The primary objective is to describe the real-life effectiveness and safety of nivolumab treatment in patients with relapsed or refractory classical Hodgkin's lymphoma. The secondary objective is to describe the therapeutic management after nivolumab monotherapy.
MethodObservational, retrospective, multidisciplinary study including all patients with relapsed or refractory classical Hodgkin's lymphoma treated with nivolumab monotherapy from November 2015 to March 2023. Patient and treatment-related variables were collected. Effectiveness was measured as overall response rate, progression-free survival, and overall survival. Safety was measured as percentage of patients with adverse effects and severity.
ResultsThirteen patients were included, median age 37.5 years (RIQ: 25.3–54.7), 84.6% male. The median number of previous lines of therapy was 3 (RIQ: 2–4.5), including autologous haematopoietic stem cell transplantation (84.6%) and brentuximab vedotin (100%). All received nivolumab 3 mg/kg/14 days, with a median of 11 cycles (RIQ: 6.5–20.5) per patient. Median time on treatment was 4.9 months (RIQ: 3–9.6) and median follow-up time was 9.2 months (RIQ: 5.6–32.3).
Complete response was achieved by 3 patients (23.1%), partial response by 3 (23.1%), stable disease by 3 (23.1%), and progression by 4 (30.8%). The objective response rate was 46.2%. Median progression-free survival was 23.9 months (95% CI: 0–49.1), median overall survival was not reached. At the study cut-off date, 5 patients had died (38.5%), 4 were in complete remission without active treatment (30.8%), and 4 were continuing treatment (30.8%).
Adverse events occurred in 76.9% of patients, 44% of severity ≥3, the most frequent being hypothyroidism and hepatotoxicity. One patient discontinued treatment due to pneumonitis, 2 suffered treatment delays (thrombocytopenia and hypertransaminemia), and 1 changed the regimen to monthly (pulmonary toxicity).
ConclusionsNivolumab in the treatment of relapsed or refractory classical Hodgkin's lymphoma has confirmed favourable effectiveness data in the study sample, expressed as objective response rate of 46.2% and a clinical benefit rate of 69.2%. Safety was acceptable, manageable, and consistent with that described in the literature.
El objetivo principal es describir la efectividad y seguridad en vida real del tratamiento con nivolumab en pacientes con linfoma de Hodgkin clásico en recaída o refractario. El objetivo secundario es describir el manejo terapéutico tras la monoterapia con nivolumab.
MétodoEstudio observacional, retrospectivo y multidisciplinar que incluyó a todos los pacientes con linfoma de Hodgkin clásico en recaída o refractario tratados con nivolumab en monoterapia desde noviembre de 2015 hasta marzo de 2023. Se recogieron variables relacionadas con el paciente y con el tratamiento. La efectividad se midió como tasa de respuestas globales, supervivencia libre de progresión y supervivencia global. La seguridad se midió como porcentaje de pacientes con efectos adversos y gravedad.
ResultadosSe incluyeron trece pacientes, mediana de edad 37,5 años (RIQ: 25,3–54,7), 84,6% hombres. La mediana de líneas terapéuticas previas fue 3 (RIQ: 2–4,5), incluyendo trasplante autólogo de progenitores hematopoyéticos (84,6%) y brentuximab vedotin (100%). Todos recibieron nivolumab 3 mg/kg/14 días, con una mediana de 11 ciclos (RIQ: 6,5-20,5) por paciente. La mediana de tiempo en tratamiento fue de 4,9 meses (RIQ: 3–9,6) y la de tiempo de seguimiento de 9,2 meses (RIQ: 5,6-32,3).
Consiguieron respuesta completa 3 pacientes (23,1%), respuesta parcial 3 (23,1%), enfermedad estable 3 (23,1%) y progresaron 4 (30,8%). La tasa de respuesta objetiva fue del 46,2%. La mediana de supervivencia libre de progresión fue 23,9 meses (IC95%: 0–49,1), la mediana de supervivencia global no se alcanzó. A la fecha de corte del estudio, cinco pacientes fueron exitus (38,5%), cuatro mantenían remisión completa sin tratamiento activo (30,8%) y cuatro continuaban en tratamiento (30,8%).
El 76,9% de los pacientes presentó efectos adversos, 44% de gravedad ≥3, siendo los más frecuentes hipotiroidismo y hepatotoxicidad. Un paciente suspendió tratamiento por neumonitis, dos sufrieron retrasos de tratamiento (trombocitopenia e hipertransaminemia) y uno modificó la pauta a mensual (toxicidad pulmonar).
ConclusionesNivolumab en el tratamiento del linfoma de Hodgkin clásico en recaída o refractario ha confirmado en la muestra de estudio datos favorables de efectividad, expresada en tasa de respuesta objetiva del 46,2% y beneficio clínico del 69,2%. La seguridad fue aceptable, manejable, y concordante con lo descrito en la bibliografía.
Hodgkin lymphoma (HL) accounts for approximately 10% of all lymphomas and 0.6% of all cancers diagnosed worldwide each year.1 Its histology is characterised by the presence of malignant Hodgkin and Reed-Sternberg (HRS) cells, surrounded by abundant non-tumour inflammatory cells. It is divided into 2 main histological subtypes: classical HL (cHL) and nodular lymphocyte-predominant HL, the latter of which is generally indolent.
