The influence of sex, age and severity on patient survival was evaluated. The marginal cost/LYG and marginal cost/survivor ratios were calculated.
Results508 patients (66 ± 13 years; 32% women) were included. Seventeen percent were admitted to the ICU. Overall survival was 77%. Age older than 71.5 years (HR = 1.08; 95% CI 1.07–1.10; p < 0.001) and ICU admission at initiation of treatment (HR = 2.01; 95% CI 1.30–3.09; p = 0.002) were identified as risk factors. The total budgetary impact of tocilizumab in the period analyzed was 206,466 euros. The patients with the highest cost per unit of health outcome were those admitted to the ICU and those over 71.5 years, with a marginal cost/LYG of €966 and a marginal cost/survivor of €1136.
ConclusionThe efficiency of treatment with tocilizumab is associated with the age and severity of the patients. The figures are lower in all subgroups than the thresholds usually used in cost-effectiveness evaluations. The results of the present study suggest that early first dose of tocilizumab is an efficient strategy.
describir el coste marginal y la supervivencia de los pacientes tratados con tocilizumab en un hospital universitario en condiciones de vida real. Y evaluar los factores que podrían influir los costes y los resultados en salud.
Metodologíaestudio observacional, retrospectivo y unicéntrico de una cohorte de pacientes adultos infectados con SARS-COV-2 tratados con tocilizumab. Se analizó, en años de vida ganados (AVG), la media de supervivencia restringida a un año. Se evaluó la influencia del sexo, la edad y la gravedad en la supervivencia de los pacientes. Se calcularon el ratio coste marginal/AVG y coste marginal/superviviente.
Resultadosse incluyeron 508 pacientes (66 ± 13 años; 32% mujeres). Un 17% ingresó en UCI. La supervivencia global fue del 77%. Se identificaron como factores de riesgo la edad mayor de 71,5 años (HR = 1,08; IC 95% 1,07–1,10; p < 0,001), y el ingreso en UCI al iniciar el tratamiento (HR = 2,01; IC 95% 1,30–3,09; p = 0,002). El impacto presupuestario total de tocilizumab en el periodo analizado ascendió a 206.466 €. Los pacientes con mayor coste por unidad de resultado en salud son los pacientes ingresados en UCI y mayores de 71,5 años, que presentan un coste marginal/AVG de 966 € y un coste marginal/superviviente de 1.136 €.
Conclusiónla eficiencia del tratamiento con tocilizumab se asocia a la edad y a la gravedad de los pacientes. Las cifras son inferiores en todos los subgrupos a los umbrales habitualmente utilizados en las evaluaciones coste-efectividad. Los resultados del presente estudio sugieren que el inicio precoz de tocilizumab es una estrategia eficiente.
The disease caused by SARS-CoV-2 has different manifestations and clinical phases (viral, pulmonary, and immunologic).1 Pharmacological treatment is based on the use of antiviral drugs, immunomodulators, and others to combat the associated complications. During the viral phase the therapeutic objective is to reduce the viral load. Remdesivir is the most widely used antiviral drug. However, evidence on its use is modest and only shows a decreased recovery time in hospitalized patients.2
The viral phase is followed by a state of systemic hyperinflammation, which, in the most severe patients, can be accompanied by cytokine release syndrome with elevated cytokines such as IL-6, IP10, MCP1, TNF-α, IL-2R, and IL-10.3,4 During this inflammatory state, monocytes, macrophages, and dendritic cells become dysregulated leading to the accumulation of macrophage infiltrates at the pulmonary level and causing hemophagocytic syndrome.5 The use of systemic corticosteroids, such as dexamethasone, has been shown to reduce mortality in critically ill patients.6 Interleukin-6 appears to play an important role in regulating the immune response in patients with COVID. The high concentration of IL-6 observed in critically ill patients makes its pharmacological antagonists potential candidates for treating this phase of the disease. However, the inflammatory response is complex and multifactorial. Other signaling pathways are involved, such as the JAK1/2-STAT pathway that participates in the macrophage activation process toward the M2 profile after IL-4R activation.7 This process generates another new therapeutic target for JAK1/2 inhibitors, such as baricitinib, in the inflammatory phase of COVID patients. During the course of the pandemic, other immunomodulatory drugs have been used, such as ruxolitinib, anakinra, and colchicine.8,9
Tocilizumab is a humanized monoclonal antibody that binds to soluble and membrane-bound IL-6 receptors (IL6-R) and is indicated for its use in rheumatoid arthritis and juvenile idiopathic arthritis. The standard doses used in rheumatoid arthritis are 4 mg/kg or 8 mg/kg intravenous every 4 weeks. Tocilizumad can also be administered via the subcutaneous route at a dosage of 162 mg once every week.10 Given the pleiotropic nature of IL-6R and its key role in controlling inflammation, it is used as a treatment for the cytokine release syndrome that occurs after chimeric antigen receptor T (CAR-T) cell therapy. Tocilizumab has been proposed as a potential therapeutic agent given the similarities between this clinical picture and that caused by SARS-COV2.11 However, the results of published studies are inconsistent and raise questions concerning the effectiveness of tocilizumab. It has been shown that tocilizumab does not improve the survival of patients with moderate disease.12,13 Nevertheless, in critically ill patients, IL-6R blockade with tocilizumab (or sarilumab) improves survival, thus demonstrating clear clinical benefit in these patients.
