To analyse and address the barriers that Pharmacy Services encounter when adopting the capacity-motivation-opportunity (CMO) pharmaceutical care model in outpatient consultations, identifying the relevant actors in the ecosystem, as well as the motivations. Finally, to make a first approach to solutions that will help us to select those that could be developed through future initiatives.
MethodA structured methodology was developed in several phases. Two teams were formed: the “Core Team,” consisting of hospital pharmacists with experience in pharmaceutical care and patient-centered care, and the “Guiding Team,” made up of professionals from various disciplines. The first phase included an online prospecting workshop to explore the phases of model adoption. Subsequently, semi-structured interviews were conducted with key actors, such as physicians, managers, and patient associations, to identify needs and barriers. Finally, a face-to-face workshop was organized to facilitate the ideation and validation of solutions.
ResultsThree categories of actors in the CMO model ecosystem were identified: interested agents (beneficiaries), interesting agents (influencers), and executive agents (implementers). Significant barriers were found, including variability in infrastructure, lack of commitment from managers, and the workload of pharmacists. However, there was also growing motivation among professionals and organizations to adopt the model. During the ideation workshop, ten initiatives were prioritized, including the use of digital technologies and ongoing training programs.
ConclusionsThe study highlights the high potential of the CMO model to improve pharmaceutical care in outpatient settings in Spain, despite the identified barriers. The proposed strategies, focused on digitalization and multidisciplinary collaboration, are essential for effective implementation. Future research is suggested to evaluate the long-term impact of these initiatives and to strengthen the involvement of patient associations and other actors in the adoption process.
analizar y abordar las barreras que los servicios de farmacia se encuentran en el momento de la adopción del modelo de atención farmacéutica capacidad-motivación-oportunidad (CMO) en consultas externas, identificando los actores relevantes en el ecosistema, así como las motivaciones. Por último, realizar un primer acercamiento a soluciones que nos ayuden a seleccionar las que podrían llevarse a desarrollo a través de iniciativas futuras.
Métodose desarrolló una metodología estructurada en varias fases, incluyendo la formación de dos equipos de trabajo: el «Equipo Rector», compuesto por farmacéuticos hospitalarios con experiencia en atención farmacéutica y humanización asistencial, y el «Equipo Core», integrado por profesionales de diversas disciplinas. La primera fase consistió en la realización de un taller de prospección online para explorar las fases de adopción del modelo. Posteriormente, se realizaron entrevistas semiestructuradas a actores clave (médicos, gerentes y asociaciones de pacientes) para identificar necesidades y barreras. Finalmente, se realizó un taller presencial que facilitó la ideación de soluciones y validación de propuestas.
Resultadosse identificaron tres categorías de actores en el ecosistema del modelo CMO: agentes interesados (beneficiarios), agentes interesantes (influenciadores) y agentes ejecutivos (implementadores). Se evidenciaron diversas barreras, como la variabilidad en la infraestructura, la falta de interés de los gestores y la sobrecarga laboral de los farmacéuticos. No obstante, se observó una creciente motivación por parte de los profesionales y organizaciones para adoptar el modelo. Durante el taller de ideación se priorizaron diez iniciativas, que incluyeron el uso de tecnologías digitales y programas de formación continua.
Conclusiónse destaca el alto potencial del modelo CMO para mejorar la atención farmacéutica en consultas externas de farmacia hospitalaria en España, a pesar de las barreras existentes. Las estrategias propuestas, centradas en la digitalización y la colaboración multidisciplinar, son clave para su implementación efectiva. Se sugiere la necesidad de futuras investigaciones para evaluar el impacto a largo plazo de estas iniciativas y fortalecer la implicación de asociaciones de pacientes y otros actores en el proceso de adopción.
Since its launch in 2014, the Strategic Map for Outpatient Care (MAPEX) project has played a pivotal role in transforming pharmaceutical care (PC) in Spain, particularly in the setting of hospital outpatient units1. The Spanish Society of Hospital Pharmacy (SEFH) initiated this project in response to the need to implement a patient-centred model, rather than a medication-focused one, with the aim of optimising health outcomes, particularly in the context of chronic patients and those with highly complex conditions in specialised care settings2. Since its inception, MAPEX has been driven by the need to redefine the role of pharmacists in the monitoring and management of drug therapy, with the objective of advancing towards comprehensive PC, focused on providing meaningful answers to patients, the healthcare system, and contemporary society answers, and always with a forward-looking vision3.
One of the milestones in its development was the first “Consensus Conference”, held in 20161. During the conference, various key actors in the healthcare sector, including hospital pharmacists, managers, patient associations, and other healthcare professionals, recognised the need to create a new PC model that would address the challenges of the emerging health and social care environment. This conference not only marked the beginning of a transformation process but also served as a platform for adopting a structured and evidence-based approach. As a result of this consensus, along with the initial situation analysis conducted up to that point, the Capacity-Motivation-Opportunity (CMO) model was proposed and defined, inspired by public health intervention methodology, but adapted to the specific needs of hospital pharmacy4.
