To determine the prevalence of PIMDINAC criteria and to implement pharmacological interventions in a population with multiple sclerosis over 55 years of age.
MethodsRetrospective, observational, open-label study, including patients with multiple sclerosis aged 55 years and older during December 2022 and February 2023. The main variable determined was the percentage of compliance with the PIMDINAC criteria.
ResultsNinety-five patients were included, with the presence of PIMDINAC criteria detected in 67.4%. The most frequently detected criterion was non-adherence to concomitant treatment (84.4%), followed by drug–drug interactions (56.2%) and potentially inappropriate medication (25%). A total of 20 pharmaceutical interventions were performed in 17 patients (17.9%). Potentially inappropriate medication was responsible for 11 interventions, non-adherence for 7, and drug–drug interactions for 2. The 81.8% of interventions were accepted, resulting in the discontinuation of 15 inappropriately prescribed drugs. The prevalence of PIMDINAC criteria in this group of patients is high. The study revealed that PIMDINAC criteria were prevalent in 67.4% of the study population, with polypharmacy playing an important role, suggesting the potential for a multidisciplinary approach, through pharmaceutical interventions to address unnecessary or duplicate treatments.
determinar la prevalencia de los criterios PIMDINAC y llevar a cabo intervenciones farmacéuticas en una población con esclerosis múltiple mayor de 55 años.
MétodoEstudio abierto, observacional, retrospectivo en el que se incluyeron pacientes con esclerosis múltiple mayores de 55 años durante diciembre de 2022 y febrero de 2023. La variable principal determinada fue el porcentaje de cumplimiento de los criterios PIMDINAC.
ResultadosSe incluyeron 95 pacientes, detectándose la presencia de criterios PIMDINAC parciales en el 67,4%. El criterio más frecuentemente detectado fue la falta de adherencia al tratamiento concomitante (84,4%), seguido de las interacciones medicamentosas (56,2%) y la medicación potencialmente inapropiada (25%). Se realizaron un total de 20 intervenciones farmacéuticas en 17 pacientes (17,9%). La medicación potencialmente inapropiada fue responsable de 11 intervenciones, la falta de adherencia al tratamiento de 7 y las interacciones medicamentosas de 2. El 81,8% fueron aceptadas, suspendiéndose 15 fármacos inadecuadamente prescritos. El estudio reveló una alta prevalencia de los criterios PIMDINAC en la población estudiada, y que la polifarmacia desempeñaba un papel importante, lo que sugiere el potencial de un enfoque multidisciplinar, en particular mediante intervenciones farmacéuticas para abordar los tratamientos innecesarios o duplicados.
Multiple sclerosis (MS) is a chronic, inflammatory, autoimmune disease of the central nervous system in which demyelination occurs as a result of an abnormal immune response. Its prevalence has increased due to improvements in diagnosis and treatment.1 The average age of diagnosis is 32 years, and it is more common in women (2:1).2 In Spain, there are about 55, 000 patients with an annual incidence of 4.2 cases/100, 000 inhabitants.3
This population is ageing in parallel with the increase in life expectancy in the general population due to disease-modifying therapies (DMTs)1 and is at risk of polypharmacy, affecting quality of life and resulting in long-term disability.4 The symptoms of MS often require additional drugs that are difficult to discontinue.5 This accumulation of drugs increases healthcare costs and poses risks such as inappropriate prescribing, drug–drug interactions (DDI), and non-adherence.6
Studies analysing these issues vary widely and, to date, few have assessed them together. To address this situation, the PIMDINAC criteria have been developed, which comprise three components: potentially inappropriate medication (PIM), potential drug interactions (DI), and non-adherence to concomitant medication (NAC).7 These criteria have only been applied to chronic diseases such as infectious diseases (HIV),7 spondylitis, rheumatoid arthritis, and psoriatic arthritis.8 Therefore, their application to MS could provide insight into polymedication and the problems associated with this group.
The aim of this study was to determine the prevalence of PIMDINAC criteria, the factors associated with their presence in an MS population over 55 years in real-world clinical practice, and to implement interventions to improve drug therapy.
MethodsRetrospective, open, observational study of patients over 55 years of age with a diagnosis of MS (McDonald criteria9) under active follow-up at a tertiary hospital pharmaceutical care unit between December 1, 2022 and February 28, 2023. Exclusion criteria were patients lost to follow-up or without signed consent. The study was approved by the Biomedical Research Ethics Committee of the Andalusian Health System (Code 0023-N-23).
Demographic variables collected were age, sex, date of diagnosis, type of MS (relapsing–remitting, primary progressive, and secondary progressive), and comorbidities according to the International Classification of Diseases code and the Expanded Disability Status Scale (EDSS).
