Linezolid is an oxazolidin commonly related to the development of haematological toxicity, being renal clearance the major factor involved in the drug clearance. The aim of this study is to evaluate the influence of increased filtration rates in the incidence of linezolid-induced haematological toxicity by comparing augmented renal clearance (ARC) patients versus normal renal function patients.
Material and methodsA retrospective, observational study was conducted on hospitalized patients treated with linezolid for 5 days or more during 2014–2019 period. Patients with a filtration rate of ≥130 mL/min versus reference patients (60–90 mL/min) were compared. Haematological toxicity was defined as a decrease of 25% in platelets, of 25% in haemoglobin, and/or 50% in neutrophils from baseline. Toxicity relevance was classified according to Common Terminology Criteria for Adverse Events v5. Incidence of haematological toxicity between groups was studied by chi-square and Fisher test. Furthermore, percentage diminution of all 3 parameters was calculated and compared by Mann–Whitney test and treatment interruption and transfusion requirements were registered.
Results30 ARC patients and 38 reference patients were included. Haematological toxicity was observed in 16.66% of ARC patients vs 44.74% of reference patients (P=.014); thrombocytopenia in 13.33% vs 36.84% (P=.051), anaemia in 3.3% vs 10.52% (P=.374) and neutropenia in 10% vs 23.68% (P=.204). Median percentage of platelets decrease in ARC patients was −10.36 (−193.33–62.03) vs 2.68 (−163.16–82.71) in reference patients (P=.333), while haemoglobin decrease was 2.50 (−12.12–25.93) vs 9.09 (−17.72–30.63) (P=.047) and neutrophils decrease was 9.14 (−73.91–76.47) vs 27.33 (−86.66–90.90) (P=.093). 10.5% of normal renal function patients reported at least 1 adverse event grade 3 or superior while 2.6% of them interrupted treatment and 5.2% had transfusion requirements. No major events or interruptions were reported in ARC patients.
ConclusionOur findings suggest a lower incidence and clinical relevance of haematological toxicity in augmented renal clearance patients. Thrombocytopenia was the major event in both populations. This might be related to a lower exposure to the drug due to the higher clearance and likely lower therapeutic efficiency. These results suggest a potential benefit of therapeutic drug monitoring on high risk patients.
Linezolid es una oxazolidina frecuentemente implicada en el desarrollo de toxicidad hematológica, siendo el aclaramiento renal el mecanismo mayoritario en su eliminación. Se evaluó la influencia de la hiperfiltración glomerular en la toxicidad hematológica inducida por linezolid en pacientes con aclaramiento incrementado frente a pacientes con función renal normal.
Material y métodosSe diseñó un estudio observacional y retrospectivo en pacientes hospitalizados tratados al menos 5 días con linezolid entre 2014 y 2019. Se comparararon pacientes con aclaramiento de creatinina incrementado (≥130 mL/min) y normal (60-90 mL/min). Se definió la toxicidad hematológica como el descenso en plaquetas y hemoglobina del 25% y en neutrófilos del 50% frente a los valores basales. Se clasificó el grado de toxicidad según Common Terminology Criteria for Adverse Events v5 y se comparó la incidencia entre ambos grupos mediante Chi-cuadrado Y Fisher. Así mismo, se calculó el porcentaje de disminución de los tres parámetros y su asociación mediante el test de Mann–Whitney y se registraron las interrupciones y transfusiones asociadas.
ResultadosSe evaluaron 30 pacientes hiperfiltradores y 38 normofiltradores. El 16,66% de hiperfiltradores presentó toxicidad hematológica frente al 44,74% (p=0,014). La plaquetopenia fue del 13,33% vs 36,84% (p=0,051), la anemia del 3,3% vs 10,52% (p=0,374) y la neutropenia del 10% vs 23,68% (p=0,204). La mediana del porcentaje de descenso plaquetario en hiperfiltradores frente a normofiltradores fue del −10,36 (−193,33-62,03) vs 2,68 (−163,16 - 82,71) (p=0,333), de hemoglobina 2,50 (−12,12 – 25,93) vs 9,09 (−17,72 – 30,63) (p=0,047) y de neutrófilos 9,14 (−73,91 – 76,47) vs 27,33 (−86,66– 90,90) (p=0,093). El 10,5% con filtrado normal presentó toxicidad grado 3 o superior, el 2,6% interrumpió el tratamiento y el 5,2% requirieron transfusiones. Ningún paciente hiperfiltrador presentó toxicidad clinicamente significativa ni interrupciones/transfusiones asociadas a ella.