The CMO model and its proposed redefinition of the concept of PC emphasises the need to go beyond medication dispensing. It focuses on optimising patients' ability to manage their treatment, their motivation to adhere to the therapeutic guidelines, and their achievement of health goals, while incorporating support from new technologies to provide timely and sustained monitoring over time5. This new definition highlights the importance of a model that addresses both individual and systemic barriers that may interfere with proper medication management, while promoting a more holistic and personalised strategy based on a multidimensional approach.
Since its introduction, the CMO model has been adapted to different patient profiles, including those with chronic conditions and others with highly complex health needs such as HIV, oncohaematological disorders, immune-mediated conditions, or respiratory diseases6–9. Various projects within the framework of the SEFH working groups have demonstrated the usefulness of the model for improving health outcomes, such as monitoring therapeutic adherence, achieving health goals, and optimising patients' quality of life. In addition, they have shown how integrating this model into daily pharmaceutical practice has positively impacted healthcare and pharmacotherapeutic management, although there remains significant room for improvement regarding its widespread adoption10,11.
The survey analysing the evolution of the MAPEX project between 2016 and 2021 demonstrated that, despite progress, significant barriers remain in the implementation of the model in practice12,13. These barriers include variability in the infrastructure available in hospital pharmacy outpatient units (HOPUs), varying levels of commitment from managers and healthcare professionals, lack of awareness of patient associations, and the limited human and technological resources typical of many hospital pharmacy services (HPSs). To address these challenges, the QPEX healthcare quality certification standard—pioneering at the global level—has been developed14. Despite the obstacles described above, the survey highlighted increasing motivation among professionals and institutions to further develop and implement the CMO model. Key areas for improvement and expansion were identified for future stages, as outlined in the prioritisation of initiatives scheduled for implementation in the period 2024 to 2027, following the project's second Consensus Conference held in 202315.
Since 2019, the SEFH has also been promoting improvements in the humanisation of care within HPSs, including, of course, outpatient units. The focus has been placed on improving care circuits, patient interactions with the healthcare system, and the overall patient experience—elements that align closely with the concept of the CMO model itself16.
Although the MAPEX project has therefore been a fundamental catalyst in the evolution of PC in Spain, providing a structured and adaptable framework that has proved useful in different clinical contexts, the implementation of the CMO model in pharmaceutical practice still poses challenges, highlighting the need to continue developing strategies that facilitate its adoption and consolidation throughout the health system.
