The Pharmacotherapeutic follow-up program (PFU) carried out by the clinical pharmacist can be categorized within 3 fundamental activities; identification, resolution and prevention of adverse drug events. These must be adjusted to the requirements and resources of each institution, developing procedures to increase PFU efficiency and to guarantee patient safety. The clinical pharmacists of UC-CHRISTUS Healthcare Network developed a Standardized Pharmacotherapeutic Evaluation Process (SPEP). The main goal of our study is to evaluate the impact of this tool through the pharmacist evaluation number and pharmacist interventions number. Secondarily to determine the potential and direct cost savings associated with the pharmacist interventions in an Intensive care unit (ICU).
MethodsA quasi-experimental study evaluated the frequency and type of pharmacist evaluation and pharmacist interventions performed by clinical pharmacists in adult patients units of UC-CHRISTUS Healthcare Network, before and after the implementation of SPEP. The distribution of variables was evaluated using the Shapiro–Wilk test and the association between the use of SPEP and the pharmacist evaluation and pharmacist interventions number was performed using the Chi-square test. The cost evaluation associated with pharmacist interventions in the ICU was carried out using methodology proposed by Hammond et al.
ResultsA total number of 1781 patients was evaluated before and 2129 after the SPEP. The pharmacist evaluation and pharmacist interventions number in the before-SPEP period were 5209 and 2246. In the after-SPEP period were 6105 and 2641, respectively. The increase in both the pharmacist evaluation and pharmacist interventions number was significant only in critical care patients. The potential cost saving in after-SPEP period in the ICU was USD 492,805. Major adverse drug events prevention was the intervention that generated the most savings with a reduction of 60.2%. The total direct savings for sequential therapy was USD 8072 in the study period.
ConclusionsThis study shows a clinical pharmacist developed tool called SPEP that increased the pharmacist evaluation and pharmacist interventions number in multiple clinical scenarios. These were significant only in critical care patients. Future investigations should make effort to evaluate the quality and clinical impact of these interventions.
El Seguimiento Farmacoterapéutico (SFT) realizado por el farmacéutico clínico puede enmarcarse dentro de 3 actividades; la identificación, resolución y prevención de eventos adversos a medicamentos. Éstas deben ajustarse a los requerimientos y recursos de cada institución, generando la necesidad de desarrollar procedimientos que aumenten la eficiencia del SFT y garantizan seguridad del paciente. Los farmacéuticos clínicos de la Red de Salud UC-CHRISTUS Chile desarrollamos un Proceso Estandarizado de Evaluación Farmacoterapéutica (PEEF). El objetivo principal del estudio fue evaluar el impacto de esta herramienta en términos del número de evaluaciones e intervenciones de los farmacéuticos clínicos y secundariamente determinar el ahorro de costos potenciales y directos asociados a las intervenciones en la Unidad de Cuidados Críticos (UCI).
MétodoEstudio cuasi-experimental que evaluó la frecuencia y tipo de evaluaciones e intervenciones realizadas por los farmacéuticos clínicos en unidades de paciente adulto de la Red UC-CHRISTUS, previo y posterior a la utilización del PEEF. La distribución de variables se evaluó mediante el test Shapiro–Wilk, la asociación entre el uso del PEEF y el número de evaluaciones e intervenciones fue realizada mediante test Chi cuadrado. La evaluación de costos asociados a las intervenciones del farmacéutico clínico en UCI se realizó utilizando la metodología propuesta por Hammond y cols.
ResultadosEl total de pacientes evaluados pre y post PEEF fue de 1.781 y 2.129, respectivamente. Las evaluaciones e intervenciones en el periodo pre-PEEF fueron 5.209 y 2.246, en el periodo post-PEEF fueron 6.105 y 2.641, respectivamente. El aumento de las evaluaciones como de las intervenciones fue significativo sólo en las unidades de mayor complejidad. La reducción potencial de costos estimados en el periodo post-PEEF en UCI fue de 492.805 dólares americanos. La intervención que más ahorro generó fue la prevención de eventos adversos mayores (reducción del 60,2%). El ahorro directo total por terapia secuencial fue de 8.072 dólares americanos en el periodo de estudio.
Conclusiones: Esta investigación demuestra que la utilización del PEEF permite aumentar el número de evaluaciones e intervenciones del farmacéutico clínico en diferentes servicios clínicos, siendo significativo en unidades de mayor complejidad. Se sugiere en futuras investigaciones evaluar la calidad y el impacto clínico de estas intervenciones.