Recent advances in radiotherapy and polychemotherapy have significantly improved cure rates for patients with cHL, with approximately 80% of newly diagnosed patients under the age of 60 years achieving durable complete remission (CR).2,3
However, a significant percentage of patients either do not achieve these responses or experience a relapse. The standard treatment for patients with relapsed or refractory cHL (R/R cHL) is salvage chemotherapy followed by autologous haematopoietic stem cell transplantation (aHSCT), which has a CR rate of 50%.4,5 Patients with R/R cHL following aHSCT have a very poor prognosis, with a median survival of 27 months.6
However, the introduction of new drugs has improved the overall survival of patients who relapse after aHSCT.7 For many years, allogeneic haematopoietic stem cell transplantation (allo-HSCT) was the only curative treatment option for these patients.8 However, the situation has changed significantly with the approval of brentuximab vedotin (BV) and, more recently, of immune checkpoint inhibitors (ICIs).9
Programmed death-ligands 1 and 2 (PD-L1/2) are highly expressed in cHL HRS cells, with increased expression associated with amplification or gain of chromosomal region 9p24.1.10 This finding makes them excellent therapeutic targets for the ICIs nivolumab and pembrolizumab. The latter drug is not currently approved in Spain for this indication.
Nivolumab is a human monoclonal antibody that binds to the programmed death receptor 1 (PD-1) and blocks its interaction with PD-L1 and PD-L2. In Spain, it is approved and reimbursed as monotherapy in adult patients with R/R cHL following treatment with aHSCT and BV.11 The results supporting its approval are based on a phase 1b study (CheckMate 039) and a phase 2 study (CheckMate 205) in 95 patients who had received aHSCT and BV. The objective response rate (ORR) was 68%, with 13% achieving CR and 55% achieving partial response (PR). The median follow-up was 22.7 months, and the median duration of response was 15.9 months. Median progression-free survival (PFS) was 14.7 months. Median overall survival (OS) had not been reached at the time of analysis, but the OS rate was 95% at 12 months.12,13 Extended follow-up of the CheckMate 205 trial showed durable responses in patients with CR, PR, and stable disease (SD), with similar 1-year OS rates.14 Chemotherapy alone or in combination with an ICI, particularly as a bridge to allo-HSCT, has shown encouraging responses in some patients who have failed to anti-PD-1 therapy.15
The safety profile of nivolumab in HL is similar to that observed in other indications. Most adverse reactions, including serious ones, typically resolve after discontinuation, dose delay, or initiation of appropriate treatment (e.g., high-dose corticosteroids or other immunosuppressants).11,16,17 Although most adverse reactions are mild to moderate, definitive discontinuation of treatment may be necessary. Notably, treatment with nivolumab may increase the risk of severe graft-versus-host disease (GVHD) and death in patients who have previously undergone allo-HSCT, especially in those with a history of GVHD. Other immune-related adverse reactions may occur in patients who have received allo-HSCT prior to nivolumab, including sinusoidal obstruction syndrome (SOS) and early-onset severe acute fever (SAF), which develop 1–7 days after allo-HSCT.15,18
The primary objective of this study was to evaluate the effectiveness (measured as overall response rate, PFS, and OS) and safety of nivolumab monotherapy in patients with R/R cHL in the real-world setting of a tertiary care hospital. The secondary objective was to describe the therapeutic management of patients following nivolumab monotherapy.
MethodsAn observational, retrospective, multidisciplinary, single-centre study was conducted, including all patients with R/R cHL treated with nivolumab monotherapy at a tertiary hospital setting from November 2015 to August 2023.
We included patients aged at least 18 years with a diagnosis of R/R cHL who had been treated with nivolumab for at least 3 months (6 cycles). We excluded patients who had received nivolumab in a clinical trial or who had non-classical HL.
The following variables were collected at the start of nivolumab treatment: age, sex, cHL subtype (nodular, mixed cellularity, lymphocyte-rich, lymphoid-depleted), number of prior lines including HSCT, first-line refractory cHL, prior treatment, HSCT and BV prior to nivolumab, time from diagnosis of cHL to first dose of nivolumab, and Ann Arbor stage.
We also collected the following treatment variables: dose, regimen and number of nivolumab cycles, duration of nivolumab monotherapy, successive lines from discontinuation to end of follow-up, HSCT after nivolumab, and type of HSCT.
Effectiveness was assessed on the basis of best response as measured by positron emission tomography/computed tomography or computed tomography. Response was classified into 4 groups according to the Lugano classification criteria19,20: metabolic complete response (CR), metabolic PR, SD, and progressive disease (PD).
The overall ORR was defined as the sum of CR and PR, while the clinical benefit rate was defined as the sum of CR, PR, and SD. We also assessed PFS and OS at the end of the study.
Safety was measured by the occurrence and severity of AEs according to the National Cancer Institute's Common Terminology Criteria for Adverse Events v5.0 (NCI CTCAE).21 In addition, we recorded the reasons for dose reduction, discontinuation, or delay in treatment. We also measured the incidence of acute GVHD, SOS, and SAF in patients treated with nivolumab following allo-HSCT.