The aim of this study was to analyse and address the barriers that HPSs face at the point of adopting the CMO model for PC in outpatient settings, to identify the relevant actors in the ecosystem and their motivations, and to explore potential solutions that could be prioritised for development through future initiatives.
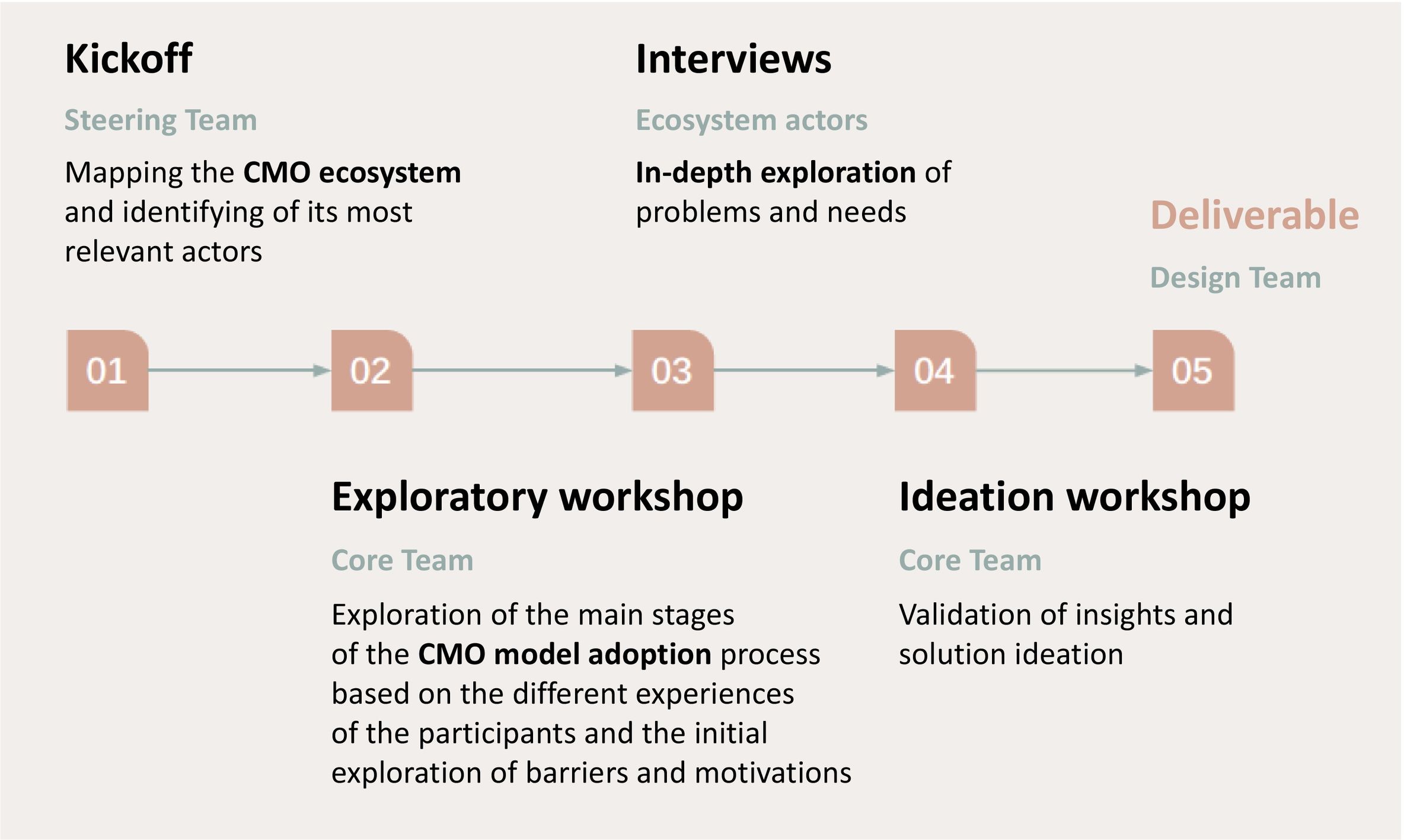
MethodThe project was conducted using a Human-Centered Design methodology with support from a strategic service design consultancy with extensive experience in this type of initiative. The roadmap was planned in several stages (see Fig. 1). Two working teams were set up: the Steering Team, made up of 2 hospital pharmacists with experience in outpatient PC and extensive knowledge of the CMO methodology and the SEFH humanisation guidelines; and the Core Team, a broader group made up of pharmacists with expertise in outpatient PC from eight national hospitals, as well as various healthcare actors, including medical specialists, hospital managers, representatives of the pharmaceutical industry and patient associations, with the aim of providing a global vision and validating the proposals developed by the Core Team. This team structure was chosen to include a range of perspectives and to ensure that the analysis and proposed solutions would be applicable in different hospital settings.
Once the two teams had been established, in the first stage, the Steering Team mapped the CMO model ecosystem and identified its main actors.
In the second stage, an online exploratory workshop was held with the Core Team aimed at identifying the main stages of the model adoption process based on the diverse experiences of the participants and to conduct an initial exploration of barriers and motivations.
The third stage consisted of a series of semi-structured interviews with key actors within the CMO model ecosystem, such as medical specialists, members of patient associations, hospital managers, heads of HPSs, and other healthcare professionals. These qualitative interviews served to identify the specific needs of each group and to explore in more depth the barriers and facilitators currently influencing adoption of the model. Information was also collected on the motivations of these actors, enabling the development of a framework tailored to the realities and expectations of each group within the healthcare ecosystem.
In the fourth stage, a face-to-face ideation workshop was held to facilitate the ideation process and validate both the information and proposals made by the team members. The workshop was designed and structured as a co-creation space in which, through a divergent thinking exercise, the participants discussed and confirmed the barriers and opportunities identified. In addition, the data obtained were analysed and solutions to the problems identified were developed.
The fifth and final stage focused on communication—which included a strategy for disseminating the results through various means, such as scientific publications, presentations at conferences, social networks, and collaboration with patient associations—to make the benefits of the model known to health managers, clinicians, and patients, and to highlight the importance of its adoption in improving health outcomes and the efficiency of the health system.