A pharmacist is the healthcare professional responsible for ensuring the safe use of medication. Clinical pharmacy is a branch of pharmacy defined as “a health science discipline, in which pharmacists provide patient care that optimizes medication therapy and promotes health, wellness and disease prevention”1. Pharmacotherapeutic follow-up (PFU) is one of the activities carried out by clinical pharmacist practitioners (CPPs) to optimize the clinical benefit of medications2,3. CPPs perform a broad variety of duties primarily aimed at optimizing medication use, with special focus on dosing, monitoring, detection of adverse drug reactions, and ensuring cost-effectiveness in order to improve health outcomes4,5. The activities and duties of a CPP are categorized into three crucial functions: identification, management and prevention of adverse drug reactions (ADRs)6,7.
Although there is solid evidence that CPP's interventions reduce the incidence of ADRs 8, they are faced with some barriers. Years of practice demonstrate that, in most cases, CPP duties are determined by the requirements and resources available in each center. Thus, it is necessary that standard operating procedures are developed for pharmacotherapeutic follow-up. This initiative would create standards of practice that will ensure the quality and safety of healthcare services6.
There is a limited number of tools available for training inexperienced CPPs in PFU activities. The FASTHUG-MAIDENS protocol helps the CPP adopt a stepwise approach to the identification of ADRs. The use of this protocol reduces anxiety and concerns among inexperienced professionals working in intensive care units (ICUs)9. In contrast, the Dader method establishes standard operating procedures for CPPs conducting PFU, whatever the level of healthcare6.
It is important to note that the tools currently available do not consider limitations related to the human and technological resources available at each center. Therefore, their applicability may be limited by the local resources available. The CPPs of the UC-CHRISTUS health network in Chile developed a Standardized Pharmacotherapeutic Follow-Up Process (PEEF) based on the activities described in the literature and adapted to the needs and resources available in our center.
The primary objective of the study was to assess the impact of PEEF on the number of drug therapy evaluations and interventions performed by CPPs. Our secondary objective was to estimate potential and direct cost savings resulting from drug therapy interventions in the Intensive Care Unit (ICU).
MethodsA quasi-experimental, before/after study was conducted to assess the frequency and types of drug therapy evaluations (DTEs) carried out by four CPPs serving at different hospital units of adult patients of the UC-CHRISTUS Clinical Hospital and at the Outpatient Unit Cancer Center of the UC-CHRISTUS health network before and after the implementation of the PEEF protocol. UC-CHRISTUS is a high-complexity hospital located in Santiago de Chile that is equipped with 420 beds, of which 331 are for adult patients. This center hosts an Outpatient Cancer Center with specialists in solid tumors and hematological cancer. The first study period extended from July 2018 to February 2019. The second study period was from May 2019 to December 2019. The study included the Clinical Units of the UC-CHRISTUS health network where a CPP was available, namely: The Medical-Surgical Unit (MSU), Intermediate Care Unit (IMCU), Intensive Care Unit (ICU), and the Outpatient Medical Oncology Unit (OMOU).
PEEF classifies drug therapy evaluation activities into nine categories, namely: 1) thromboprophylaxis (TPF): initiation, discontinuation, dose adjustment or change of anticoagulant; 2) gastric stress ulcer prophylaxis (GSUP): initiation, discontinuation, dose adjustment or change of antisecretory agent; 3) dosing and therapeutic drug monitoring (TDM): dose adjustment for renal function, renal replacement therapy (RRT), liver function and monitoring of plasma drug concentrations; 4) administration: indication of the form of administration, stability and storage of drugs; 5) drug interactions: initiation, discontinuation or change of dose of one or more drugs due to a clinically relevant interaction; 6) monitoring of toxicities and adverse drug reactions (ADRs): prevention, management or reporting of ADRs to the national regulatory authority; 7) sequential therapy (ST): change from intravenous to enteral formulation; 8) indication/reconciliation: initiation, discontinuation or resuming a medication for acute or chronic condition; 9) information: response to queries through a literature search or administrative management.
Drug therapy evaluation (DTE) was defined as the process of reviewing and analyzing the drug therapy of a patient. Drug therapy intervention (DTI) was defined as a suggestion from the CPP to the clinical team. An intervention was considered to have been accepted if it resulted in a change in the indication or use of a drug therapy.
Quantitative variables are presented as means and standard deviation or as interquartile range. Qualitative variables are expressed as frequencies. The distribution of variables was assessed using Shapiro–Wilk test. The association between the use of PEEF and the number of drug therapy evaluations and interventions was assessed using Chi square test. A p value < 0.05 was considered statistically significant.
