To review and analyse the available literature on peripheral administration of noradrenaline (NA) with the aim of providing recommendations to ensure correct use and patient safety.
MethodsSystematic review on the databases PubMed, ISI Web of Science, SCOPUS, and Science Direct, using the following search terms: (“Noradrenaline” [Mesh]) AND (“Norepinephrine” [Mesh]) AND (“Vasopressors” [Mesh]) AND (“Peripheral infusions” [Mesh]) OR (“Extravasations” [Mesh]). A total of 1040 articles were identified. Animal studies and studies written in languages other than English were excluded. Finally, 83 articles were included.
ResultsNA can be administered peripherally. The risk of extravasation should be taken into account, with phentolamine being the first pharmacological line of treatment. It has also been related to the appearance of thrombophlebitis, cellulitis, tissue necrosis, limb ischaemia, and gangrene, although its incidence seems to be low. The use of peripheral NA in children seems to be carried out without obvious complications. The use of standard concentrations is suggested to reduce the risk of errors. It is recommended to use 0.9% saline as the default diluent for peripheral NA.
ConclusionsPeripheral infusions of NA could be a safe and beneficial option in early resuscitation provided that a number of guidelines are followed that reduce the likelihood of complications associated with this route.
Revisar y analizar la literatura disponible sobre la administración de noradrenalina (NA) por vía periférica con el fin de aportar recomendaciones que garanticen el correcto uso y la seguridad del paciente.
MétodoRevisión sistemática en las bases de datos PubMed, ISI Web of Science, SCOPUS y Science Direct, utilizándose los siguientes términos de búsqueda: («Noradrenaline» [Mesh]) AND («Norepinephrine» [Mesh]) AND («Vasopressors» [Mesh]) AND («Peripheral infussions» [Mesh]) OR («Extravasations» [Mesh]). Se identificaron 1040 artículos. Se excluyeron estudios en animales y estudios escritos en idiomas diferentes al inglés. Finalmente se incluyeron 83 artículos.
ResultadosLa administración de NA se puede realizar por vía periférica. Se debe tener en cuenta el riesgo que existe de extravasación, siendo la fentolamina la primera línea farmacológica de tratamiento. También se ha relacionado con la aparición de tromboflebitis, celulitis, necrosis tisular, isquemia de miembros y gangrena, aunque su incidencia parece ser baja. El uso de NA periférica en niños parece llevarse a cabo sin complicaciones evidentes. Se sugiere el empleo de unas concentraciones estándares para reducir el riesgo de errores. Se recomienda emplear solución salina 0,9% como diluyente predeterminado para la NA periférica.
ConclusionesLas infusiones periféricas de noradrenalina podrían ser una opción segura y beneficiosa en la reanimación temprana siempre y cuando se sigan una serie de directrices que reduzcan la probabilidad de las complicaciones asociadas a esta vía.