Data were also collected on DMTs, drugs that improve walking speed (fampridine) and/or are indicated in moderate-to-severe spasticity (dronabinol/cannabidiol), dose, start date, and end date. The treatments included were those available in hospitals in Spain at the time of the study. In addition, data were collected on concomitant treatment and the reason for prescription (comorbidity, symptomatic treatment of MS, symptomatic treatment of acute process, or supplementation). We analysed polypharmacy (5 or more drugs, including DMTs), major polypharmacy (10 or more drugs, including DMT), complexity index (high or low, calculated according to the Medication Regimen Complexity Index,10) and anticholinergic burden (Anticholinergic Drug Burden Index.11)
The prevalence of PIMDINAC criteria was classified as follows: total (joint presence of PIM+DI+NAC) and partial (isolated presence of some criteria). PIM was identified using STOPP-START 2014 criteria.12 The Lexicomp13 database was used identify DDIs of grade D (potential) or X (contraindications) between DMTs and concomitant medications. Non-adherence to concomitant home medications and DMTs was assessed according to correct dispensing (more than 90%) in the previous 6 months.
Based on the data, pharmaceutical interventions were made by contacting the patients directly or by contacting the prescribers. The type of notification, the PIMDINAC criteria involved, and the outcome were recorded.
All analyses were performed using R Commander. Quantitative variables are expressed as measures of central tendency and dispersion (median and interquartile range [IQR]); qualitative variables are expressed as absolute frequencies and percentages. Categorical variables were compared using Chi-squared or Fisher's exact test. Quantitative variables were compared using the t test or Mann–Whitney U-test. Sample size was calculated based on the total number of patients who met the inclusion criteria (102), resulting in a sample size of 94, with a 95% confidence interval and a 3% margin of error.
ResultsA total of 423 patients with MS were seen in the pharmaceutical care unit, of whom 102 met the inclusion criteria. Seven patients were excluded: 5 could not be followed up, and 2 refused consent. In total, 95 patients were included. Table 1 shows the demographic characteristics of the study patients.
Demographic characteristics of the patients with multiple sclerosis.
| Demographic characteristics | |
| Sex ratio, female/male | 65/30 |
| Age, median | 61 (IQR 58–65) |
| Age at diagnosis, median | 45.2 (IQR 38.5–50.2) |
| Type of sclerosis, n (%) | |
| Recurrent-remitting | 68 (71.6) |
| Secondary progressive | 18 (19) |
| Primary progressive | 9 (9.4) |
| EDSS scale, median | 6 (IQR 3–6.7) |
| Comorbidities, n (%) | |
| Hypertension | 36 (37.9) |
| Dyslipidaemia | 31 (32.6) |
| Hypothyroidism | 13 (13.7) |
| Anxiety and/or depression | 10 (10.5) |
| DM | 9 (9.5) |
| No comorbidities | 8 (8.2) |
| Migraine | 7 (7.4) |
| Arthrosis | 5 (5.2) |
| Asthma | 3 (3.1) |
| Fibromyalgia | 3 (3.1) |
| DMT, n (%) | |
| Interferon | 24 (25.2) |
| Teriflunomide | 14 (14.7) |
| Dimethyl fumarate | 12 (12.6) |
| Fingolimod | 10 (10.5) |
| Glatiramer acetate | 8 (8.4) |
| Natalizumab | 7 (7.4) |
| Siponimod | 5 (5.2) |
| Ocrelizumab | 4 (4.2) |
| Patients with symptomatic treatment dispensed at the hospital pharmacy, n (%) | |
| Fampridine | 18 (18.9) |
| Dronabinol/cannabidiol | 7 (7.3) |
| Duration (months) of DMT, median | 95.5 (IQR 56.8–97.3) |
| Number of drugs excluding DMT, median | 6 (IQR 3–9) |
| Number of drugs including DMT, median | 7 (IQR 4–11) |
| Polypharmacy (including DMT) | 65 (68.4) |
| Major polypharmacy (including DMT) | 32 (33.7) |
| High treatment complexity index | 38 (40) |
| Most frequent concomitant medication (%) | |
| Omeprazole | 34.7 |
| Acetaminophen | 30.5 |
| Baclofen | 27.4 |
| Gabapentin | 22.10 |
| Simvastatin | 18.90 |
| Diazepam | 17.9 |
| Main causes of concomitant medication prescription (%) | |
| Comorbidities | 53.4 |
| Symptoms associated with MS | 25.5 |
| Acute symptoms | 13 |
| Supplementation | 7.9 |
| Anticholinergic load according to the ADBI, n (%) | |
| No risk | 19 (20) |
| Moderate risk | 21 (22.1) |
| High risk | 55 (57.9) |
Abbreviations: DM, diabetes mellitus; EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; DMT, disease modulating therapy; ADBI, Anticholinergic Drug Burden Index.