Evaluation of costs in the post-intervention periodThe reduction of costs resulting from drug therapy interventions of the CPP in the ICU was estimated using the method designed by Hammond et al.10 This method has only been validated for ICU patients; therefore, results cannot be generalized to other patients. Costs savings resulting from sequential therapy (ST) were estimated based on data provided by our hospital. The drug therapies selected for analysis included omeprazol, levetiracetam and paracetamol. These drugs were chosen on the basis of the significant cost difference between the intravenous and oral forms. Data on drug therapy use was extracted from the pharmacy information system.
ResultsA total of 1781 and 2129 patients were included in the pre-intervention and post-intervention periods, respectively. The distribution by Unit was (pre-intervention/post-intervention): MSU (371/389), IMCU (531/729), ICU (293/397) and OMOU (586/614).
Based on historical data of our hospital, the MSU is the unit with the highest number of beds available per day (160), as compared to the IMCU and ICU, with about 30 beds available. About 48 patients are admitted to the OMOU daily. The majority of the patients admitted to the MSU, IMCU and ICU are polypharmacy patients (using more than 7 drugs), with an average age of 60 years, whereas the mean age of OMOU patients is near 50 years. With respect to the length of stay, it was shorter in the IMCU, with a mean of 3 days, as compared to the MSU and the ICU, with a mean length of stay of 7 to 9 days.
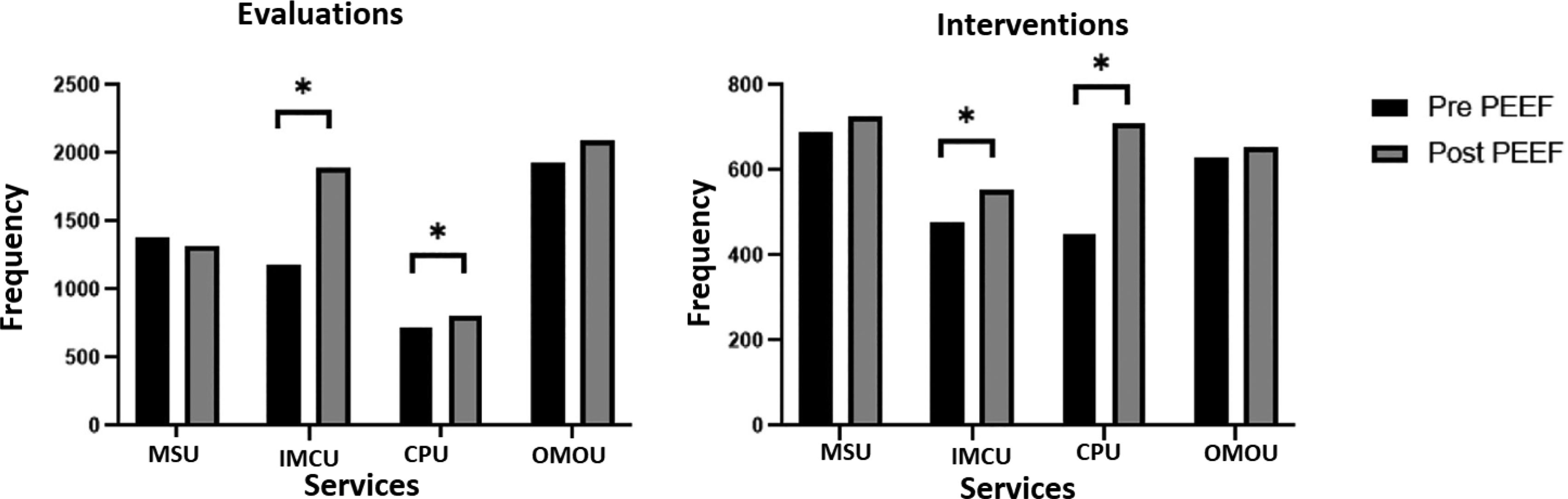
Total drug therapy evaluations and interventionsA total of 5209 drug therapy evaluations (DTEs) and 2246 interventions (DTIs) were performed during the pre-intervention period, as compared to 6105 and 2641 in the post-intervention period, respectively. As shown in Fig. 1, the Chi square test revealed a statistically significant increase in the number of DTEs and DTIs in the post-intervention period in the ICU and the IMCU (p value < 0.01 for the two units), as opposed to the MSU and OMOU (p value = 0.23 and p value = 0.97, respectively). A total of 713 DTEs and 450 DTIs were performed in the ICU, respectively, in the pre-intervention period vs 821 and 708 in the post-intervention period, respectively. In the IMCU, there were 1184 DTEs and 476 DTIs in the pre-intervention period vs 1894 and 552 in the post-intervention period.