Data were obtained from medical records using Orion Clinic, and pharmacotherapeutic patient management information was obtained using Farmis-Oncofarm.
Centrally distributed quantitative variables are expressed as mean and 95% confidence interval (95% CI), and asymmetrically distributed quantitative variables are expressed as median and interquartile range (IQR). Categorical variables are presented as absolute and relative frequencies. A p-value of <.05 was used as the cut-off for statistical significance. The Kaplan–Meier method was used to calculate PFS and OS. All statistical analyses were performed with SPSS (IBM, Chicago).
The study was submitted to and approved by the Medical Reseach Ethics Commitee of the hospital (code 2023–435-1 [EOm]; approved 10 May 2023). To protect the patients' confidential data, they were identified by a numerical code in accordance with the Organic Law on Data Protection 3/2018, Regulation 2016/679 of the European Parliament, and of the Council of 27 April, 2016.
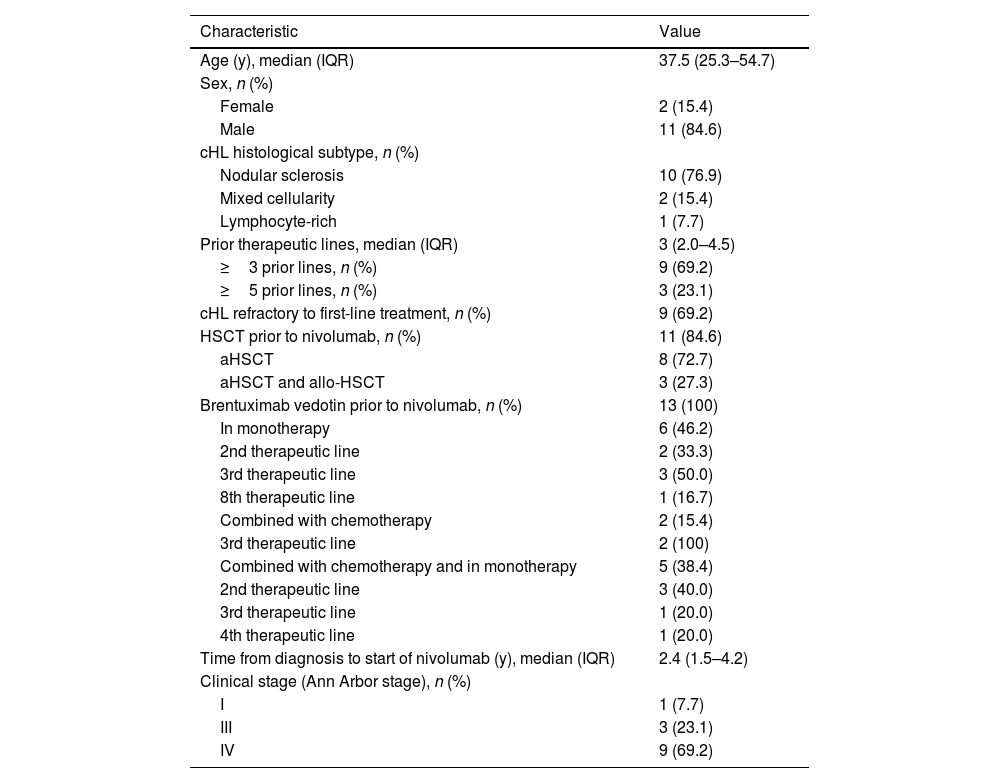
ResultsThe study included 13 patients. Table 1 describes their demographic and clinical characteristics at the start of nivolumab treatment.
Patient characteristics at the start of nivolumab treatment (n=13).
| Characteristic | Value |
|---|---|
| Age (y), median (IQR) | 37.5 (25.3–54.7) |
| Sex, n (%) | |
| Female | 2 (15.4) |
| Male | 11 (84.6) |
| cHL histological subtype, n (%) | |
| Nodular sclerosis | 10 (76.9) |
| Mixed cellularity | 2 (15.4) |
| Lymphocyte-rich | 1 (7.7) |
| Prior therapeutic lines, median (IQR) | 3 (2.0–4.5) |
| ≥3 prior lines, n (%) | 9 (69.2) |
| ≥5 prior lines, n (%) | 3 (23.1) |
| cHL refractory to first-line treatment, n (%) | 9 (69.2) |
| HSCT prior to nivolumab, n (%) | 11 (84.6) |
| aHSCT | 8 (72.7) |
| aHSCT and allo-HSCT | 3 (27.3) |
| Brentuximab vedotin prior to nivolumab, n (%) | 13 (100) |
| In monotherapy | 6 (46.2) |
| 2nd therapeutic line | 2 (33.3) |
| 3rd therapeutic line | 3 (50.0) |
| 8th therapeutic line | 1 (16.7) |
| Combined with chemotherapy | 2 (15.4) |
| 3rd therapeutic line | 2 (100) |
| Combined with chemotherapy and in monotherapy | 5 (38.4) |
| 2nd therapeutic line | 3 (40.0) |
| 3rd therapeutic line | 1 (20.0) |
| 4th therapeutic line | 1 (20.0) |
| Time from diagnosis to start of nivolumab (y), median (IQR) | 2.4 (1.5–4.2) |
| Clinical stage (Ann Arbor stage), n (%) | |
| I | 1 (7.7) |
| III | 3 (23.1) |
| IV | 9 (69.2) |
allo-HSCT, allogeneic haematopoietic stem cell transplantation; IQR, interquartile range; aHSCT, autologous haematopoietic stem cell transplantation; HSCT, haematopoietic stem cell transplantation.