The COVID-19 pandemic continues to have a major impact on health care systems and the economic impact of therapeutic strategies needs to be assessed.15 The main costs of hospitalization are expenditures on drugs and on laboratory and imaging diagnostic tests.16 For these reasons, it is relevant to conduct an economic analysis of the cost of the therapeutic options used in routine clinical practice.
The main objective of this work was to describe the marginal costs and survival of patients treated with tocilizumab in a university hospital under real-life conditions and to assess the factors that could influence costs and health outcomes.
Material and methodsWe conducted a retrospective single-centre observational study of a cohort of hospitalized patients with COVID-19 who received at least one dose of tocilizumab during the study period (February 2020–February 2021). The study was approved by the Clinical Ethics Committee of the Hospital Clínico Universitario de Valencia (hereafter, the Hospital).
Inclusion criteria were as follows: patients older than 18 years with a diagnosis of SARS-CoV-2 infection confirmed by PCR or antigen test; or clinical evidence of COVID-19 pneumonia and admission to inpatient units. Patients were stratified according to their level of severity: a) critical patients (hospitalization in intensive care units [ICU]); and b) non-critical patients (conventional hospitalization in medical units [HU]). Patients were classified at the time of the first administration of tocilizumab. Patients who received tocilizumab for rheumatologic or hematologic indications during the study period were excluded. Patients weighing less than 75 kg received 400 mg tocilizumab and those weighing more than 75 kg received 600 mg as a single dose, although successive doses were allowed if required by the clinical situation. We did not set a maximum number of tocilizumab doses. Tocilizumab was dispensed and recorded on an individualized basis.
Survival was assessed from the time of administration of the first dose of tocilizumab until the end of follow-up or death due to COVID-19. Patients who died from causes unrelated to the event of interest were scored as censored. When exact survival times were not known or the event did not take place, the data were also scored as censored. Survival was analyzed using the Kaplan–Meier method and 1-year restricted median survival time (RMST). COX regression analysis was performed to identify possible risk factors associated with survival: age, sex, and hospitalization area (ICU/HU). To categorize the variable age, a ROC curve was previously constructed to identify the cut-off point with the highest sensitivity and specificity as determined using the Youden index. Statistical analyses were conducted using SPSS software v.22.
The cost analysis was conducted from the perspective of funders, with the acquisition cost of tocilizumab in euros being considered to be the only marginal cost. Depending on the availability of the different forms, we used 80 mg, 200 mg, and 400 mg vials of tocilizumab at a cost of €114, €285, and €573, respectively. No discount rate was applied, since the time horizon was restricted.
Life-years gained (LYG) were calculated as the product of the RMST multiplied by the number of patients in each subgroup analyzed. The number of survivors was calculated as the product of the probability of survival at the end of the study multiplied by the number of patients in each subgroup analyzed. Efficiency was estimated by calculating the marginal cost/LYG ratio and the marginal cost/survivor ratio. Research project file number 85/21 was approved on April 31, 2021, by the Clinical Ethics Committee of the Hospital.
ResultsIn total, 508 patients fulfilled the inclusion criteria. Mean age was 66 ± 13 years and 32% were female. Eighty-seven patients (17%) were classified as critically ill at the time of the first administration of tocilizumab. Overall survival for all the study patients was 77%.