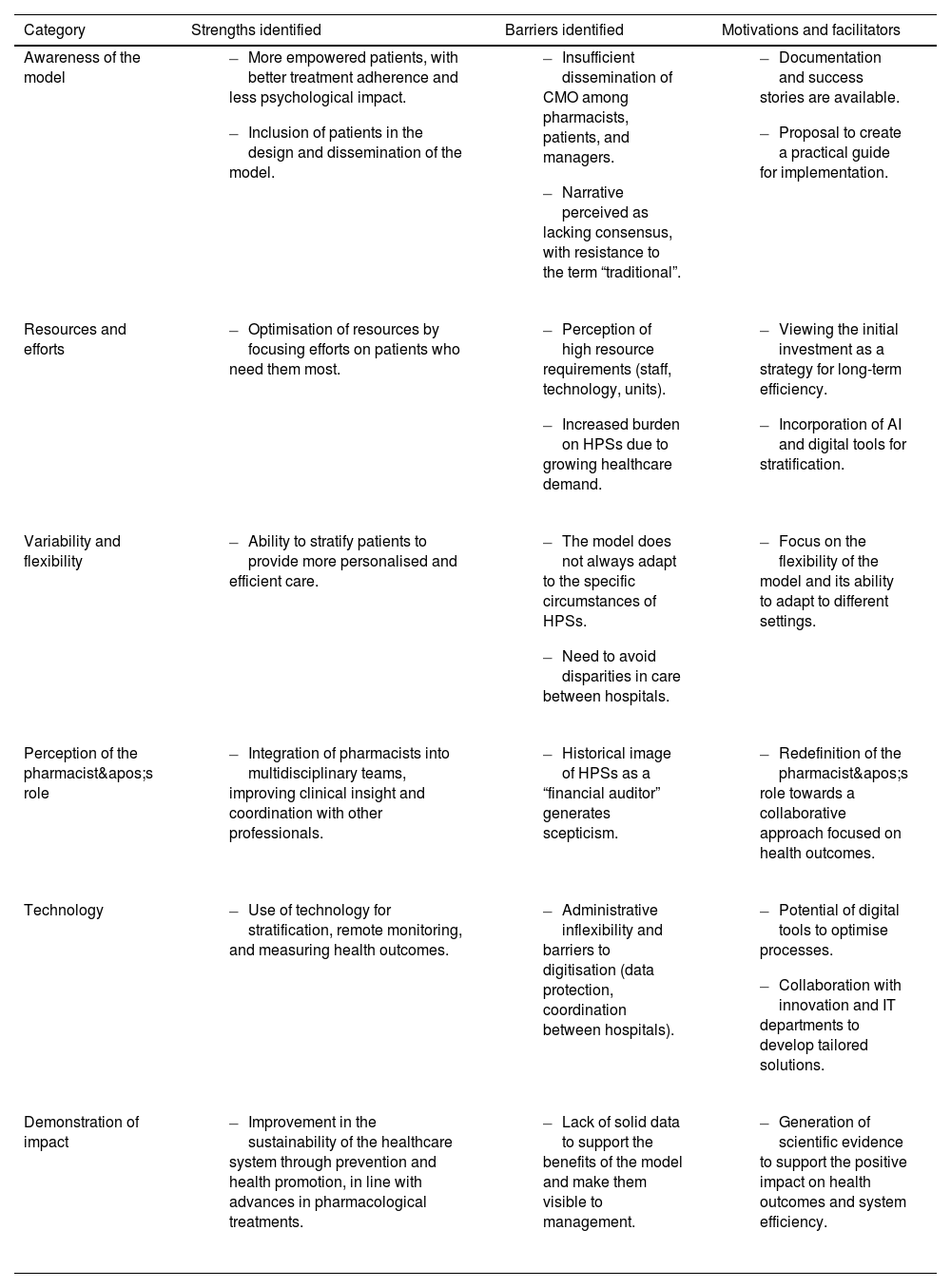
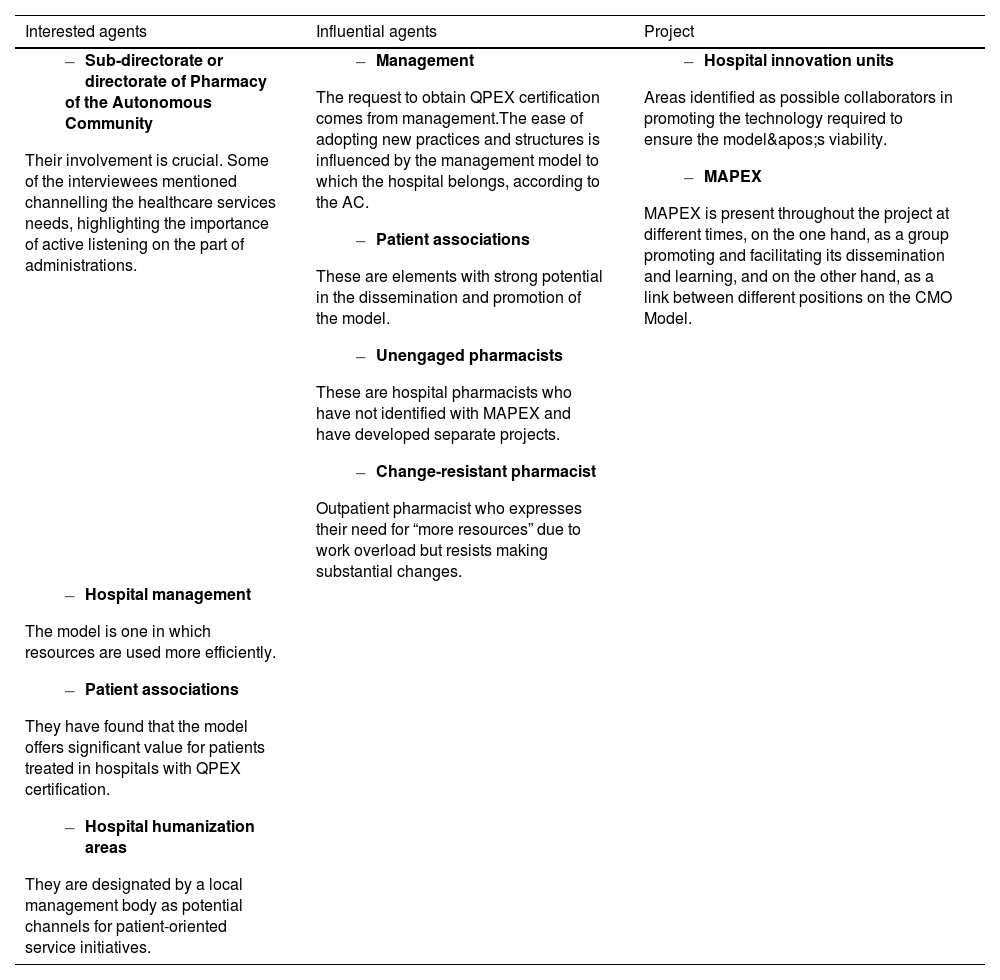
ResultsTable 1 shows the barriers, strengths, and motivations identified for implementing the CMO model. The key diagram defining the CMO model ecosystem within the current healthcare system identified the main actors within it, dividing them into 3 categories: interested agents, defined as the beneficiaries of the model; influential agents, those who influence its adoption; and executive or project agents, responsible for applying the model. This classification is fundamental to understanding the interactions between the different groups and designing specific strategies. A survey was conducted with each agent to identify their barriers and motivations for adopting the model. Table 2 shows a summary of these issues.