Noradrenaline (NA) is a catecholamine (α1 and β1 adrenoreceptor agonist) with vasoconstrictive properties used to restore blood pressure in patients with various forms of shock, and is currently the first-line vasopressor.1–3 In Spain, NA bitartrate is commercially available at concentrations of 0.5 and 1 mg/mL of base NA (1 and 2 mg/mL of NA bitartrate, respectively). This vasopressor is dosed by patient weight, so although the volume of the dose may vary considerably depending on the type used, there are no differences between these 2 dilutions.4
Vasopressors are typically infused centrally. However, in recent years, some, such as phenylephrine and dopamine, have been administered peripherally, unlike NA.5 A post-hoc analysis of the Australasian Resuscitation In Sepsis Evaluation study conducted in 2022 showed that septic shock patients given peripheral NA (pNA) started vasoactive therapy approximately 2.5 h earlier and without increased risk of complications.6 A recent systematic review of vasopressors found that NA was the most commonly administered agent (702 episodes), followed by phenylephrine (546), dopamine (108), metaraminol (74), and vasopressin-adrenaline (<5 episodes).7 There are concerns regarding pNA because its’ in vivo vasoconstrictor potency is 76% greater than that of phenylephrine.8
In critical care settings, typical concentrations of NA bitartrate are 200 μg/mL and sometimes 400 μg/mL. Administration via central venous catheters has been recommended in the past because vasoactive drugs can cause local damage and tissue necrosis if extravasation occurs.4
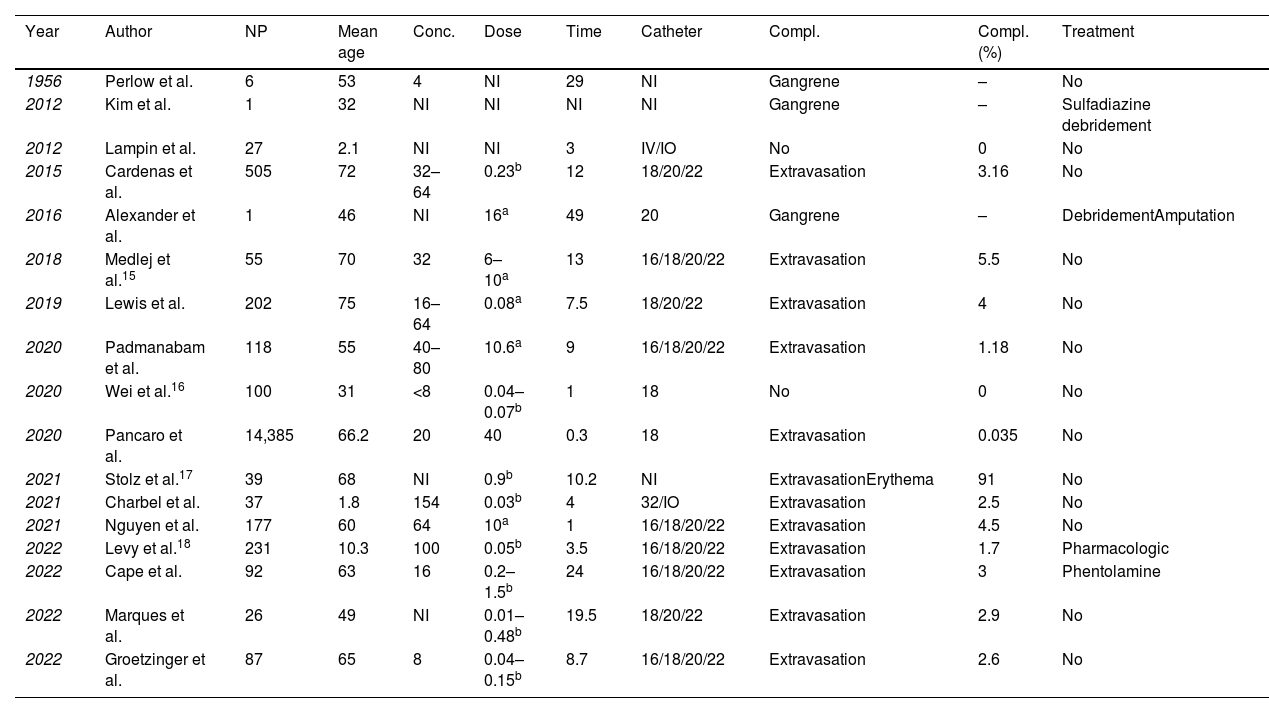
Previous observational studies and clinical guidelines have recommended that vasopressors such as NA should be administered peripherally for short periods of time. Although increasingly popular, there is little medical evidence to support this practice.9 No studies have directly compared the 2 routes in terms of extravasation and tissue damage, making it difficult to assess pNA effectively.10Table 1 shows a list of articles addressing pNA in adult and paediatric populations. It has been suggested that this mode of administration reduces the time required to treat and control haemodynamic instability, which, in turn, has been associated with a reduction in morbidity and mortality.11–14
Published articles on peripheral administration of noradrenaline in adult and paediatric populations.