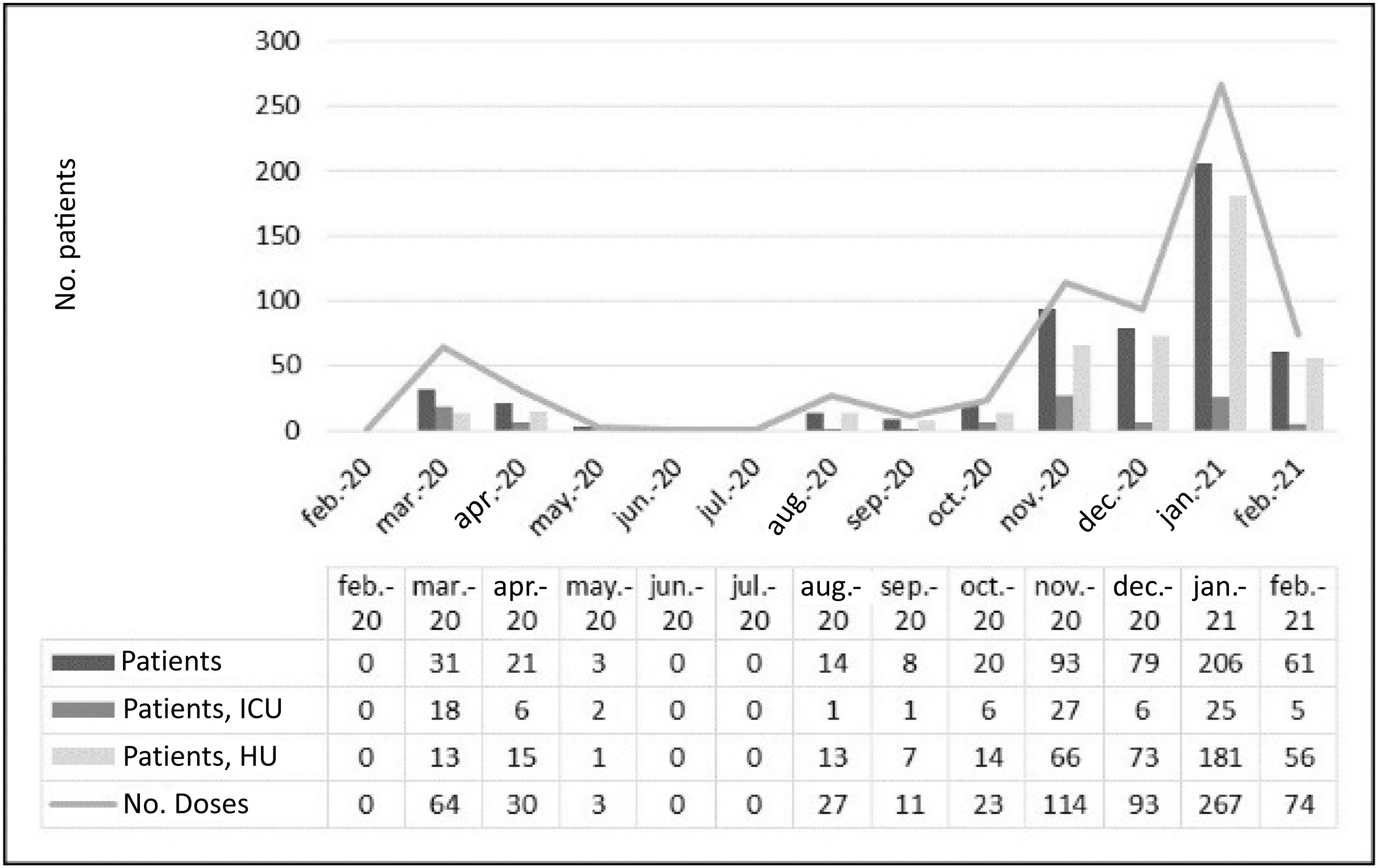
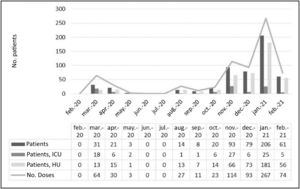
During the study period, the Hospital was differentially affected by the successive waves of the pandemic (Fig. 1). The number of patients treated with tocilizumab was clearly higher from November 2020 to January 2021, which was also the worst period of the pandemic. The proportion of patients admitted to the ICU was much higher during the first wave (March 2020 – May 2020) than in the second (August 2020 – February 2021). However, regarding survival, no statistically significant differences (P = 0.97) were found between the two periods (74% and 77%, respectively).