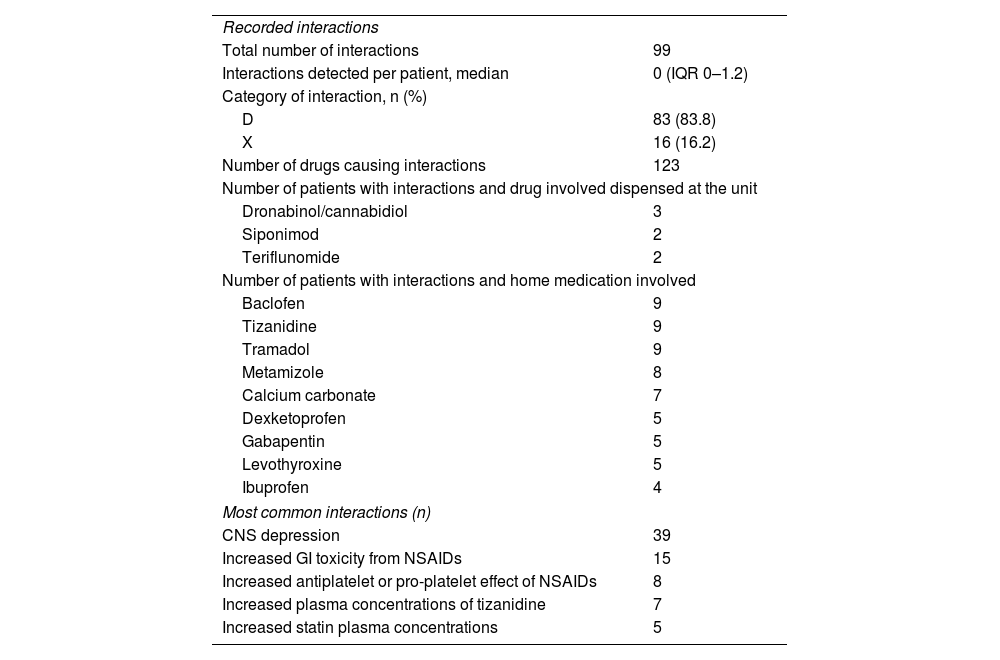
The presence of PIMDINAC criteria was detected in 67.4% of patients, with 10.5% having all the PIMDINAC criteria. Overall, NAC was identified in 84.4% of patients, DI in 56.2% (Table 2), and PIM in 25%. Of the patients, 26.6% had DI+NAC, 4.6% had PIM+NAC, and 1.6% had PIM + DI. Eighteen patients met the STOPP-START criteria (18.9%), of whom 16 were eligible to discontinue medication, including benzodiazepines (n=7; 43.7%), cholecalciferol and calcium supplementation (n=6; 37.5%), and non-steroidal anti-inflammatory drugs (n=3; 18.7%). Untreated hypertension was observed in 2 patients (2.1%).
Drug–drug interaction characteristics and pharmaceutical interventions.
| Recorded interactions | |
| Total number of interactions | 99 |
| Interactions detected per patient, median | 0 (IQR 0–1.2) |
| Category of interaction, n (%) | |
| D | 83 (83.8) |
| X | 16 (16.2) |
| Number of drugs causing interactions | 123 |
| Number of patients with interactions and drug involved dispensed at the unit | |
| Dronabinol/cannabidiol | 3 |
| Siponimod | 2 |
| Teriflunomide | 2 |
| Number of patients with interactions and home medication involved | |
| Baclofen | 9 |
| Tizanidine | 9 |
| Tramadol | 9 |
| Metamizole | 8 |
| Calcium carbonate | 7 |
| Dexketoprofen | 5 |
| Gabapentin | 5 |
| Levothyroxine | 5 |
| Ibuprofen | 4 |
| Most common interactions (n) | |
| CNS depression | 39 |
| Increased GI toxicity from NSAIDs | 15 |
| Increased antiplatelet or pro-platelet effect of NSAIDs | 8 |
| Increased plasma concentrations of tizanidine | 7 |
| Increased statin plasma concentrations | 5 |
Abbreviations; IQR, interquartile range; NSAIDs, non-steroidal anti-inflammatory drugs; D, potential interaction; GI, gastrointestinal; CNS, central nervous system; DMT, disease modulating therapy; X, contraindications.
A statistically significant association was found between PIMDINAC, polypharmacy, and number of drugs (p<.05), but not with major polypharmacy. A statistically significant relationship was found between the number of drugs and the treatment complexity index. No relationship (p>.05) was found between anticholinergic burden and age, EDSS, number of interactions, and number of medications.