Strengths, barriers, and motivations identified in the Capacity-Motivation-Opportunity model for pharmaceutical care.
| Category | Strengths identified | Barriers identified | Motivations and facilitators |
|---|---|---|---|
| Awareness of the model |
|
|
|
| Resources and efforts |
|
|
|
| Variability and flexibility |
|
|
|
| Perception of the pharmacist's role |
|
|
|
| Technology |
|
|
|
| Demonstration of impact |
|
|
|
CMO, Capacity-Motivation-Opportunity; AI, Artificial Intelligence; HPS, Hospital Pharmacy Services; IT, Information Technology.
Actors in the system and their motivation or barriers to implementation.
| Interested agents | Influential agents | Project |
|---|---|---|
|
|
|
|
AC, Autonomous Community; MAPEX, Strategic Map for Outpatient Care; QPEX, MAPEX Quality Certification Standard for Outpatient Care.
As a result of the exploratory workshop, the stages of the model adoption process were defined, identifying in each of them the “key steps or moments”, sub-steps, tools, process steps requiring significant effort, barriers, motivations or levers for implementation, and specific cases of success.
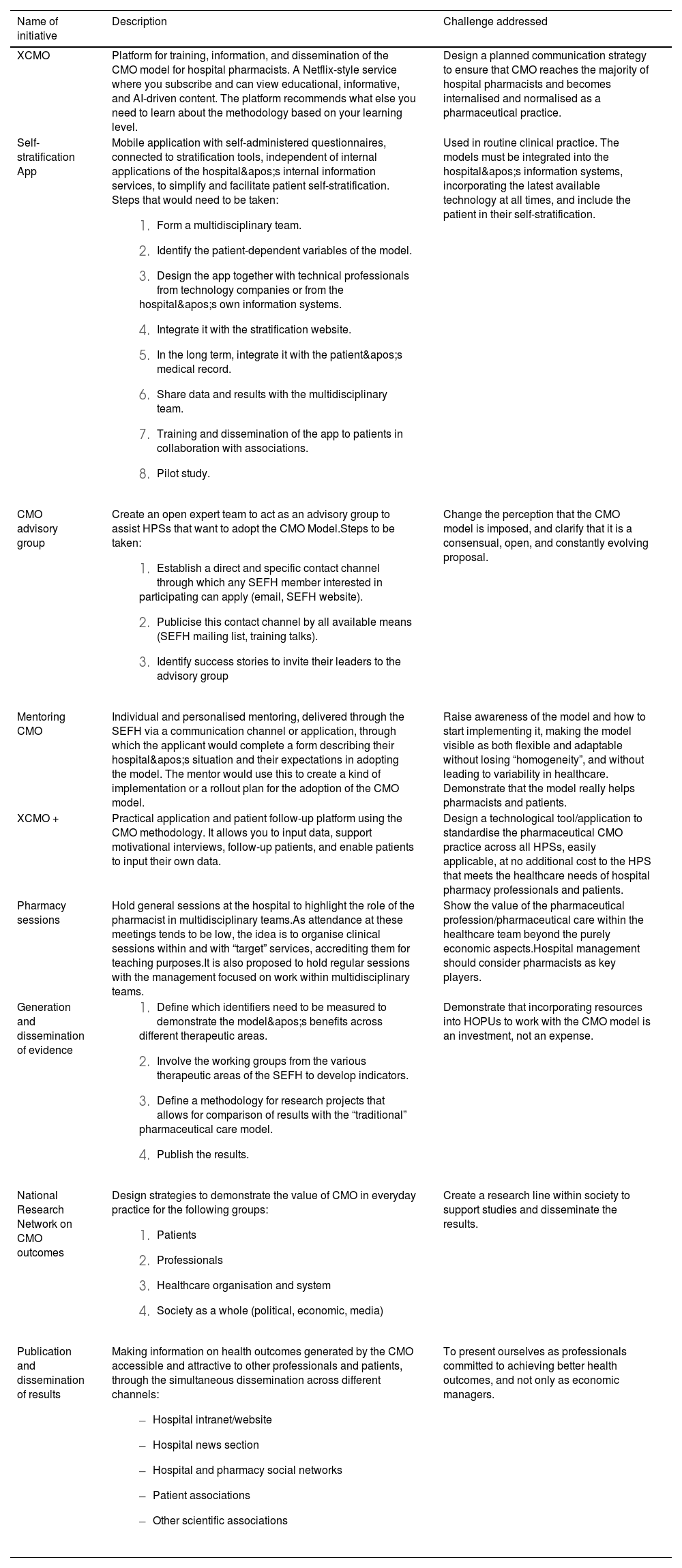
Finally, the battery of solutions developed during the ideation workshop was prioritised according to their feasibility and applicability in pharmaceutical practice. Table 3 provides a summary of the top ideas.