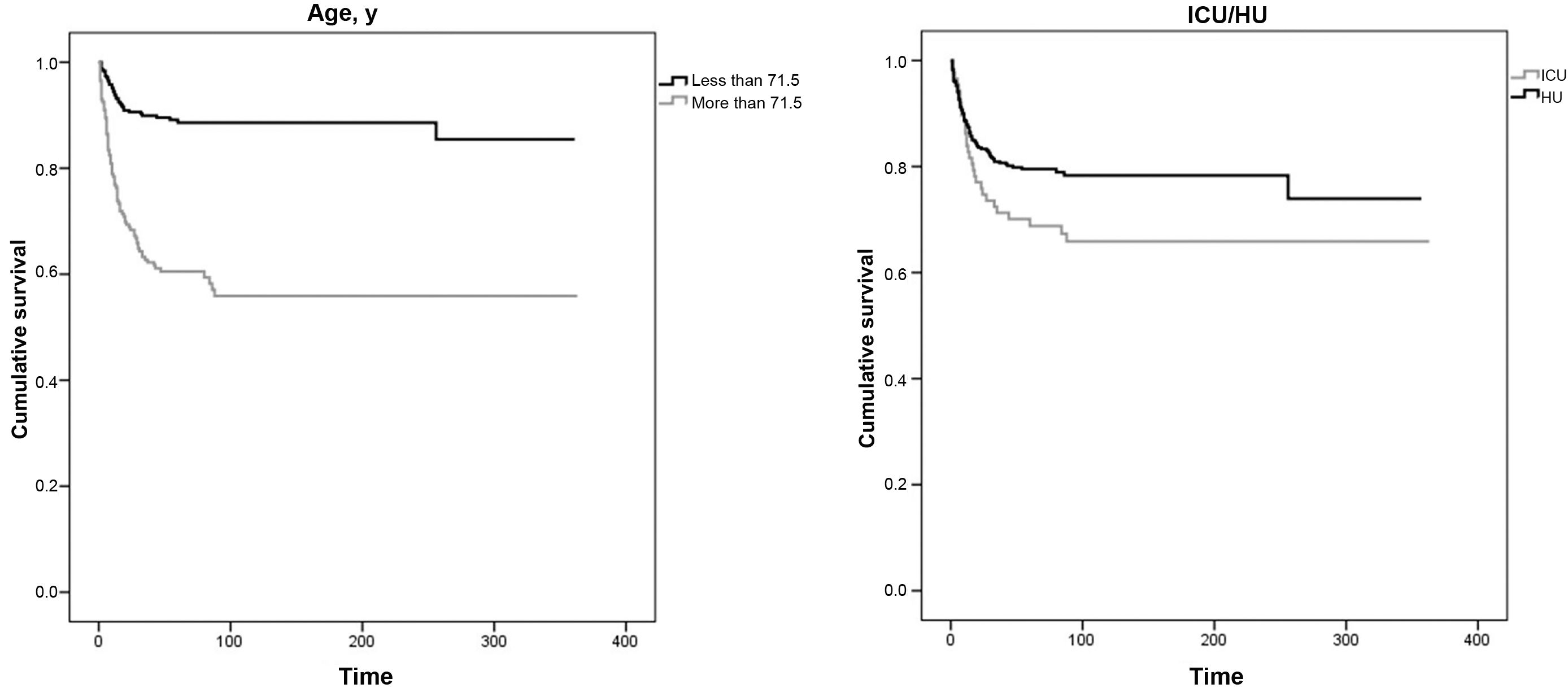
The COX regression analysis (Fig. 2) identified patients' level of severity and age as risk factors for death. The sex variable did not appear to affect mortality in the study population. Over the time period analyzed (1 year), the median survival rate was not reached in any of the groups. Admission to the ICU or HU was used as a surrogate variable for clinical severity (hazard ratio [HR] = 2.01, 95% confidence interval [95% CI]: 1.30–3.09; P = 0.002]). Regarding age, the aforementioned ROC curve analysis indicated that the optimal cut-off point was 71.5 years (sensitivity and specificity = 0.701). The COX regression analysis showed that being more than 71.5 years of age had an HR of 1.08 (95% CI: 1.07–1.10; P < 0.001).
Survival analysis by age (leftmost figure) and by severity of the patients' condition (rightmost figure) according to admission to intensive care units or conventional hospitalization units.
Note: Over the time horizon analyzed (1 year), the median survival rate was not reached in any of the groups. Clinical severity of the process (ICU/HU): HR = 2.01 (95%CI: 1.30–3.09; P = 0.002); Age more than 71.5 years: HR = 1.08 (95%CI: 1.07–1.10; P < 0.001).