ConclusiónNuestro estudio sugiere una menor incidencia y relevancia clínica de toxicidad hematológica en pacientes con aclaramiento incrementado. La plaquetopenia es la condición más frecuente en ambos. Esto probablemente esté relacionado con la menor exposición al fármaco condicionada por la mayor eliminación plasmática y presumiblemente menor efectividad antimicrobiana. Los resultados corroboran el potencial beneficio de la monitorización farmacocinética en poblaciones de riesgo.
Linezolid is an antimicrobial agent belonging to the group of oxazolidinones1 with activity against a broad spectrum of Gram-positive bacteria.2 It is currently used in hospital settings as the empirical therapy of choice for skin and soft tissue infections,3 nosocomial pneumonia, and ventilator-associated pneumonia4 due to its good coverage of multidrug-resistant microorganisms, particularly methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium, which are frequently implicated in nosocomial infections.4
Regarding its safety profile, haematologic toxicity is the most common adverse effect; the literature cites thrombocytopenia as the most frequently described reaction with a prevalence ranging from 15% to 50%.5
Approximately, 30–40% of linezolid is excreted unchanged in urine, making renal function a significant factor in interpatient variability in drug clearance, despite standardized dosing. Current studies have found an association between decreased renal function (<60 mL/min/1.73 m2)6 and a high incidence of haematologic toxicity,7 primarily as a result of increased exposure.
On the other hand, the phenomenon of augmented renal clearance (ARC) is characterized by increased creatinine clearance (CrCl) in patients with risk factors.8 This condition has traditionally been described in critically ill patients and is commonly defined as an increase in CrCl of more than 130 mL/min/1.73 m2,8 potentially leading to faster drug elimination and therefore lower exposure. Furthermore, specific physiological characteristics (male sex, age) or pathological situations (trauma, surgery, haematologic malignancy, etc), may predispose individuals to this condition.8 The objective of this study was to evaluate whether ARC is associated with a lower incidence of linezolid-induced haematologic toxicity, to quantify decreases in 3 haematologic parameters (platelets [PL], haemoglobin [Hb], and neutrophils [NT]), and to identify other potential causes associated with this clinical condition.
Materials and methodWe conducted an observational retrospective single-centre study that included patients of more than 18 years who were admitted to conventional hospitalization wards and started on treatment with linezolid. Hospital and outpatient follow-up were conducted in all patients until completion of treatment.
Two types of patients were included in the study based on their glomerular filtration rate (GFR), which was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Male patients with a GFR of more than 130 mL/min/1.73 m2 and female patients with a GFR of more than 120 mL/min/1.73 m2 were categorized as hyperfiltrators (HF),10 whereas patients with a GFR ranging from 60 to 90 mL/min/1.73 m2 were categorized as normofiltrators (NF).11 The study only included therapies of at least 5 days duration in patients who had undergone control laboratory tests recorded at the start and completion of linezolid treatment, while allowing for a difference of ±2 days in both tests.
Exclusion criteria were as follows: patients with a GFR of less than 60 mL/min/1.73 m2 without laboratory tests; patients admitted to the Intensive Care Unit; and patients with baseline Hb and PL counts of less than 10 mg/dL and less than 100 × 103/mm3, respectively.
The demographic variables collected included sex, age, weight, height, body surface area, and body mass index (BMI). Renal function was assessed using the GFR and serum creatinine and urea levels. Infection-related variables included the location of the infection, as well as C-reactive protein, ferritin, and procalcitonin levels. Pharmacological variables were recorded such as dosage, duration, route of administration, and interruptions due to toxicity.
The following potential triggers of haematologic toxicity in the population were also recorded: chronic diseases, concomitant immunosuppressive therapy, chemotherapy (CT) in the last 6 months, and the number of drugs with the potential to induce platelet toxicity.
At the start and completion of linezolid therapy, we recorded blood counts (PL, Hb, and NT) and calculated the percentage decrease. Haematologic toxicity was defined as a decrease from baseline in at least 1 of the 3 parameters: 25% decrease in PL, 25% decrease in Hb, and 50% decrease in NT. The grade of toxicity was characterized according to the Common Terminology Criteria for Adverse Events (CTCAE) version 515. The need for associated transfusions was also recorded. Electronic prescription software and a pseudonymized data collection notebook were used in all such cases.
