This case is based on a drug interaction between nirmatrelvir/ritonavir (approved drug for COVID-19) and voriconazole is presented, possibly derived from the bidirectional effect of ritonavir on the 2 main voriconazole metabolizing enzymes (cytochrome P450 3A and 2C19) ritonavir inhibits the former and induces the latter respectively.
According to the main pharmacotherapeutic information databases, in the interaction between both drugs, a decrease in the area under the curve of voriconazole is expected due to the inducing effect of its metabolism; however, in the case we present, unexpectedly, a paradoxical effect occurs, according to what is described in literature, with the result of sustained supratherapeutic levels of voriconazole.
Given the short treatment period with nirmatrelvir/ritonavir (5 days), the induction effect of ritonavir proposed in the studies on which the recommendations are based, where treatment with ritonavir is longer, does not occur.
Presentamos el caso de una interacción farmacológica entre nirmatrelvir/ritonavir (fármaco aprobado para la infección por COVID-19) y voriconazol, derivada del efecto bidireccional del ritonavir sobre las 2 principales enzimas metabolizadoras del voriconazol (citocromo P450 3A y 2C19) de forma que, ritonavir inhibe la primera e induce la segunda respectivamente.
De acuerdo con las principales bases de datos de información farmacoterapéutica, en la interacción entre ambos fármacos, se espera una disminución en el área bajo la curva del voriconazol por el efecto inductor de su metabolismo, sin embargo, en el caso que presentamos ha ocurrido el efecto opuesto, se dan niveles supraterapéuticos de forma mantenida, lo cual es un efecto paradójico según la literatura.
Dado el corto periodo de tratamiento con nirmatrelvir/ritonavir (5 días), no llega a manifestarse el efecto inductor del ritonavir propuesto en los estudios en los que se basan las recomendaciones, donde el tratamiento con ritonavir es más prolongado.
Nirmatrelvir/ritonavir is a combination antiretroviral therapy for COVID-19 indicated for mild–moderate cases with a high risk of progression to severe disease 1.
We present a case of drug-to-drug interaction between nirmatrelvir/ritonavir and voriconazole resulting in a substantial increase in voriconazole concentrations. Interaction occurs when the antifungal agent is metabolized by the cytochrome P450 (CYP) 3A and CYP2C19. Additionally, ritonavir has dual effects as a CYP3A inhibitor and a CYP2C19 inducer as well 2. Hence, bidirectional interaction may occur as a result of the co-administration of voriconazole and nirmatrelvir/ritonavir. CYP2C19 metabolizer status will determine the extent and direction of this interaction.
In our context, genotypic characterization is not performed in routine practice, which generates uncertainty about dosage recommendations.
Case reportWe present the case of a 65 year-old immunocompromised woman with Stage 3-A non-Hodgkin's follicular lymphoma without other medical history of interest. Although the patient had received three doses of COVID-19 vaccine (last in September 2021), she required hospitalization two times (February and March 2022) for SARS-CoV-2 infection, omicron 21 K variant (BA.1.1). During the first hospitalization, remdesivir and sotrovimab were administered for five days. During the second hospitalization, the patient received remdesivir for 10 days.
The patient was discharged despite not having a negative PCR (polymerase chain reaction) result. On 06/04/2022, the patient was re-admitted with large bilateral SARS-COV-2 pneumonia. At 10 days from admission, PCR was positive for Aspergillus terreus in serum and beta-D-glucan in serum. On 21/04/2022, intravenous voriconazole therapy was initiated following the Summary of Product Characteristics (loading dose 6 mg/kg/12 h followed by 4 mg/kg/12 h). Genotypic characterization revealed that the patient was an intermediate metabolizer of voriconazole (CYP2C19 *2/*17 polymorphism). The frequency of this type of phenotype is 18–45% in the Caucasian population. The therapeutic recommendation is using a standard starting dose 3.