Abbreviations: ICU, intensive care unit; HU, hospital medical units
At the end of the study period, patients of more than 71.5 years of age (total = 309; 70%) had a statistically much lower survival rate (59%; P < 0.001) than that observed (89%) in younger patients.
The survival analysis stratified according to the severity of the clinical picture (ICU or HU), showed that patients admitted to the ICU had a statistically lower survival rate (68%; P = 0.037) than that of patients hospitalized in HUs (79%).
Mortality at day 90 after tocilizumab administration was 33% in patients admitted to ICUs and 21% in patients admitted to HUs. Mortality was 41% in patients more than 71.5 years of age vs 11% in patients less than 71.5 years of age.
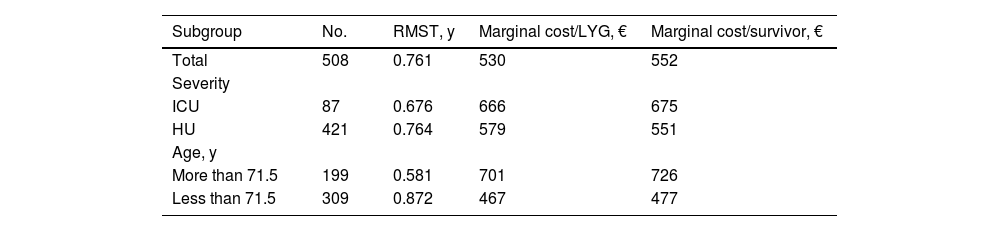
Over the study period, the acquisition cost of tocilizumab remained almost the same. During this period, the total budgetary impact of tocilizumab was €206,466. However, the marginal cost per LYG or per survivor varied by subgroup (Table 1). The greatest differences in efficiency were found between the subgroup of patients of more than 71.5 years (€701/LYG and €726/survivor) and patients of less than 71.5 years (€467/LYG and €478/survivor).
One-year restricted mean survival time for all patients and each subgroup by severity and age, and marginal cost/life-years gained and marginal cost/survivor in euros by subgroup.
| Subgroup | No. | RMST, y | Marginal cost/LYG, € | Marginal cost/survivor, € |
|---|---|---|---|---|
| Total | 508 | 0.761 | 530 | 552 |
| Severity | ||||
| ICU | 87 | 0.676 | 666 | 675 |
| HU | 421 | 0.764 | 579 | 551 |
| Age, y | ||||
| More than 71.5 | 199 | 0.581 | 701 | 726 |
| Less than 71.5 | 309 | 0.872 | 467 | 477 |
Abbreviations: RMST, restricted median survival time; LYG, life-years gained; ICU, intensive care unit; HU, hospital medical unit.
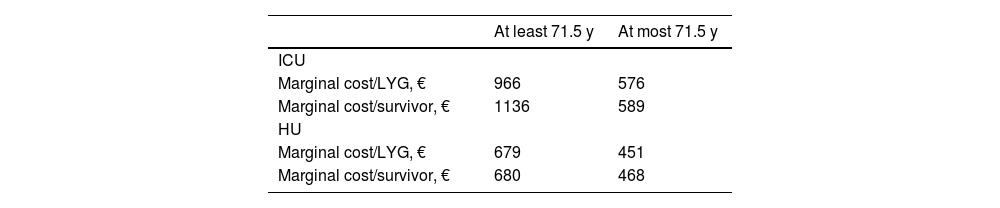
The stratified analysis showed that the highest costs per unit of health outcome were associated with patients admitted to the ICU and patients of more than 71.5 years of age (marginal cost/LYG = €966; marginal cost/survivor = €1136) (see Table 2).
Efficiency of treatment with tocilizumab. Stratified analysis by age and level of severity, marginal cost/life-years gained, and marginal cost/survivor.
| At least 71.5 y | At most 71.5 y | |
|---|---|---|
| ICU | ||
| Marginal cost/LYG, € | 966 | 576 |
| Marginal cost/survivor, € | 1136 | 589 |
| HU | ||
| Marginal cost/LYG, € | 679 | 451 |
| Marginal cost/survivor, € | 680 | 468 |
Note: Patients at the highest risk of mortality and patients more than 71.5 years of age admitted to the ICU had the highest marginal cost/LYG ratio (€966) and highest marginal cost/survivor ratio (€1136).
Abbreviations: LYG, life-years gained; ICU, intensive care unit; HU, hospital medical unit.