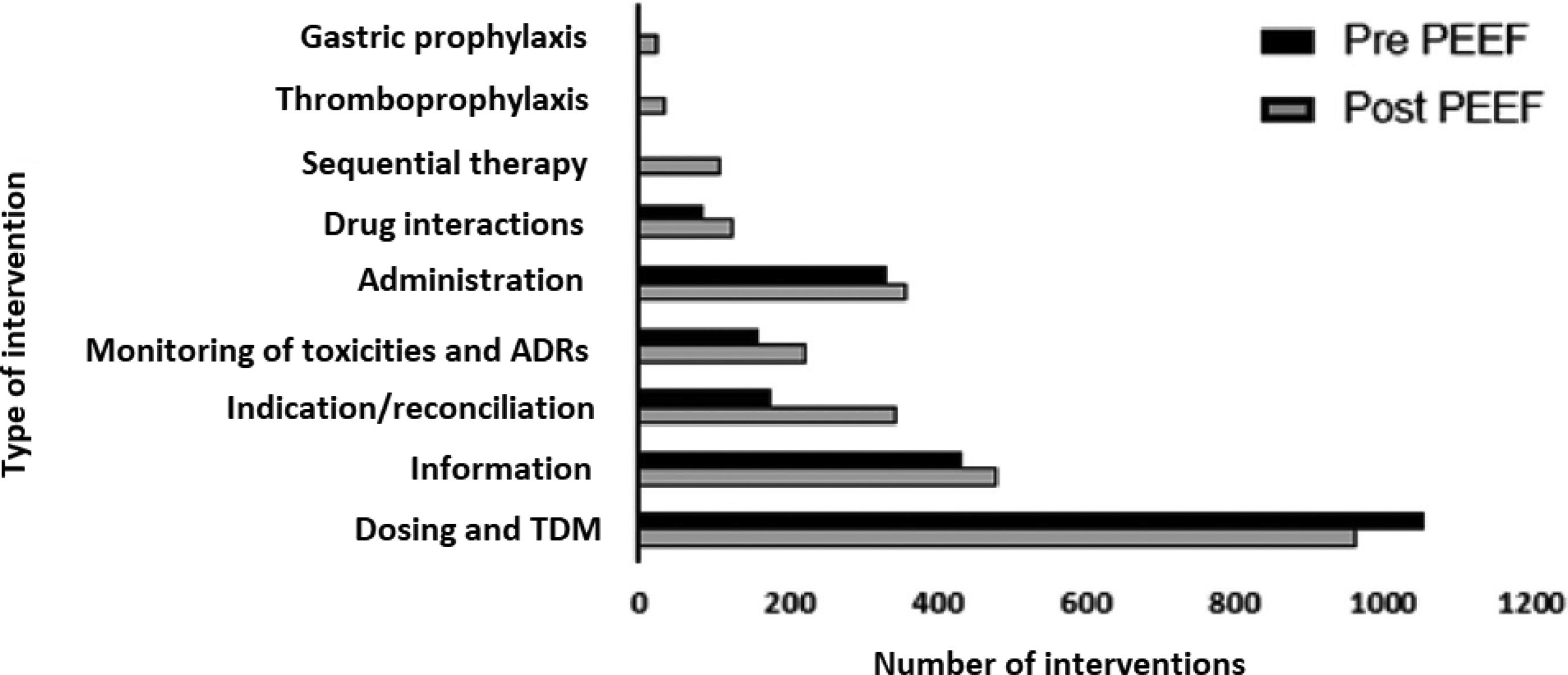
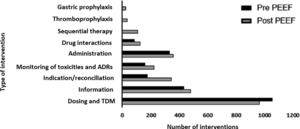
In relation to the frequency and type of interventions performed during the two periods (Fig. 2), an increase was observed in the frequency of DTIs following the implementation of the PEEF, except for dose adjustments and TDM. No data was available about DTIs of GSPU, TPF and ST during the pre-intervention period.