Data analysis was performed using the Minitab statistical software package version 21.1.0. Quantitative variables are expressed using the median as the measure of central tendency, and qualitative variables are expressed as absolute and relative frequencies. The normal distribution was assessed using the Kolmogorov–Smirnov test. Associations between quantitative variables were determined using the Student t-test, and qualitative variables were analysed using the Chi-square test or Fisher test for small samples. We calculated the percentage decrease in the 3 haematologic parameters (PL, Hb, and NT) and analysed associations using the Mann–Whitney test. Correlations between this percentage decrease and the GFR and the duration of antibiotic therapy were evaluated using Spearman's rank correlation coefficient. Finally, we determined other aetiologies, besides renal function, to analyse possible associations between other factors described in the literature as triggers of haematologic toxicity. In all analyses, a P-value of <.05 was used as a cut-off for statistical significance.
This study was authorized by the Clinical Research Ethics Committee of our hospital.
ResultsThe study included 68 patients (30 HF patients and 38 NF patients), who were treated between January 2014 and December 2019. The median duration of linezolid treatment was 8 (5–28) days in HF patients and 8 (5–25) days in NF patients. Table 1 shows the baseline laboratory parameters, infectious focus, and route of linezolid administration for all patients. All patients received linezolid 600 mg/12 h.
Characteristics of the study population and therapies received prior to the development of haematologic toxicity or concurrent therapies with the potential for its development.
| Characteristic | Hyperfiltrators (N=30) | Normofiltrators (N=38) | P-value |
|---|---|---|---|
| General | N (%) | N (%) | |
| Male | 18 (60) | 27 (71.05) | .338 |
| Median (IQR) | Median (IQR) | ||
| Age, y | 41 (18–62) | 57.50 (21–79) | .028 |
| Weight, kg | 66 (36–90) | 73 (38.5–108.9) | .115 |
| Height, cm | 164 (144–175) | 169 (145–184) | .070 |
| BSA, m2 | 1.75 (1.3–2) | 1.78 (1.32–2.31) | .095 |
| BMI, kg/m2 | 21.56 (14.98–32.79) | 25.05 (15.62–45.3) | .272 |
| Renal data | |||
| GFR, mL/min/1.73 m2 | 130 (124.04–153.07) | 72.50 (60–90) | <.001 |
| Creatinine, mg/dL | 0.47 (0.3–0.81) | 1.13 (0.7–1.45) | .348 |
| Urea, mg/dL | 19.5 (11–28) | 53 (32–81) | <.001 |
| Baseline blood count | |||
| Platelets, U × 109/L | 280 (137–454) | 263 (102–500) | .438 |
| Haemoglobin, g/dL | 11.6 (10–14.4) | 11.95 (10–15.8) | .465 |
| Neutrophils, U/μL | 6.8 (0.7–22) | 7.5 (1.6–18) | .062 |
| Inflammatory parameters | |||
| CRP, mg/dL | 5.08 (0.19–30.85) | 7.97 (0.41–44.73) | .432 |
| PCT, ng/mL | 0.13 (0.094–8.44) | 0.49 (0.27–17.42) | .119 |
| Ferritin, ng/mL | 263.5 (17–623.3) | 535 (20–728) | .114 |
| Infectious focus | N (%) | N (%) | |
| Respiratory | 13 (43.33) | 12 (31.58) | .318 |
| SST | 9 (30) | 15 (39.57) | .417 |
| Bacteraemia | 5 (16.65) | 10 (26.32) | .340 |
| Abdominal | 3 (10) | 1 (2.63) | .313 |
| Route of administration | N (%) | N (%) | |
| Oral | 10 (33.33) | 24 (63.16) | .014 |
| IV | 10 (33.33) | 7 (18.42) | .158 |
| IV/Oral | 10 (33.33) | 7 (18.42) | .158 |
| Chronic disease | N (%) | N (%) | |
| Cardiac | 2 (6.67) | 7 (18.42) | .359 |
| Hypertension | 4 (13.33) | 9 (23.68) | .123 |
| Diabetes | 3 (10) | 10 (26.31) | .069 |
| Dyslipidaemia | 1 (3.33) | 7 (18.42) | .124 |
| Viral infection | – | 4 (10.52) | .450 |
| Pulmonary | 5 (16.67) | 3 (7.89) | .450 |