Proposed prioritised initiatives.
| Name of initiative | Description | Challenge addressed |
|---|---|---|
| XCMO | Platform for training, information, and dissemination of the CMO model for hospital pharmacists. A Netflix-style service where you subscribe and can view educational, informative, and AI-driven content. The platform recommends what else you need to learn about the methodology based on your learning level. | Design a planned communication strategy to ensure that CMO reaches the majority of hospital pharmacists and becomes internalised and normalised as a pharmaceutical practice. |
| Self-stratification App | Mobile application with self-administered questionnaires, connected to stratification tools, independent of internal applications of the hospital's internal information services, to simplify and facilitate patient self-stratification. Steps that would need to be taken:
| Used in routine clinical practice. The models must be integrated into the hospital's information systems, incorporating the latest available technology at all times, and include the patient in their self-stratification. |
| CMO advisory group | Create an open expert team to act as an advisory group to assist HPSs that want to adopt the CMO Model.Steps to be taken:
| Change the perception that the CMO model is imposed, and clarify that it is a consensual, open, and constantly evolving proposal. |
| Mentoring CMO | Individual and personalised mentoring, delivered through the SEFH via a communication channel or application, through which the applicant would complete a form describing their hospital's situation and their expectations in adopting the model. The mentor would use this to create a kind of implementation or a rollout plan for the adoption of the CMO model. | Raise awareness of the model and how to start implementing it, making the model visible as both flexible and adaptable without losing “homogeneity”, and without leading to variability in healthcare. Demonstrate that the model really helps pharmacists and patients. |
| XCMO + | Practical application and patient follow-up platform using the CMO methodology. It allows you to input data, support motivational interviews, follow-up patients, and enable patients to input their own data. | Design a technological tool/application to standardise the pharmaceutical CMO practice across all HPSs, easily applicable, at no additional cost to the HPS that meets the healthcare needs of hospital pharmacy professionals and patients. |
| Pharmacy sessions | Hold general sessions at the hospital to highlight the role of the pharmacist in multidisciplinary teams.As attendance at these meetings tends to be low, the idea is to organise clinical sessions within and with “target” services, accrediting them for teaching purposes.It is also proposed to hold regular sessions with the management focused on work within multidisciplinary teams. | Show the value of the pharmaceutical profession/pharmaceutical care within the healthcare team beyond the purely economic aspects.Hospital management should consider pharmacists as key players. |
| Generation and dissemination of evidence |
| Demonstrate that incorporating resources into HOPUs to work with the CMO model is an investment, not an expense. |
| National Research Network on CMO outcomes | Design strategies to demonstrate the value of CMO in everyday practice for the following groups:
| Create a research line within society to support studies and disseminate the results. |
| Publication and dissemination of results | Making information on health outcomes generated by the CMO accessible and attractive to other professionals and patients, through the simultaneous dissemination across different channels:
| To present ourselves as professionals committed to achieving better health outcomes, and not only as economic managers. |
CMO, Capacity-Motivation-Opportunity; MAPEX, Strategic Map for External Patient Care; SEFH, Spanish Society of Hospital Pharmacy; HPS, Hospital Pharmacy Services; HOPUs, Hospital Outpatient Pharmacy Units.
The results of this study underline the importance and potential of the CMO model in hospital pharmacy, particularly in the setting of HOPUs. The identification of the key actors and the classification into interested agents, influential agents, and executive agents provides a clear view of the dynamics and interactions needed for the effective and widespread adoption of the model.
Within the analysed healthcare ecosystem, and in line with what is expected from future hospitals17,18, the Sub-directorates or Directorates of Pharmacy of the Autonomous Communities have been identified as key actors in the implementation of the model, as they have a comprehensive view of the needs and challenges of pharmaceutical services within the different regions. However, their relationship with hospital services is a critical issue, as it can be decisive but also problematic if there is no active listening on their part. For the model to be successfully implemented, it is essential that health authorities not only listen but also respond proactively to the needs expressed by the HPSs. Therefore, the interests of regional administrations should be aligned with clinical needs and the interests of hospital pharmacists. Any lack of connection could lead to resistance and hinder the implementation of the model.