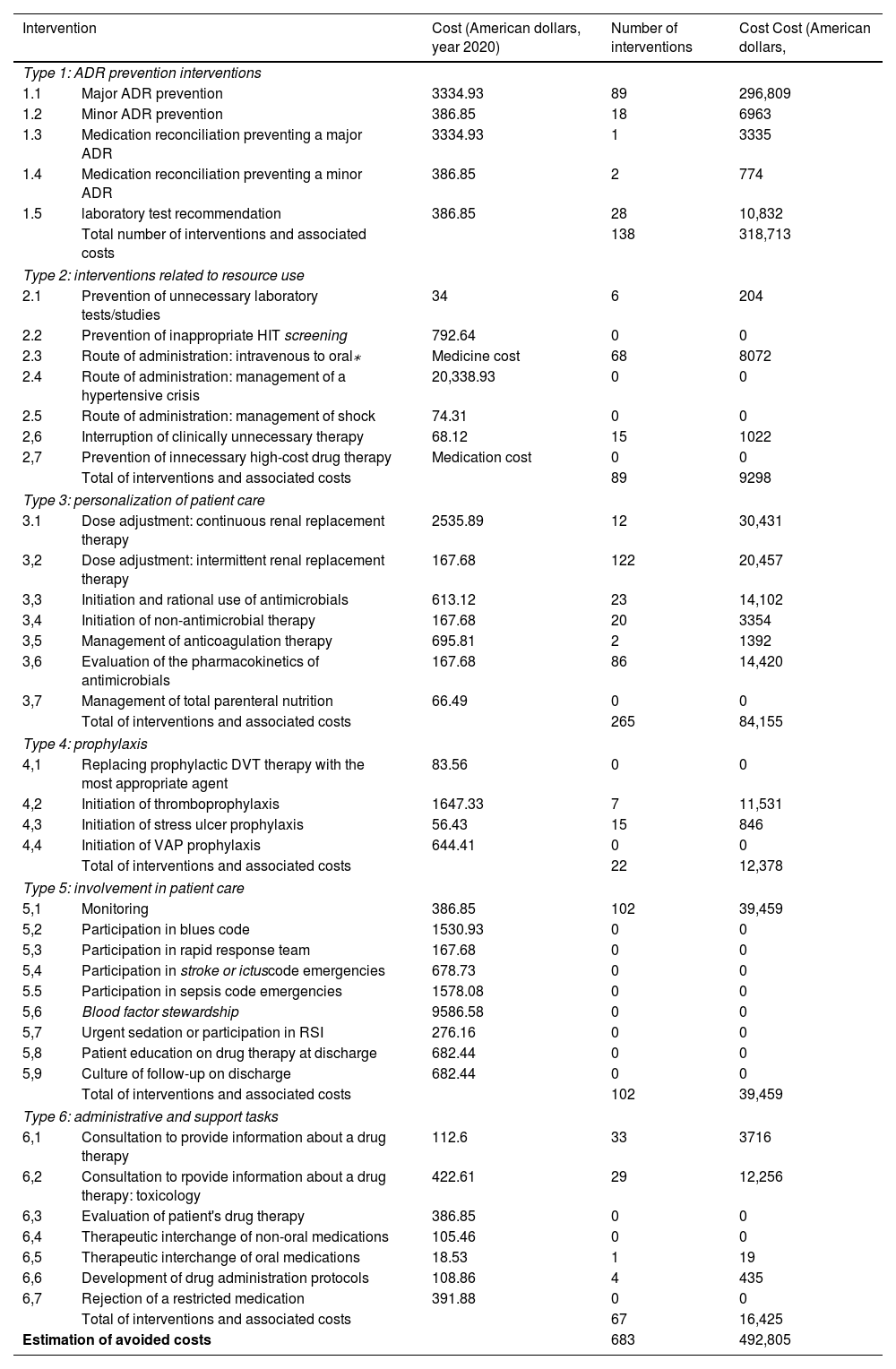
Cost analysis of drug therapyOf the total of interventions carried out by the CPP in the ICU, 683 were approved (96.5%), as shown in Table 1, based on the method developed by Hammond et al.10. Of the interventions accepted, 265 (38.8%) involved patient care personalization; 138 (20.2%) were categorized as interventions for the prevention of adverse drug reactions; 102 (14.9%) as involvement in patient care; 89 (13%) as interventions on resource use; 67 (9.8%) as administrative or support interventions; and 22 (3.2%) as prophylaxis.
Cost savings associated with CPP's activity in the ICU by type of intervention⁎.