Twenty pharmaceutical interventions were performed in 17 patients (17.9%). Overall, PIM criteria were the reason for 11 interventions (discontinuation due to duplication or lack of need), NAC for 7, and DI for 2. More than half of the interventions were performed through contact with the prescribers responsible for the patient (13), primary care physicians (4), or by contacting the patient directly (3). Of the 11 patients with PIM criteria, 9 (81.8%) were accepted by the patients' treating physicians, resulting in the discontinuation of 15 drugs.
DiscussionPrevious studies have found a high prevalence of concomitant MS and polypharmacy,4 affecting between 15% and 59% of these patients,14 with age and comorbidities being the main risk factors.5 Polypharmacy is more frequent in older adults. Comorbidities can also occur in younger individuals, resulting in a higher risk of polymedication at around the age of 55 years4,14 which is why this age limit has been established.
A recent systematic review shows that the most commonly prescribed medications are antispasmodics, antidepressants, anticonvulsants, and treatments for osteoporosis, coinciding with the results of this study. The safety and benefits of concomitant medication remain a challenge; strategies to address this issue include routine review, discontinuation of ineffective medication, avoidance of duplication, use of non-pharmacological interventions, training in deprescribing, and patient involvement.6
There was a significant presence of PIMDINAC criteria in the sample, and was even higher among certain individuals. Based on the results, pharmaceutical interventions were conducted, focusing on PIMs agreed with the responsible medical team. Interventions were limited to patients who attended regular consultations. Despite numerous DDIs, interventions in this area were not prioritised as some medications were taken as needed during symptom exacerbations. These interventions were useful in safely and effectively deprescribing inappropriate medications, highlighting the key role of pharmacists in managing polypharmacy and their integration into multidisciplinary teams.15 Díaz-Acedo et al. found a similar prevalence of PIMDINAC criteria among patients with chronic diseases and patients with HIV, although DDIs and NAC were higher in patients with HIV.6 A previous study7 also found that more than half of the patients were non-adherent to treatment and had been prescribed potentially inappropriate medication, supporting the association between polypharmacy, advanced age, and the PIMDINAC criteria. Given the differences in the demographic and pathophysiological characteristics of the patients, comparing these studies with ours could introduce bias in the interpretation of the results.
This study is limited by its single-centre design, small sample size, short duration, and indirect estimation of adherence. Non-prescription and herbal medicines were excluded, which could have affected the assessment of polypharmacy and drug interactions. Heterogeneity was observed between the polymedicated and non-polymedicated groups.
Analysis of the PIMDINAC criteria suggests a significant association between non-adherence and the number of drugs, as well as treatment complexity. The use of these criteria can help to identify at-risk patients and design individualised strategies for optimising pharmacotherapeutic treatment. The results suggest a multidisciplinary and multidimensional approach to pharmacy team performance that promotes high-quality pharmaceutical interventions, particularly deprescribing, with the aim of establishing these methods as standard practice for managing these patients. Further research is needed to develop effective tools to measure polypharmacy and mitigate its effects. Future lines of research should focus on developing longitudinal studies to clarify the causal relationships between polypharmacy and patient health outcomes. Additionally, there is a need to implement comprehensive pharmaceutical reviews of patient treatment with a focus on deprescribing in clinical practice.
Ethical responsibilitiesThe study was conducted in accordance with the Declaration of Helsinki, and was approved by the Ethics and Research Committee of the Andalusian Health Service (Ethics Portal Protocol Code 0023-N-23).
Informed consentInformed consent was obtained from all the study participants.
FundingNone declared.
Fuentes de ApoyoNo.
Author contributionsMaría Rosa Cantudo Cuenca: study conceptualization, definition of intellectual content, literature search, and manuscript revision. María del Mar Sánchez Suárez and Alicia Martín Roldán: study design, literature search, experimental studies, data collection, and manuscript preparation, review, and editing. Alberto Jiménez Morales: manuscript review and editing. María del Mar Sánchez Suárez: data analysis and statistical analysis.
Responsibility and transfer of rightsIn the event of publication, the authors grant exclusive rights of reproduction, distribution, translation, and public communication (by any media or sound, audiovisual or electronic support) of this work to Farmacia Hospitalaria and, by extension, to the SEFH. To this end, a letter of assignment of rights will be signed at the time of submitting the paper through the online manuscript management system.
CRediT authorship contribution statementMaría Del Mar Sánchez Suárez: Writing – original draft, Methodology, Formal analysis, Data curation. Alicia Martín Roldán: Writing – review & editing, Validation, Formal analysis, Data curation. Maria Rosa Cantudo Cuenca: Writing – review & editing, Supervision, Investigation, Conceptualization. Alberto Jiménez Morales: Writing – review & editing, Supervision.