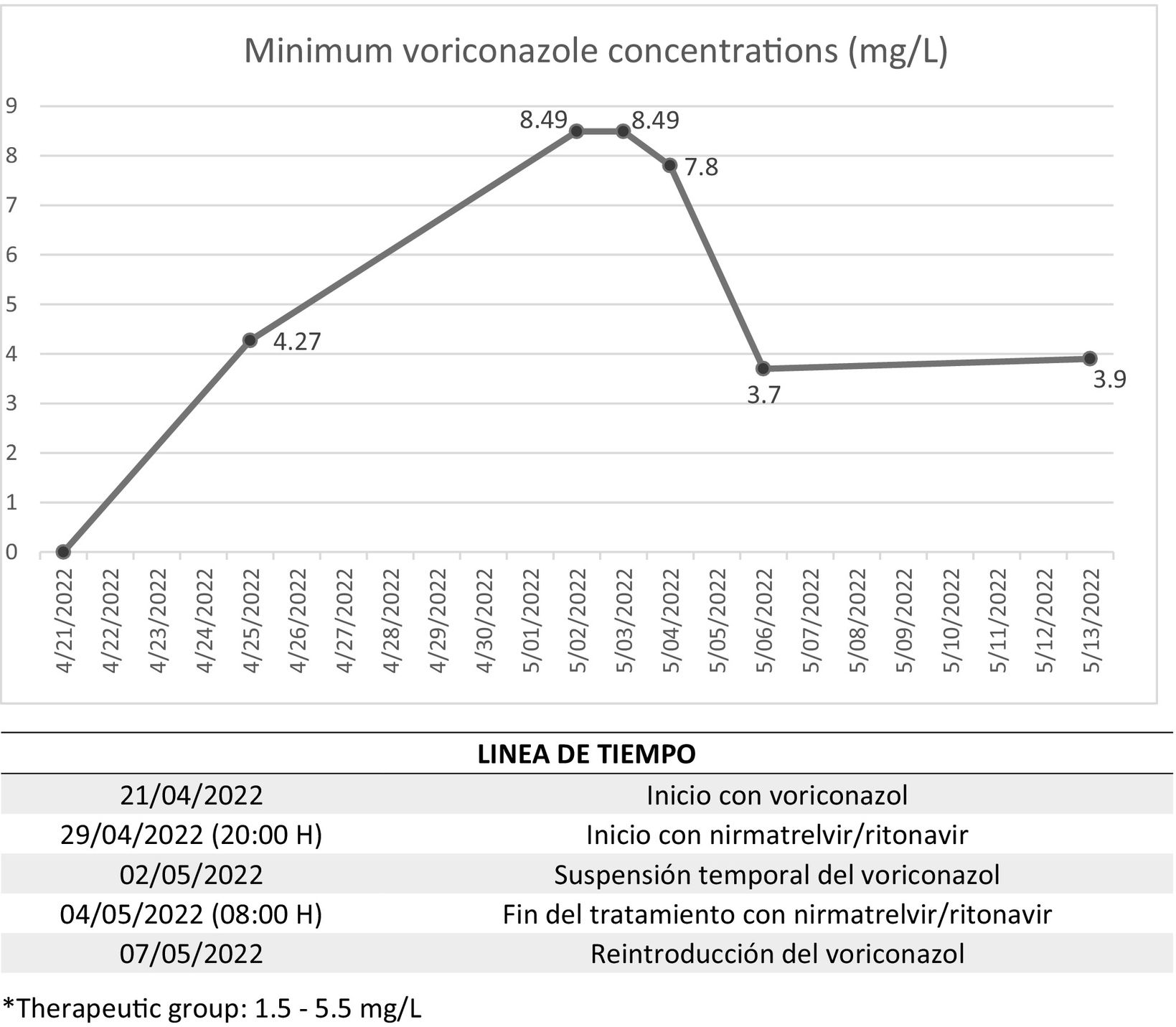
At 5 days, minimum voriconazole concentration was determined and confirmed to be in therapeutic range (4.27 mg/L; therapeutic range: 1.5–5.5 mg/L). Since the clinical and analytical status of the patient improved, and following our dosage nomogram4, the dose was reduced and the patient was switched to oral therapy.