Hospital management also plays a key role in directly influencing the adoption of new practices, as has already been demonstrated with QPEX quality certification. The ease of implementing the CMO model can vary depending on each hospital's management model. Implementation depends largely on how each management prioritises and manages resources. If hospitals have rigid systems or are overloaded, the adoption of innovative models like CMO may be slower.
However, the key players in the implementation of the CMO model are hospital pharmacists, who act as the main drivers of change in pharmaceutical outpatient care. Their knowledge of pharmacotherapy, direct contact with patients, and ability to coordinate with other healthcare professionals place them in a strategic position to lead the adoption of the model. However, their success in implementing the CMO model requires a supportive environment where hospital management provides the necessary resources, training, and digitisation. In this sense, although the initiative comes from pharmacists, support from hospital management is key to ensuring its consolidation and widespread adoption.
For all these reasons, the hospital management model and quality improvement strategies, such as QPEX certification, influence the speed and depth of implementation of the CMO model. It will be interesting to see, especially in the light of progress in implementing the standard, how healthcare incentives at the managerial level can help to overcome these structural and care-related barriers.
Patient associations have significant potential in disseminating and promoting the CMO model, as they can serve as a bridge between hospital services and the community. Although these associations can support implementation, they need to be well informed about the model and its benefits in order to communicate them effectively to patients. The role of associations as agents of change and their active involvement in the CMO approach can increase acceptance and boost patient demand for the model. A lack of alignment between associations and hospitals, or more specifically, the HPSs themselves, may hinder its adoption.
The so-called “unengaged pharmacists” or those who are resistant to change—who are invariably present—can be a factor in resistance to the implementation of the CMO model. This may be due to overload on many occasions, particularly among those who work in outpatient units. To overcome these barriers, strategies need to be developed that address the main concerns of this professional group. These actions may include providing specific training on the model and its benefits, dispelling myths and misconceptions, and ensuring that the necessary resources are in place so that its implementation does not increase the workload. In addition, sharing successful experiences from other settings, where CMO has been shown to significantly improve traditional pharmaceutical care, can be key to motivating active participation and facilitating the transition to the new model19,20. Resistance among pharmacists should therefore be mitigated through training and, whenever possible, the provision of adequate resources and the reduction or adjustment of workload. In the future, it will also be interesting to explore how effective change management at the local level can modify these professionals' perceptions of CMO and their future involvement, both for current professionals and those who are in training.
Finally, the hospitals' innovation and humanisation units have also been identified as key actors within the ecosystem. These units are positioned as key collaborators in implementing the technology needed to support the CMO methodology and its patient-centred approach. Their role is crucial in the technology-related changes required to fully integrate the model into hospital information systems.
Collaboration between innovation and humanization units and HPSs is not always smooth, as the interests and priorities of the innovation units may not fully align with the specific needs of hospital pharmacists and may, in fact, be more focused on other healthcare innovations in different areas. It will be useful to analyse how strategic alliances between innovation units and HPSs can accelerate implementation and build bridges between professionals and patients.
The prioritised initiatives and proposals share some features such as digitisation and the use of new technologies (e.g. XCMO, the Self-stratification App, and XCMO+), driven by the need to create platforms and applications that facilitate training, self-stratification, and patient follow-up. This emphasis on digitisation and the use of technological tools to facilitate the implementation of the CMO model also mirrors trends in the healthcare sector's digital transformation21–23. There is also a clear commitment to training (e.g. CMO Mentoring), prioritising it alongside knowledge sharing. All these aspects are designed to enable hospital pharmacists and other healthcare professionals to understand and effectively apply the model, which is crucial for the widespread implementation of the CMO model in HPSs. Similarly, multidisciplinary integration is a key focus, highlighting the need for active collaboration between pharmacists and other healthcare professionals, as mentioned under “Pharmacy Sessions” and “Generation and Dissemination of Evidence”. The CMO model therefore seeks not only to improve PC but also to strengthen the integration of pharmacists into multidisciplinary teams.
Finally, the importance of generating data and tangible results, as noted under “Generation and Dissemination of Evidence” or the “National Research Network”, emphasises the need to measure health outcomes to demonstrate the positive impact of the CMO model.
These initiatives are practicable—a key aspect that has been taken into consideration—given that many similar digital tools are already in use in other healthcare sectors. However, integration with current hospital systems and interoperability with electronic health records could present significant technical challenges, although these can be overcome with the appropriate technology partners24–26. It is worth highlighting the “Expert groups and mentoring” and “CMO advisory group” initiatives, which could be readily implemented, as they mainly require the mobilisation of human resources and the organisation of training activities.
All the initiatives, such as “Pharmacy sessions” and “Publication and dissemination of results”, aim to improve the visibility of the pharmacist's role, but they require a coordinated effort between HPSs, hospital management, and patient associations.