| Intervention | Cost (American dollars, year 2020) | Number of interventions | Cost Cost (American dollars, | |
|---|---|---|---|---|
| Type 1: ADR prevention interventions | ||||
| 1.1 | Major ADR prevention | 3334.93 | 89 | 296,809 |
| 1.2 | Minor ADR prevention | 386.85 | 18 | 6963 |
| 1.3 | Medication reconciliation preventing a major ADR | 3334.93 | 1 | 3335 |
| 1.4 | Medication reconciliation preventing a minor ADR | 386.85 | 2 | 774 |
| 1.5 | laboratory test recommendation | 386.85 | 28 | 10,832 |
| Total number of interventions and associated costs | 138 | 318,713 | ||
| Type 2: interventions related to resource use | ||||
| 2.1 | Prevention of unnecessary laboratory tests/studies | 34 | 6 | 204 |
| 2.2 | Prevention of inappropriate HIT screening | 792.64 | 0 | 0 |
| 2.3 | Route of administration: intravenous to oral⁎ | Medicine cost | 68 | 8072 |
| 2.4 | Route of administration: management of a hypertensive crisis | 20,338.93 | 0 | 0 |
| 2.5 | Route of administration: management of shock | 74.31 | 0 | 0 |
| 2,6 | Interruption of clinically unnecessary therapy | 68.12 | 15 | 1022 |
| 2,7 | Prevention of innecessary high-cost drug therapy | Medication cost | 0 | 0 |
| Total of interventions and associated costs | 89 | 9298 | ||
| Type 3: personalization of patient care | ||||
| 3.1 | Dose adjustment: continuous renal replacement therapy | 2535.89 | 12 | 30,431 |
| 3,2 | Dose adjustment: intermittent renal replacement therapy | 167.68 | 122 | 20,457 |
| 3,3 | Initiation and rational use of antimicrobials | 613.12 | 23 | 14,102 |
| 3,4 | Initiation of non-antimicrobial therapy | 167.68 | 20 | 3354 |
| 3,5 | Management of anticoagulation therapy | 695.81 | 2 | 1392 |
| 3,6 | Evaluation of the pharmacokinetics of antimicrobials | 167.68 | 86 | 14,420 |
| 3,7 | Management of total parenteral nutrition | 66.49 | 0 | 0 |
| Total of interventions and associated costs | 265 | 84,155 | ||
| Type 4: prophylaxis | ||||
| 4,1 | Replacing prophylactic DVT therapy with the most appropriate agent | 83.56 | 0 | 0 |
| 4,2 | Initiation of thromboprophylaxis | 1647.33 | 7 | 11,531 |
| 4,3 | Initiation of stress ulcer prophylaxis | 56.43 | 15 | 846 |
| 4,4 | Initiation of VAP prophylaxis | 644.41 | 0 | 0 |
| Total of interventions and associated costs | 22 | 12,378 | ||
| Type 5: involvement in patient care | ||||
| 5,1 | Monitoring | 386.85 | 102 | 39,459 |
| 5,2 | Participation in blues code | 1530.93 | 0 | 0 |
| 5,3 | Participation in rapid response team | 167.68 | 0 | 0 |
| 5,4 | Participation in stroke or ictuscode emergencies | 678.73 | 0 | 0 |
| 5.5 | Participation in sepsis code emergencies | 1578.08 | 0 | 0 |
| 5,6 | Blood factor stewardship | 9586.58 | 0 | 0 |
| 5,7 | Urgent sedation or participation in RSI | 276.16 | 0 | 0 |
| 5,8 | Patient education on drug therapy at discharge | 682.44 | 0 | 0 |
| 5,9 | Culture of follow-up on discharge | 682.44 | 0 | 0 |
| Total of interventions and associated costs | 102 | 39,459 | ||
| Type 6: administrative and support tasks | ||||
| 6,1 | Consultation to provide information about a drug therapy | 112.6 | 33 | 3716 |
| 6,2 | Consultation to rpovide information about a drug therapy: toxicology | 422.61 | 29 | 12,256 |
| 6,3 | Evaluation of patient's drug therapy | 386.85 | 0 | 0 |
| 6,4 | Therapeutic interchange of non-oral medications | 105.46 | 0 | 0 |
| 6,5 | Therapeutic interchange of oral medications | 18.53 | 1 | 19 |
| 6,6 | Development of drug administration protocols | 108.86 | 4 | 435 |
| 6,7 | Rejection of a restricted medication | 391.88 | 0 | 0 |
| Total of interventions and associated costs | 67 | 16,425 | ||
| Estimation of avoided costs | 683 | 492,805 | ||
ADRs: Adverse drug reaction; HIT: Heparin Induced Thrombocytopenia; VAP: Ventilator-associated pneumonia; RSI: Rapid Sequence Intubation; DVT: Deep venous thrombosis.
Based on the method developed by Hammond et al.10, the potential reduction of estimated costs in the post-intervention period was $492,805 (Table 1). The most substantial cost savings were achieved with the following interventions: prevention of major adverse drug reactions, with a reduction of $296,809 (60.2%); ADR monitoring, with $39,459 (8%); and dose adjustment in continuous RRT, $30,431 (6.2%).