| Solid organ tumour | 7 (23.33) | 3 (7.89) | .093 |
| Lymphoma | 2 (6.67) | 1 (2.63) | .579 |
| Autoimmune | 4 (13.33) | 3 (7.89) | .690 |
| Neurological | 4 (13.33) | 1 (2.63) | .161 |
| Psychiatric | 2 (6.67) | 1 (2.63) | .579 |
| Solid organ transplant | 1 (3.33) | 7 (18.42) | .069 |
| Liver disease | 2 (6.67) | 2 (5.26) | 1,000 |
| Concomitant therapies | |||
| CT <6 mo | 6 (20) | 4 (10.52) | .317 |
| Corticosteroids | 1 (36.67) | 8 (21.05) | .154 |
| IS | 3 (10) | 8 (21.05) | .323 |
| Therapies with potential platelet toxicity | |||
| 0 | 8 (27.59) | 8 (21.05) | .587 |
| 1 | 13 (43.33) | 13 (34.21) | .442 |
| 2 | 5 (16.67) | 13 (34.21) | .103 |
| 3+ | 4 (13.33) | 4 (10.53) | .724 |
GFR, glomerular filtration rate; BMI, body mass index; BSA, body surface area; CRP, C-reactive protein; PCT, procalcitonin; SST, skin and soft tissue; IV, intravenous; CT, chemotherapy; IS, immunosuppressants.
All analyses were conducted using the Student t-test, Fisher test, or Chi-square test. A P-value of <.05 was used as a cut-off for statistical significance.
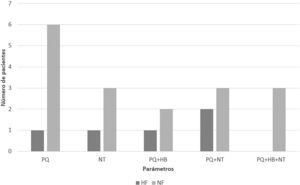
Haematologic toxicity was experienced by 16.66% of HF patients vs 44.74% of NF patients (P=.014). The incidence of platelet toxicity was 13.33% vs 36.84% (P=.051), respectively. Anaemia was experienced by 3.3% of HF patients vs 10.52% of NF patients (P=.374). Moreover, the incidence of neutropenia was 10% vs 23.68% (P=.204), respectively. Fig. 1 shows the distribution of the type of toxicity experienced by each patient.
Grade 3 toxicity or higher was not experienced by any HF patient vs 10.5% of NF patients. In the NF group, 1 patient had grade 3 anaemia, 2 patients had grade 3 neutropenia, and 1 patient had grade 4 neutropenia. In terms of clinical impact in the NF group, 1 patient discontinued linezolid treatment and 2 patients required transfusions as a result of toxicity. No other clinically relevant adverse events characteristic of linezolid16 were reported in the medical records of any of the 68 patients studied.
Table 1 provides information on the study population regarding chronic disease, prior therapies (chemotherapy), and concomitant drugs with the potential to induce haematologic toxicity according to the literature. Table 2 shows the results on patients with predisposing factors to haematologic toxicity17 and the incidence of toxicity.
Results on patients with factors predisposing them to haematologic toxicity who experienced toxicity vs patients who did not experience toxicity.
| Factor under study | Toxicity (N=22) | No toxicity (N=46) | P-value |
|---|---|---|---|
| N (%) | N (%) | ||
| >7 d | 16 (72.72) | 35 (76.08) | .764 |
| >10 d | 7 (31.81) | 26 (56.52) | .056 |
| IS | 7 (31.81) | 16 (34.78) | .808 |
| Thrombogenic drugs | 16 (72.72) | 36 (78.26) | .614 |
| CT <6 mo | 6 (27.27) | 4 (8.70) | .066 |
| Onco-haematologic | 6 (27.27) | 7 (15.21) | .236 |
| Solid organ transplant | 5 (22.72) | 3 (6.52) | .100 |
| Liver disease | 2 (9.09) | 2 (4.34) | .589 |
IS, immunosuppressants; CT, chemotherapy.
All analyses were conducted using the Fisher test or Chi-square test. A P-value of <.05 was used as a cut-off for statistical significance.
Table 3 shows the median percentage decrease in PL, Hb, and NT from baseline to the end of treatment according to 2 clinical situations: renal function (HF patients vs NF patients); and a comparison of 2 patient populations who experienced toxicity in at least 1 of the 3 parameters versus those who did not experience any toxicity regardless of their CrCl.