All patients initially received nivolumab 3 mg/kg every 14 days, with a median of 11 cycles (IQR: 6.5–20.5) per patient. The median treatment duration was 4.9 months (IQR: 3.0–9.6). Total follow-up time was 93.9 months, with a median of 9.2 months (IQR: 5.6–32.3).
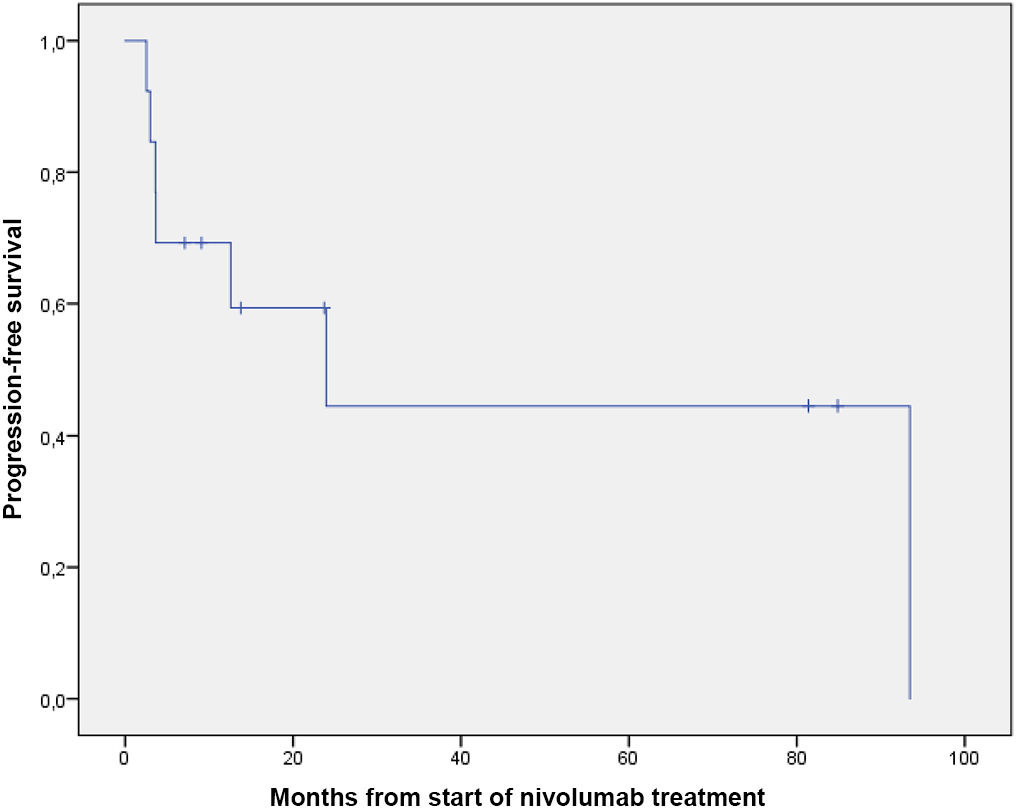
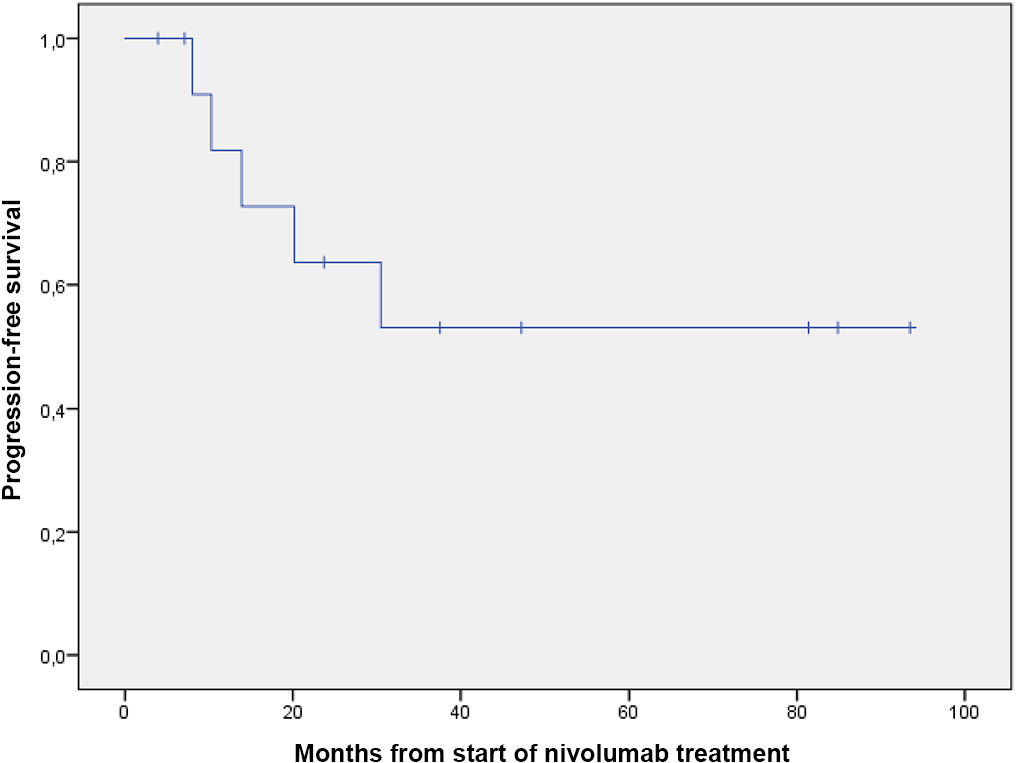
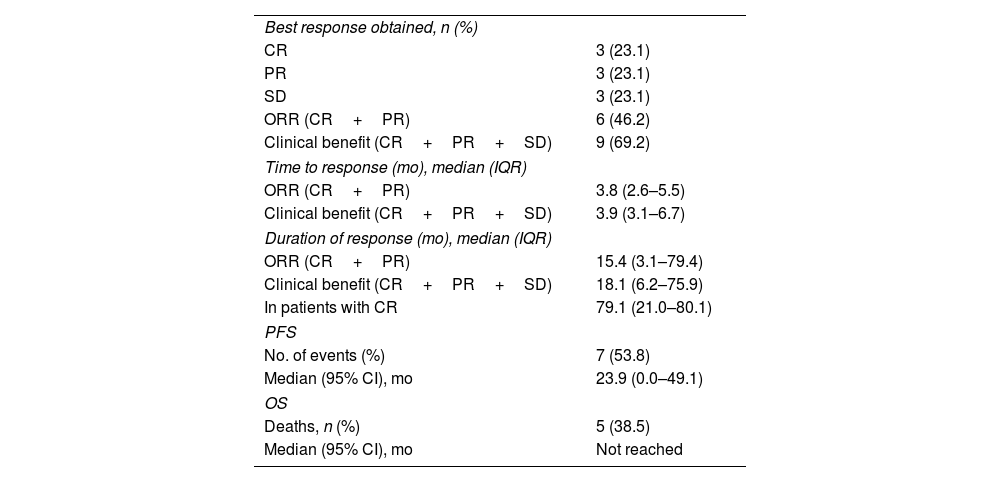
Table 2 shows the results for effectiveness, and Figs. 1 and 2 show survival curves.
Results for effectiveness.
| Best response obtained, n (%) | |
| CR | 3 (23.1) |
| PR | 3 (23.1) |
| SD | 3 (23.1) |
| ORR (CR+PR) | 6 (46.2) |
| Clinical benefit (CR+PR+SD) | 9 (69.2) |
| Time to response (mo), median (IQR) | |
| ORR (CR+PR) | 3.8 (2.6–5.5) |
| Clinical benefit (CR+PR+SD) | 3.9 (3.1–6.7) |
| Duration of response (mo), median (IQR) | |
| ORR (CR+PR) | 15.4 (3.1–79.4) |
| Clinical benefit (CR+PR+SD) | 18.1 (6.2–75.9) |
| In patients with CR | 79.1 (21.0–80.1) |
| PFS | |
| No. of events (%) | 7 (53.8) |
| Median (95% CI), mo | 23.9 (0.0–49.1) |
| OS | |
| Deaths, n (%) | 5 (38.5) |