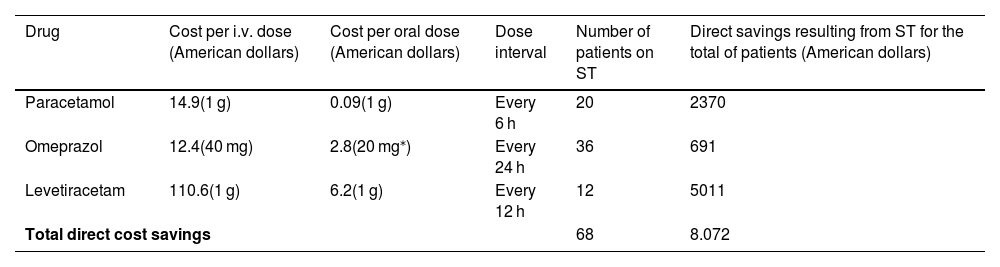
On another note, the total direct cost savings resulting from ST associated with the three medications evaluated was $8072 (Table 2). The most significant reduction resulted from switching from endovenous to oral levetiracetam.
Direct savings resulting from sequential therapy for 2 days of treatment.
| Drug | Cost per i.v. dose (American dollars) | Cost per oral dose (American dollars) | Dose interval | Number of patients on ST | Direct savings resulting from ST for the total of patients (American dollars) |
|---|---|---|---|---|---|
| Paracetamol | 14.9(1 g) | 0.09(1 g) | Every 6 h | 20 | 2370 |
| Omeprazol | 12.4(40 mg) | 2.8(20 mg⁎) | Every 24 h | 36 | 691 |
| Levetiracetam | 110.6(1 g) | 6.2(1 g) | Every 12 h | 12 | 5011 |
| Total direct cost savings | 68 | 8.072 | |||
IV: Intravenous; ST: sequential therapy; P.O: Oral/enteral route.
This study presents a new tool for the standardization of CPP activities in hospital services of different complexity, aimed at achieving a more efficient pharmaceutical care. These new operating procedures were named PEEF.
The FASTHUG-MAIDENS mnemonic was one of the tools used as a reference for the development of the PEEF. This method provides a structured, standardized approach to pharmaceutic evaluations by CPPs at the ICU9,11. PEFF integrates the general topics included in FASTHUG-MAIDENS that can be used by the CPP in different hospital units, including thromboprophylaxis, GSUP, drug therapy indication/reconciliation, dosing and TDM, and drug interactions12–18. PEFF also includes 4 categories of activities performed by CPPs that have been proven to have a significant clinical and economic impact. These topics include: drug administration, monitoring of toxicities and ADRs, reporting to the clinical team and ST19–24.
Following the implementation of PEEF, an increase was observed in the number of DTEs in three of the four services included in the study (Fig. 1). This increase occurred despite the rise in the number of patients treated in each service in the post-intervention period. The slight decrease in DTEs observed in the MSU may be explained by the fact that it is the unit with the lowest complexity and with the largest volume of patients, as compared to the other units. Thus, reaching 9 PEEF points in the MSU may require a longer time, which directly affects the volume of patients that can be evaluated in the same period of time.
There was an increase in DTIs in the four clinical services (Fig. 1). However, this increase was statistically significant only in high and intermediate complexity units. The reason for such an increase in the ICU and IMCU may be that the CPP can contact the medical team at any time. These units are closed services where the treating physicians are always available, which may facilitate participation of the CPP in medical consultations. Additionally, the complexity of these patients (polypharmacy, high-risk medication, supportive treatment, severity) required the intervention of the CPP more frequently25,26.
In contrast, in the MSU, where the number of beds available is five times higher than in the ICU/IMCU, the CPP had to select the patients that would benefit the most from their evaluation. Moreover, the CPP did not have access to the information system or electronic medical records. Finally, treating physicians were not always physically present, as they moved across the different wards that compose the MSU. This situation hindered communication and the implementation of drug therapy interventions.
DTEs did not increase significantly in the OMOU after the implementation of PEEF, since the CPP had the same opportunity to perform DTEs as in the pre-intervention period. The OMOU is a closed service where the same number of patients is admitted every day. In the post-intervention period, the volume of DTIs did not increase significantly in this Unit. This can be explained because only 6 in 9 points of the PEEF are applicable to ambulatory patients. An adapted version of PEEF for ambulatory patients is required to expand the number of pharmacy activities and services offered.
In relation to the type of DTI, the implementation of PEEF was associated with an increase in DTIs, except for dose-adjustment and TDM interventions (Fig. 2). This exception may be due to the fact that the primary activity and goals of the CPP prior to the implementation of the PEEF included adjusting doses to organ failure, and TDM. During this period, dose adjustment training guidelines were developed for physicians and nurses. The availability of guidelines, added to close cooperation with the CPP, provided more autonomy to the medical and nursing staff in drug-dosing decision-making.