Percentage decrease in the 3 parameters evaluated (PL, Hb, and NT) from baseline to the end of treatment.
| Parametera haematological | Normal CrCl (N=38) | Increased CrCl (N=30) | P-value |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Platelets (U × 109/L) | 2.68 (−163.16–82.71) | −10.36 (−193.33–62.03) | .333 |
| Haemoglobin, g/dL | 9.09 (− 17.72–30.63) | 2.50 (− 12.12–25.93) | .047 |
| Neutrophils, U/μL | 27.33 (−86.66–90.90) | 9.14 (−73.91–76.47) | .093 |
| Parameterbc haematological | Toxicity (N=22) | No toxicity (N=46) | P-value |
| Median (IQR) | Median (IQR) | ||
| Platelets (U × 109/L) | 33.34 (−128.04–82.71) | −22.05 (−193.3–17.74) | <.001 |
| Haemoglobin, g/dL | 12.50 (−7.40–30.63) | 2.68 (−12.12–18.1) | .007 |
| Neutrophils, U/μL | 50 (−40–90.91) | 12.29 (−73.91–73.52) | .004 |
Spearman's correlation coefficient was used to analyse correlations between the GFR and the percentage decrease in haematological parameters in the 68 patients. The results were as follows: PL (R=−.087, P=.493); Hb (R=−0.286, P=.03); NT (R=−0.087, P=.514). The same test was used to analyse correlations between the total duration of treatment and the percentage decrease in haematological parameters in the 68 patients. The results were as follows: PL (R=−0.046, P=.714), Hb (R=0.069, P=.606), NT (R=0.136, P=.221).
DiscussionAccording to the literature, although the exact aetiology of ARC remains unknown it is a prevalent condition in critically ill patients.10 On the other hand, Sime et al. associated this condition with the use of vasoactive medications and fluid therapy.18 Balk proposed the theory of systemic inflammatory response syndrome (SIRS), in which patients with proinflammatory clinical conditions, such as neoplasia, sepsis, trauma, and recent major surgery with the potential for cytokine release, have decreased vascular resistance and increased cardiac output.19 Both these factors are involved in increased glomerular filtration.8 In a systematic review, Bilbao-Meseguer et al. identified male sex and age as predisposing factors,10 with medians of around 40 years in the patients described in the studies versus more than 50 years in populations with decreased CrCl, which is a characteristic observed in our study population10 (Table 1). Patients admitted to Intensive Care were excluded from our study due to their high complexity and typical polytherapy; thus, only patients on hospital wards were studied.
González del Castillo et al. studied haematological toxicity induced by linezolid in the general population and found an association between a higher incidence of thrombocytopenia and prolonged treatment duration, hepatopathies, renal failure, cancer, low BMI, and a low baseline platelet count.5 Hanai et al. identified 3 risk factors for the incidence of thrombocytopenia: CrCl, haemodialysis, and treatment duration, and 1 factor for the incidence of anaemia: duration.17 Regarding these factors, we excluded patients with decreased CrCl or with thrombocytopenia <100 × 103/mm3 at baseline. No statistically significant association was found between any of the factors commonly described in the literature,5 nor for concomitant therapies with the potential for triggering toxicity (drugs with thrombocytopenic potential,12–14 immunosuppressants, and chemotherapy) and the incidence of toxicity in the entire population (Table 2). This result is probably related to the relatively small sample size.
On the other hand, the correlation between percentage decreases in CrCl and the duration of antibiotic therapy, which was calculated using Spearman's correlation coefficient, was insufficient to establish conclusive linear relationships in any of the comparisons. A significant negative association was only found between decreased Hb and CrCl (i.e., higher CrCl reduces the impact of linezolid treatment on Hb). However, this association was quite weak in our study population.
Despite a literature search, no study was found that exclusively focussed on populations without renal impairment; thus, there were no references by which to estimate the expected prevalence. In our population, the only statistically significant finding was related to the overall incidence of haematological toxicity, whereas statistical significance was not reached regarding its incidence in relation to each of the other parameters (PL, Hb, and NT). However, Fig. 1 shows that PL in NF patients was the parameter most affected by toxicity. Furthermore, the incidence of PL toxicity observed in this subgroup corresponds to that described in other toxicity studies conducted in the general population20; however, the incidence observed in our HF population was considerably lower and was similarly observed in the decrease in Hb. Therefore, the lack of significance in our study regarding the evaluation of PL, Hb, and NT separately is probably related to the small sample size, which was due to the low prevalence of ARC in hospitalized patients and the restrictive inclusion criteria of our study.