| Median (95% CI), mo | Not reached |
SD, stable disease; 95% CI, 95% confidence interval; CR, complete response; IQR, interquartile range; PR, partial response; OS, overall survival; PFS, progression-free survival; ORR, objective response rate.
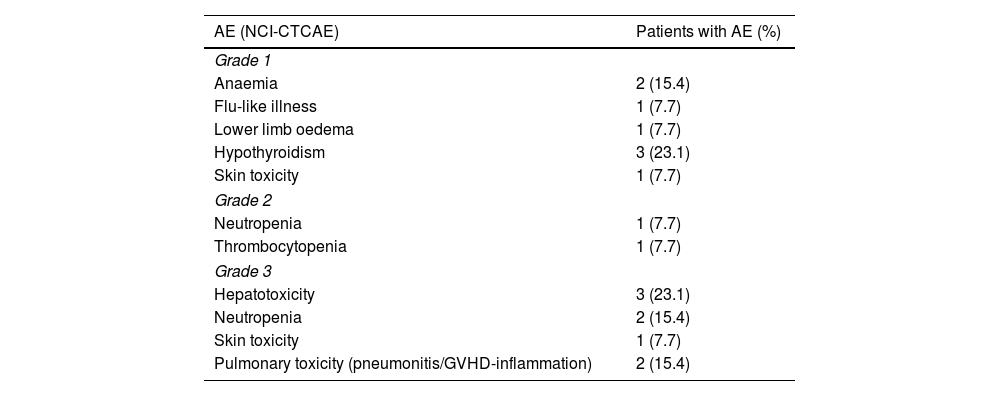
In terms of safety, 76.9% of patients (10 out of 13) experienced an AE of any grade. A total of 18 treatment-related AEs were reported. Table 3 shows the AEs and their severity according to NCI-CTCAE criteria. There were 10 grade 1 and 2 AEs (55.6%) and 8 grade 3 AEs (44.4%). One patient discontinued treatment due to toxicity (pneumonitis), 2 patients had temporary treatment delays or interruptions (thrombocytopenia and hypertransaminemia), and 1 patient had the interval between cycles changed from fortnightly to monthly (pulmonary toxicity). The management of AEs was as follows: 2 patients received filgrastim for neutropenia, 1 patient received epoetin for anaemia, and 2 patients received corticosteroids for immune-mediated toxicity (cutaneous and pulmonary).