Data for TPF, GSUP and ST was only available for the post-intervention period. Prior to the intervention, this type of interventions were not recorded and were categorized into other categories as an indication or administration. The implementation of PEEF did not only enable the correct categorization of these types of interventions, but they also started to be routinely recorded in a structured way, which led to an increase in DTIs10.
Cost analysisIn relation to cost savings, we could only assess the impact of DTIs in the ICU, since the method used was specifically developed for ICU patients10.
The estimated cost savings amounted to $492,805 in a period of 8 months following the implementation of PEEF. These results were consistent with those reported by Muñoz D. et al.27, who estimated a potential cost savings of $ 263,498 over a 12-month period in the ICU of a high-complexity hospital in southern Chile. Differences in net cost savings can be due to the weekly working hours of CPPs in the ICU, 44 and 22 h, respectively, and the number of beds available. The DTI that generated the most substantial cost savings was the prevention of major adverse drug reactions, defined in our study as dose adjustment, switch or discontinuation of a drug therapy due to the occurrence of drug interactions. The most frequent DTI was individualization of patient care, mainly dose adjustment in kidney failure, intermittent and continuous RRT and TDM.
Although the method developed by Hammond et al. has been used in previous studies based on the method of Hammond et al., this method assumes healthcare costs that are not generalizable to other hospitals. Such is the case of ST. In our study, we could only estimate cost savings resulting from changes of formulations. However, costs savings associated with the prevention of complications, as described by Hammond et al., could not be calculated (medical supplies, development of thrombophlebitis, catheter infection, etc)10,28–30.
Limitations and strengthsOne of the limitations of this study is that DTIs were recorded differently in the two study periods. In the pre-intervention period, the type of PI was recorded and described in more general terms. This hindered the categorization of interventions, which made it more difficult to estimate the associated cost savings in this period.
In addition, CPPs did not receive any training in the use of PEEF, what could have led to differences in pharmacy practices. For these reasons, data for March and April 2019 were excluded from analysis, as it is a period of adaptation to new pharmaceutical activities.
Finally, this study was conducted in a private healthcare care, which may affect the generalizability of results to public healthcare centers.
A strength of this study is that it involves a pharmacotherapeutic follow-up and evaluation tool for CPPs attending patients with different levels of complexity. The before/after design of the study enabled us to estimate the impact of PEEF through comparison with a pre-intervention period.
ConclusionsThis study demonstrates that the PEEF increases the number of DTEs and DTIs carried out by CPPs in different clinical services, with a significant increase having been observed in high-complexity units. However, further studies are warranted to assess the quality and impact of these interventions on clinical outcomes.
This study reveals the substantial reduction of potential and direct costs resulting from the incorporation of the CPP to the ICU. Additional studies based on methods similar to that of Hammond et al. that are representative of the national reality are needed.
Contribution to the scientific literatureStandard training and education are not provided to CPs. As a result, CPs perform a variety of tasks determined by the needs of each center and the complexity of the service where they work. In addition, the economic impact of their interventions is not assessed. These activities do not necessarily include the basic activities of a CP, which results in a lack of standardization of pharmaceutical activities. This situation hinders the performance of studies to assess efficiency and quality and compare the pharmaceutical services provided by CPPs.
This study demonstrates that adherence to PEEF may increase the number of evaluations and interventions performed by the CPP in medical services where a model of pharmaceutical services has not been clearly defined. In addition, this tool could be used in economic evaluation models to estimate cost reductions associated with pharmaceutical interventions.
FundingNone.
Ethical considerationsThis study was approved by the Ethics Committee CEC-Salud UC of the Pontificia Universidad Católica de Chile (ID: 210731003).
Contributions| Antonio González | Waldo Gutiérrez | Tamara Fuenzalida | Felipe Lizana | Mariela Gutiérrez | Nicolas Severino | |
| Conception | X | X | X | X | X | X |
| Design | X | X | X | X | X | X |
| Definition of intellectual content | X | X | X | – | – | X |
| Literature search | X | X | – | X | – | X |
| Clinical trials | NA | NA | NA | NA | NA | NA |
| Experimental trials | NA | NA | NA | NA | NA | NA |
| Data collection | X | X | X | – | – | X |
| Data analysis | X | X | X | X | – | X |
| Statistical analysis | X | X | – | – | – | X |
| Manuscript drafting | X | X | X | X | – | X |
| Manuscript edition | X | X | X | X | – | X |
| Manuscript review | X | X | X | X | X | X |
| Guarantor | X | X | X | X | X | X |
We thank the Service of Pharmacy of Hospital Clínico UC-CHRISTUS and of the Centro Ambulatorio de Oncología de la Red de Salud UC-CHRISTUS in Chile.