In this regard, the well-known resistance to change must be addressed at the strategic, macro, micro, and local levels. A significant obstacle may lie in the perception that the CMO model is an “imposition”, as mentioned under “CMO Advisory Group”. It will therefore be crucial to establish clear channels of communication and demonstrate with evidence that the model brings real benefits to both professionals and patients. As mentioned, the integration of new technologies and applications into existing hospital systems could pose a challenge, especially in hospitals with outdated IT infrastructure or stringent security protocols.
Despite these aspects, all the initiatives are in line with current healthcare trends, as they take digital transformation into account27–29. The focus on creating digital tools for patient monitoring, education, and stratification is also aligned with growing trends in healthcare systems. Furthermore, the incorporation of artificial intelligence in training (as in XCMO) is a plus point, mirroring the current focus on personalised learning and process automation.
The measurement of health outcomes to generate evidence and assess clinical impact is in line with the increasing focus on prioritising outcomes-based medicine, an approach that is gaining ground in healthcare planning in many countries, including Spain. The key aspect of a multidimensional approach, focused on humanisation and the patient experience, should also be borne in mind. Involving patients in their own care, as exemplified by the Self-stratification App, reflects the trend of empowering patients and encouraging their active participation in managing their health, in alignment with patient-centred healthcare policies.
The development of the proposed tools will require specialised technical teams to design, implement and maintain the platforms and applications. Institutional support, such as the backing of hospital management, is essential to ensure that computer systems and organisational structures adapt to the new processes introduced by the CMO model. Continuous training will of course be necessary, with accessible training programmes delivered at different levels of need and appeal, motivating pharmacists to acquire and apply CMO knowledge in their daily practice.
It is relevant to recognise the limitations inherent to this qualitative study. Although attempts were made to control for inter-hospital variability by selecting centres with different levels of CMO model adoption and by including participants with different perspectives (i.e. for or against the model), the results reflect contextualised experiences and are not intended to be statistically generalizable. It would therefore be relevant to conduct further qualitative and quantitative studies to complement these results and allow for a more in-depth exploration of the applicability of the model in different settings.
Future research should focus on the long-term evaluation of the proposed initiatives and their impact on pharmaceutical practice. It would also be useful to explore strategies to more actively involve patient associations and other key actors in the process of adopting the CMO model.
In conclusion, the results show that there is great potential for adopting the CMO model and levering its value to improve PC in the outpatient units of HPSs in Spain. Despite the barriers identified, the motivation and commitment of professionals and institutions suggest that, with the right strategies in place, a more widespread and effective adoption of the model is achievable in the near future.
Contribution to the scientific literatureThe main contribution of this study to the scientific literature lies in its analysis of the challenges associated with the adoption and implementation of the CMO (Capacity-Motivation-Opportunity) model in pharmaceutical care in Spain, especially in the setting of outpatient units. The study used a structured methodology, including the identification of key actors, to provide a comprehensive framework for understanding the dynamics affecting the adoption of the model. This study not only highlights existing barriers, such as variability in infrastructure and lack of commitment from managers, but also underscores the potential of this model to transform PC, emphasising the importance of a multidisciplinary and patient-centred approach.
It also provides a set of concrete and strategic initiatives, prioritised through a collaborative ideation process, that can facilitate the implementation of the CMO model in pharmaceutical practice. It offers healthcare professionals practical tools to improve health outcomes by identifying digital technologies and continuing education programmes as key elements in overcoming barriers to its adoption. This contribution is not only relevant to the field of hospital pharmacy but could also serve as a benchmark in other settings and healthcare systems that are seeking to optimise PC through innovative and patient-centred approaches.
CRediT authorship contribution statementAna María Álvarez-Díaz: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Formal analysis, Conceptualization. Carlos Crespo Diz: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Formal analysis, Conceptualization. Emilio Monte Boquet: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Investigation, Formal analysis, Conceptualization. José Antonio Marcos Rodríguez: Writing – review & editing, Visualization, Validation, Supervision, Methodology, Investigation. Luis Margusino Framinan: Writing – review & editing, Visualization, Validation, Supervision, Methodology, Investigation. Elena Sánchez Yañez: Writing – review & editing, Visualization, Validation, Supervision, Methodology, Investigation. Manuel Vélez-Díaz-Pallarés: Writing – review & editing, Visualization, Validation, Supervision, Methodology, Investigation. Esther Vicente Escrig: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Conceptualization. Ramón Morillo-Verdugo: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization.
FundingThe authors declare that this study was conducted using the Spanish Society of Hospital Pharmacy's own funds.
The authors declare that no competing of interest.
We would like to thank all those who participated in this initiative, particularly the interviewees. We also extend our thanks to Irene Porro, Antonella Imbriaco, Jesús Sotelo, and Gelo Alvarez de Oopen for their help with the methodology used to conduct the project. Finally, we would like to thank all the collaborators of the MAPEX-SEFH project, as well as the Board of Directors and the Governing Board of the SEFH for their support of the project.