Adverse events.
| AE (NCI-CTCAE) | Patients with AE (%) |
|---|---|
| Grade 1 | |
| Anaemia | 2 (15.4) |
| Flu-like illness | 1 (7.7) |
| Lower limb oedema | 1 (7.7) |
| Hypothyroidism | 3 (23.1) |
| Skin toxicity | 1 (7.7) |
| Grade 2 | |
| Neutropenia | 1 (7.7) |
| Thrombocytopenia | 1 (7.7) |
| Grade 3 | |
| Hepatotoxicity | 3 (23.1) |
| Neutropenia | 2 (15.4) |
| Skin toxicity | 1 (7.7) |
| Pulmonary toxicity (pneumonitis/GVHD-inflammation) | 2 (15.4) |
AE, adverse event; GVHD, graft-versus-host disease; NCI-CTCAE, National Cancer Institute's Common Terminology Criteria for Adverse Events.
Complications were common in patients treated with nivolumab after allo-HSCT (n=4), with 50% developing acute GVHD, 25% SOS, and 25% SAF. Haematopoietic progenitor cells were derived from HLA-identical (n=2) or haploidentical (n=2) peripheral blood of related donors. A reduced intensity conditioning regimen was used in all cases: thiotepa 10 mg/kg for 2 days, busulfan 6.4 mg/kg for 2 days, and fludarabine 150 mg/m2 for 3 days. GVHD prophylaxis consisted of post-HSCT intravenous cyclophosphamide (days +3 and +4) together with sirolimus and mycophenolate mofetil from day 5 post-HSCT. The median time between the last dose of nivolumab and allo-HSCT was 73.5 days (IQR: 41.5–94.8).
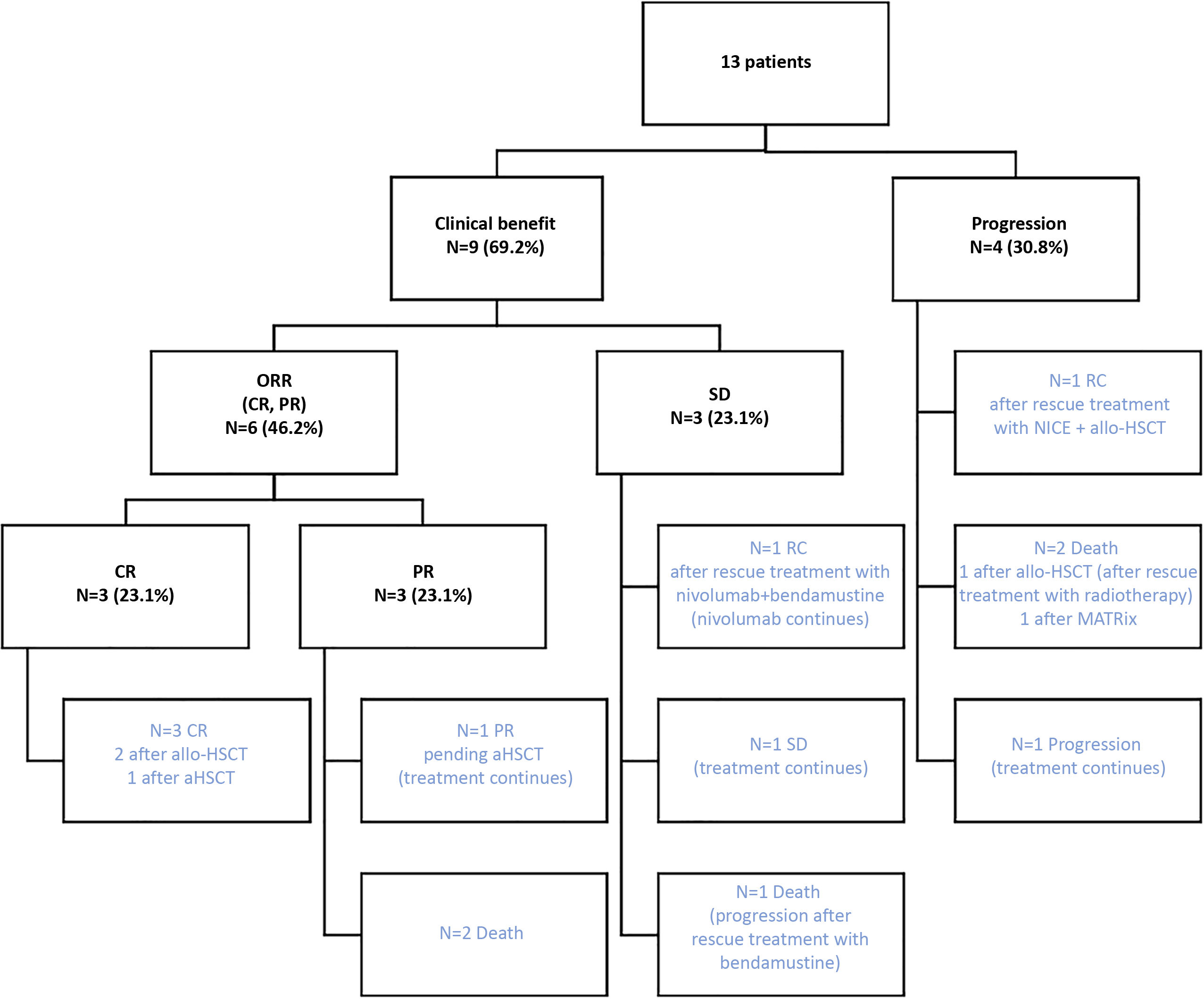
Fig. 3 depicts the results of the effectiveness of nivolumab monotherapy, as well as the subsequent therapeutic management of the patients, and their final situation. At the study cut-off date, 5 patients had died (38.5%), 4 were in CR without active treatment (30.8%), and 4 were still being treated (30.8%) (see Fig. 3).