Post-marketing studies on linezolid have found a high prevalence of thrombocytopenia within the first days of therapy, which stands in contrast to the late onset of anaemia21 (up to 10–14 days after start). Early onset is due to the slow onset of myelosuppression in most patients,6 and so regimens longer than 14 days of treatment are not recommended.6Fig. 1 depicts this clinical situation, in that none of the study patients experienced isolated anaemia. Therefore, Crass et al. proposed a standardized dose reduction and pharmacokinetic monitoring in patients with decreased CrCl and an anticipated duration of antibiotic therapy of more than 14 days.6
In our study, the toxicity grade and its implications, such as treatment interruption and the need for transfusions, were of clinical significance in NF patients (anaemia and neutropenia), but not in HF patients. Table 3 shows the percentage decreases in both populations; significant differences were only found in relation to the decrease in Hb. It should be noted that the decreases in all 3 parameters appear to be smaller in HF patients. In the second comparison, regardless of renal function, a significant percentage decrease (Table 3) was observed between patients with at least 1 affected parameter (PL, Hb, and NT) versus patients without any affected parameter, which could be explained by the generalized myelosuppressive effect of linezolid.6
One limitation of this type of study is the technique used for determining GFR. Currently, inulin levels are considered the gold-standard to measure the GFR,8 but this approach has a high cost–benefit ratio.7 Creatinine clearance can be calculated via 24-h urine collection.22 We do not routinely perform this technique in most of our hospital patients, preferring to determine serum creatinine levels (CKD-EPI, MDRD-4). However, the CKD-EPI and MDRD-4 equations tend to underestimate clearance in HF patients,10 which would suggest that the clearance rate was higher than that described in our study population (Table 1).
The literature on ARC mainly refers to pharmacokinetic studies in critically ill patients receiving linezolid in terms of effectiveness. Barrasa et al. demonstrated the influence of ARC on subtherapeutic levels of linezolid, which was not observed in patients with normal or decreased CrCl.9 Wang et al. confirmed that the standard dosage (1200 mg/d) was insufficient to reach the Minimum Inhibitory Concentration in part of their study population.23 The lower exposure to the drug described in these patients sheds light on the lower incidence and haematological toxicity grade observed in our population. Luque et al. developed a pharmacokinetic dosing algorithm to ensure safety and effectiveness.24
Currently, the standard dosage of linezolid (600 mg/12 h) is used both orally and intravenously regardless of GFR and interindividual variability,6 and so patients would probably benefit from individualized dosage adjustments based on their clinical condition, in addition to monitoring plasma concentrations of linezolid in at-risk populations.
Therefore, our study suggests that the incidence of haematological toxicity is lower in patients with ARC than in patients with normal CrCl. In addition, the clinical toxicity grade and implications also appear to be lower in patients with ARC. The lower incidence of linezolid-induced toxicity in patients with ARC could be related to lower exposure to the drug, which is due to the high renal elimination rate. Given this possibility, we recommend the concurrent study of the antimicrobial efficacy of linezolid in this population.
As described in the literature, obtaining subtherapeutic plasma levels could result in inadequate therapy with the consequent risk of therapeutic failure. Therefore, we believe that individualized dosing and pharmacokinetic monitoring are likely to be of benefit in patients with ARC.
Ethical responsibilitiesThe present study was authorized by the Ethical Committee in Medicinal Research (CEIM).
All authors accept the responsibilities defined by the International Committee of Medical Journal Editors (available at: http://www.icmje.org/).
The authors exclusively transfer the rights of reproduction, distribution, translation, and public communication (by any means or audio-visual or electronic support) of our work to Farmacia Hospitalaria and, by extension, to the Spanish Society of Hospital Pharmacy (SEFH), in the event of its publication. For this purpose, a letter ceding such rights will be signed at the time of submitting the manuscript through the online manuscript management system.
FundingNone declared.
Presentation at CongressesEuropean Association of Hospital Pharmacists (EAHP), Vienna, March 2022.
Contribution to the scientific literatureThe results of our study contribute, in terms of clinical safety, to the limited publications on ARC that have found associations between this clinical situation and lower exposure to linezolid due to subtherapeutic plasma levels as determined by laboratory assays. In addition, these patients had an impaired immune response. Our study also suggests that, in routine clinical practice, the haematological toxicity grade and its incidence are lower in ARC patients. It provides the first comparison described in the literature using a reference population without renal impairment.