On 29/04/2022, given the persistence of SARS-COV-2 infection and although five days have passed since the onset of symptoms, nirmatrelvir/ritonavir was prescribed for off-label use. At 60 h after concomitant administration of voriconazole plus nirmatrelvir/ritonavir, minimum levels of voriconazole doubled (8.49 mg/L), and the Hospital Pharmacy recommended that voriconazole should be discontinued and close monitoring performed. Minimum value remained constant 24 h after discontinuation of voriconazole and only decreased by 8.1% at 48 h. Finally, levels reached therapeutic range (3.7 mg/L) 48 h after completion of 5-day treatment with nirmatrelvir/ritonavir and 4 days after voriconazole discontinuation (Fig. 1).
With respect to the toxicity of voriconazole, alanine transaminase exceeded 1.4 times the upper limit of normality.
This drug-to-drug interaction was reported to the Spanish Pharmacovigilance System for Medicinal Products for Human Use (SEFV-H).
DiscussionThe main drug information databases, Lexicomp®, IBM Micromedex®, and Liverpool COVID-19 Interactions®, contraindicate concomitant use of voriconazole and ritonavir at doses of 400 mg/12 h and recommend avoiding concomitant use with ritonavir 100 mg/12 h. These recommendations are all based on the study by Liu et al.2, who documented that the area under the curve of voriconazole decreased by 82% and 39% with ritonavir administered at 400 mg/12 h and 100 mg/12 h, respectively. This phenomenon is explained by the authors to result from the inductive effect of ritonavir on CYP2C19. The authors also reported that 3.9% of their patients experienced the opposite effect (increase in minimum levels of voriconazole). According to the authors, this effect is explained by the inhibitory effect of ritonavir on CYP3A in patients presumed to be slow metabolizers (genotypic characterization was not performed), since levels of voriconazole were significantly higher before ritonavir was introduced.
We report the case of an intermediate metabolizer that apparently tolerated monotherapy with standard doses of voriconazole. Thus, the first level analyzed (prior to initiation of nirmatrelvir/ritonavir), along with the level obtained at 9 days after completion of nirmatrelvir/ritonavir therapy, was in therapeutic range.
In relation to the CYP2C19 inductive effect of ritonavir reported by Liu et al.2, it is worthy of note that voriconazole levels were determined 20 days after initiation of ritonavir (10 days alone and 10 concomitantly with voriconazole). This time lapse is long enough for the inductive effect to manifest, since enzymatic systhesis is estimated to take from some days to some weeks 5. However, in our case, the duration of nirmatrelvir/ritonavir therapy was only 5 days.
In contrast, in our case, voriconazole levels reached therapeutic range 48 h after nirmatrelvir/ritonavir therapy was discontinued, and 4 days after voriconazole was discontinued). Stader et al.6, designed a model to analyze the duration of CYP3A inhibition following lopinavir/ritonavir withdrawal (400/100 mg every 12 h) by using midazolam as the model drug (CYP3A substrate). The authors observed that, for the age range of 60–69 years, 51% and 76% of the effect had disappeared at 24 h and 48 h, respectively.
Therefore, the findings of this clinical case demonstrate that close monitoring of voriconazole concentrations should be performed to prevent toxicity or lack of response. In addition, therapies should be tailored to the patient's specific situation, as recommendations on drug reference databases are not always consistent in terms of the extent or direction of interaction. Further studies are needed to gather more scientific evidence and improve our knowledge on the management of potential nirmatrelvir/ritonavir interactions.
Ethical considerationsIn compliance with current laws and regulations, all reasonable measures will be adopted to guarantee the confidentiality of the personal data of the patient.
FundingNone.
AuthorshipAuthor 4 contributed to data collection. Author 1 contributed to data analysis, conception and design of the study. Authors 1, 3 and 4 participated in the thorough literature search. Author 1 drafted the manuscript and, with the help of authors 2 and 4, developed the intellectual content and conception of the manuscript. Finally, Author 5 reviewed and proofread the manuscript.