Effectiveness of nivolumab monotherapy and subsequent therapeutic strategy.
ORR, objective response rate; CR, complete response; PR, partial response; aHSCT, autologous haematopoietic stem cell transplantation; allo-HSCT, allogeneic haematopoietic stem cell transplantation; NICE, nivolumab + ifosfamide/carboplatin/etoposide; MATRix, methotrexate/cytarabine/rituximab/thiotepa.
Five patients underwent transplantation after treatment with nivolumab, 4 underwent allo-HSCT, and 1 underwent aHSCT. All of them were in CR prior to transplantation, and 1 was rescued with 2 cycles of nivolumab in combination with ifosfamide/carboplatin/etoposide (NICE protocol). These patients were in CR after transplantation and at the end of the study, except for 1 patient who died from a post-transplant complication.
Two patients (15.4%) were rescued with an immunochemotherapy regimen. The patient with SD was treated with nivolumab 3 mg/kg every 14 days, with bendamustine 90 mg/m2 on days 1 and 2 every 28 days for 4 cycles, then continued on nivolumab monotherapy. The patient was in CR at the study end date. The other patient experienced disease progression and received salvage treatment with 2 cycles of NICE plus followed by allo-HSCT.
DiscussionThe present study analysed real-world data on nivolumab monotherapy in patients diagnosed with R/R cHL after aHSCT and BV treatment. The effectiveness and safety results are comparable to those reported in pivotal clinical trials.12,13
The median age of our sample was similar to that of the pivotal trial cohorts (37 years), and its variability was within the described range. Our sample had a lower percentage of women compared to the pivotal trials (30%–40%), which may be due to its small size. Most patients were heavily pretreated (69% had ≥3 prior lines and 23% had ≥5 lines compared with 52% with ≥5 lines in the pivotal trials), and most were in advanced stages (92% in stages III and IV compared with 85% in the pivotal trials).
In our study, the ORR of 46.2% was lower than the 68% observed in cohort B of the pivotal CheckMate 205 trial, who received similar treatment to the 13 patients in our study. In addition to the extended follow-up results from the phase II CheckMate 205 trial,14 5 large studies have published real-world results on the use of nivolumab in patients with R/R cHL.22–26 These studies reported ORRs and CRs ranging from 60% to 69% and from 15% to 45%, respectively. These rates were higher than those observed in the present study (ORR: 46.2%; CR: 23.1%). As reported by Martínez et al., the GELTAMO group conducted a retrospective real-world study in our centre with 74 patients treated with nivolumab.26 The ORR was 58% and the CR rate was 30.6%, both of which were higher than in the present study.
The median time to best response (CR+PR+ES) was 3.9 months, which is longer than the 2 months reported in the pivotal trials but similar to the 3 months reported by the GELTAMO group. The median duration of response in responders (CR+PR) was 15.4 months (IQR: 3.1–79.4), which is comparable to the 16.6 months (range: 0–71) reported in the CheckMate 205 trial (cohort B). For patients who achieved a CR, the median duration of response was 79.1 months, compared to 30.3 months reported in the CheckMate 205 trial (cohort B).
Median PFS was 23.9 months (95% CI 0–49.1), which is higher than the 14.8 months (95% CI 11.0–19.8) reported in the CheckMate 205 trial (cohort B). In addition, 12-month PFS was 57%. Although median OS was not reached, 61.5% of patients were still alive at the study end date.
The reasons for discontinuation of nivolumab monotherapy were consolidation with transplantation in 3 patients (23.1%), disease progression in 5 patients (38.5%), and toxicity in 1 patient (7.7%). Of the 6 patients who discontinued due to disease progression or toxicity, 5 died (38.5% of the study population). In the GELTAMO study, the main reasons for discontinuation were consolidation with transplantation (41.7%) and disease progression (37.5%).26 In total, 52.8% of patients in the GELTAMO study underwent HSCT, compared with 38.5% in the present study (1 patient with aHSCT and 4 with allo-HSCT).