The results show that negative prognostic factors for survival were being more than 71 years of age and the severity of the clinical condition at the start of tocilizumab treatment. It is relevant to assess these factors given the disparities in efficacy results obtained in clinical trials, which have reported similar mortality rates with tocilizumab treatment vs placebo or best standard treatment.12,13,17–19 Other efficacy parameters, such as the decreased risk of ICU admission or mechanical ventilation, also show heterogeneous results. Nevertheless, the REMAP-CAP investigators found that treatment with tocilizumab improved outcomes, with a significant decrease in mortality in critically ill patients receiving respiratory or cardiovascular support.14 Mortality in the critically ill patients in our study was slightly higher than that reported in the REMAP-CAP study. This result may be due to the high care pressure on the ICU and the level of disease severity in the patients over the study period. A post-hoc analysis found a mortality rate of 11% on day 90 in patients treated with tocilizumab without mechanical ventilation.20 The authors suggested that tocilizumab could be a good option for these types of patients when they also have C-reactive protein (CRP) values of less than 150 mg/L. Taking into account the mortality rates of patients less than 71.5 years of age in our study, it is to be expected that this population would be the one with the best survival results.
The disparate results obtained in these studies is due to the variability of the inclusion criteria, time of initiation of treatment with tocilizumab, and other factors, such as associated treatments received by the patients.21 Therefore, it is relevant to document the reality of patient prognosis in routine clinical practice. One of the key points was the inclusion of systemic corticosteroid therapy following evidence of improved survival in July 2020.6 Clinical management was another factor that distinguished the different waves of the disease. This factor was more uncertain during the first wave, when therapeutic strategies such as hydroxychloroquine, interferon-beta, or the combination lopinavir/ritonavir were applied. These approaches finally fell into disuse after having demonstrated a lack of efficacy.22,23
Due to their poorer prognosis, the population with the highest cost per LYG were critically ill patients of more than 71.5 years of age. However, the potential benefits of treatment in terms of reduced ICU stay and decreased total hospital admission time could potentially outweigh the higher marginal cost observed. The mean age of our cohort was similar to that reported in the REMAP-CAP study.14 These results are in line with those recently reported in a meta-analysis that included more than 10,000 patients.14 This meta-analysis found that the risk of death was lower in the tocilizumab group than in the patients receiving placebo or best standard patient care. It also found treatment benefit with tocilizumab in patients of more than 70 years of age: this age is similar to that found in our study as a cut-off point. The meta-analysis also found a mortality rate of 40% at day 28 in this group of patients: this result is also similar to the mortality rate found in our cohort.
Although in absolute terms the difference in costs may appear insignificant, in relative terms the cost/survivor ratio is 140% higher in ICU patients older than 71.5 years than that in HU patients less than 71.5 years of age. In the setting of a global pandemic context, this difference may become of increasing relevance in terms of its budgetary impact, particularly in the face of possible tocilizumab shortages that could arise in the future.
In addition to the direct costs associated with the acquisition of tocilizumab, other direct health care costs could be modeled or analyzed, especially those due to reductions in the risk of admission to the ICU and reductions in hospital stay.24 However, this possibility is beyond the scope of the present study, which addressed the efficiency of use of tocilizumab in real-life clinical practice and quantified the marginal cost/LYG and marginal cost/survivor in real clinical practice. The results should be taken into account when selecting the optimal treatment at each moment and for each patient. This process could include other treatment options, such as baricitinib, that have also been shown to improve the prognosis of COVID-19 patients.25
In summary, the efficacy of treatment with tocilizumab in COVID-19 patients remains uncertain in reducing the severity of the disease and in improving survival.26 The results according to clinical severity at the start of treatment show differences in survival between patients receiving tocilizumab in ICUs and those receiving treatment in HUs. Similarly, survival rates were higher in patients less than 71.5 years of age.
We found an association between efficiency of treatment and age and the patients' level of severity. However, the values were much lower than the thresholds typically used in cost-effectiveness assessments in all the subgroups in the study. Consequently, the results of the present study suggest that early initiation of tocilizumab is an efficient strategy.
Presentation at scientific meetingsThe abstract of this study has been previously presented and accepted at the 26th EAHP Congress.
Ethical responsibilitiesThe research project with file number 85/21 was approved by the CEIm of the approved on 31/05/2021 by the CEIm of the Hospital Clínico Universitario of Valencia.
Declaration of authorshipAs the main author of this study, I declare that I participated in its design, and data collection and analysis, and in its writing, the preparation of the tables and figures, and the preparation of the document for its submission.
The second author, Manuel Alós Almiñana, participated in its design, and supervised the statistical analysis, the results, and writing the final article, having participated in its revision and in the supervision of the study.