Two of our patients (15.4%) received rescue treatment with immunochemotherapy and achieved CR by the end of the study. Romero et al.27 described 3 cases of heavily pretreated patients with cHL refractory to nivolumab monotherapy who were successfully rescued by adding chemotherapy to nivolumab as a bridge to allo-HSCT. All patients had unfavourable clinical features, such as 3 or 4 prior treatment lines—including BV and aHSCT—refractoriness to the line of treatment prior to nivolumab, or rapid disease progression. Despite these challenges, they achieved CR after the addition of chemotherapy, consolidated with allo-TAPH, and remain in CR.
Chemotherapy in combination with PD-1 inhibitors has shown encouraging results,28,29 although there are still few studies of this combination in patients who fail to respond to nivolumab. There are several ongoing trials of new combinations of chemotherapy and PD-1 inhibitors in both R/R cHL and in first-line therapy.30 The results suggest that anti-PD-1 therapy may reprogram the immune system by activating effector cells and inhibiting immunosuppressive cells, potentially overcoming chemoresistance.
Overall, 76.9% of our patients experienced AEs, 44% of which were grade ≥3. The most common AEs were hypothyroidism, hepatotoxicity, pulmonary toxicity, anaemia, and neutropenia. Definitive discontinuation of nivolumab treatment was required for only 1 patient, who developed pneumonitis (approximately. 8%). These results are similar to those described in pivotal clinical trials, where AEs were observed in 77.1% of patients, but with fewer grade ≥3 AEs (19.5%). These results show a higher incidence of AEs than those reported in real-world studies. Martinez et al.26 reported treatment-related AEs in 56.8% of patients (grade ≥3 in 9.4%), while Manson et al.24 reported 37% of patients with grade 3 AEs and 20.5% with serious AEs. The most common ≥3 haematological toxicity was neutropenia at 15.4%, which was higher than the 2.3% observed in the pivotal trials.
The most common immune-related AEs were grade 3 hepatotoxicity in 23.1% of patients and grade 3 pulmonary toxicity in 15.4%, compared with 0.8% and 3.4%, respectively, in the pivotal trials.12,13 Although the results of retrospective studies should be interpreted with caution, they suggest that the real-world safety profile of nivolumab may differ from that observed in the pivotal clinical trials, which did not include patients at high risk of AEs, such as those with a history of autoimmune disease or prior allo-HSCT. In our study, 3 patients had received allo-HSCT prior to treatment with nivolumab. Nevertheless, it should be noted that no patient in our sample died due to treatment toxicity.
The incidence of acute GVHD after allo-HSCT was higher than in the pivotal trials (50% vs 27.4%), as was the incidence of SOS (25% vs 2%) and SAF (25% vs 12%). However, these results may be biased by the small number of patients who received allo-HSCT. In the real-world study conducted by the GELTAMO group, the cumulative incidence of grade II to grade IV acute GVHD was 33.3% (grade III to grade IV in 2 of 74 patients).26
The present study has several limitations, the first of which is the small sample size. In addition, the data collection was retrospective, which may mean that the information recorded in the clinical histories was incomplete. Finally, we must consider in mind the limitations of using ORR as the main variable and the lack of robust data on patient-relevant variables.
In conclusion, in a small sample of patients analysed in a real-world setting, the ORR with nivolumab monotherapy was approximately 46%, with a clinical benefit rate approaching 70%. Although the incidence of AEs was high (44% with grade ≥3), only 1 patient required definitive discontinuation of treatment. Nivolumab achieved a very durable response in patients who achieved CR (79 months). Nivolumab in combination with chemotherapy rescued 2 trial patients with a very poor prognosis, both achieving CR. This study provides an opportunity to analyse and learn from clinical practice, as it involved real patients who differ from the highly selected cohorts of clinical trials.
Contribution to the scientific literatureThe prognosis for patients with R/R cHL after aHSCT and BV treatment is poor and there are few therapeutic alternatives.
There is a body of literature supporting the efficacy and safety of nivolumab in R/R cHL. However, much of the published research has been conducted under the highly controlled conditions typical of experimental studies. Observations made in the context of routine clinical practice can add to previous results and provide post-marketing evidence, but should never replace the evidence obtained from clinical trials.
This study aimed to provide a descriptive overview of the effectiveness and safety of nivolumab treatment in R/R cHL in clinical practice.
FundingNone declared.
Ethics statementThis study was submitted to and approved by the hospital's Research Committee. The authors declare that the patients' confidential data were protected in accordance with the Organic Law on Data Protection 3/2018, Regulation 2016/679 of the European Parliament, and of the Council of 27 April, 2016.
CRediT authorship contribution statementLaura Lorente Fernández: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Samuel Romero Domínguez: Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Asunción Albert Marí: Writing – review & editing, Writing – original draft, Validation, Supervision, Data curation. Esperanza Núñez Benito: Writing – original draft, Visualization, Validation, Data curation, Conceptualization. Eduardo López Briz: Writing – original draft, Validation, Methodology, Conceptualization. José Luis Poveda Andrés: Visualization, Validation, Conceptualization.