| Year | Author | NP | Mean age | Conc. | Dose | Time | Catheter | Compl. | Compl. (%) | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| 1956 | Perlow et al. | 6 | 53 | 4 | NI | 29 | NI | Gangrene | – | No |
| 2012 | Kim et al. | 1 | 32 | NI | NI | NI | NI | Gangrene | – | Sulfadiazine debridement |
| 2012 | Lampin et al. | 27 | 2.1 | NI | NI | 3 | IV/IO | No | 0 | No |
| 2015 | Cardenas et al. | 505 | 72 | 32–64 | 0.23b | 12 | 18/20/22 | Extravasation | 3.16 | No |
| 2016 | Alexander et al. | 1 | 46 | NI | 16a | 49 | 20 | Gangrene | – | DebridementAmputation |
| 2018 | Medlej et al.15 | 55 | 70 | 32 | 6–10a | 13 | 16/18/20/22 | Extravasation | 5.5 | No |
| 2019 | Lewis et al. | 202 | 75 | 16–64 | 0.08a | 7.5 | 18/20/22 | Extravasation | 4 | No |
| 2020 | Padmanabam et al. | 118 | 55 | 40–80 | 10.6a | 9 | 16/18/20/22 | Extravasation | 1.18 | No |
| 2020 | Wei et al.16 | 100 | 31 | <8 | 0.04–0.07b | 1 | 18 | No | 0 | No |
| 2020 | Pancaro et al. | 14,385 | 66.2 | 20 | 40 | 0.3 | 18 | Extravasation | 0.035 | No |
| 2021 | Stolz et al.17 | 39 | 68 | NI | 0.9b | 10.2 | NI | ExtravasationErythema | 91 | No |
| 2021 | Charbel et al. | 37 | 1.8 | 154 | 0.03b | 4 | 32/IO | Extravasation | 2.5 | No |
| 2021 | Nguyen et al. | 177 | 60 | 64 | 10a | 1 | 16/18/20/22 | Extravasation | 4.5 | No |
| 2022 | Levy et al.18 | 231 | 10.3 | 100 | 0.05b | 3.5 | 16/18/20/22 | Extravasation | 1.7 | Pharmacologic |
| 2022 | Cape et al. | 92 | 63 | 16 | 0.2–1.5b | 24 | 16/18/20/22 | Extravasation | 3 | Phentolamine |
| 2022 | Marques et al. | 26 | 49 | NI | 0.01–0.48b | 19.5 | 18/20/22 | Extravasation | 2.9 | No |
| 2022 | Groetzinger et al. | 87 | 65 | 8 | 0.04–0.15b | 8.7 | 16/18/20/22 | Extravasation | 2.6 | No |
Abbreviations: Catheter, catheter calibre; Compl, type of complications; Compl (%), complication rate; Concent, noradrenaline concentration (μg/mL); IV, intravenous; IO, intraosseous; NP, number of patients; NI, no information; Time, time in hours.
For the reasons outlined above, NA is considered a high-risk medication, and so safety strategies are required by units that habitually use it (e.g., hospital pharmacy services). Broadly speaking, there is a lack of standardised protocols on these pharmaceuticals. It is commonly accepted that guidelines should always be followed, so consensus protocols involving multidisciplinary teams, including doctors, pharmacists, and nurses, are crucial to prevent the occurrence of adverse effects. Quality assurance programs are needed to prevent issues such as the co-administration of vasopressors with other drugs to avoid inadvertent boluses.
The aim of this review was to analyse and summarise the evidence from the available medical literature on pNA in adults and children, aiming to establish consensus practices that ensure correct use and patient safety.
MethodologyA systematic review of pNA was conducted using the PubMed, ISI Web of Science, SCOPUS, and Science Direct databases. The search was conducted in October 2023 with a 10-year time horizon (2013–2023). The search used the following Mesh terms: (“Noradrenaline” [Mesh]) AND (“Norepinephrine” [Mesh]) AND (“Vasopressors” [Mesh]) AND (“Peripheral infusions” [Mesh]) OR (“Extravasations” [Mesh]).
All publications from the last 10 years were included, regardless of article type or additional filters.
We excluded animal studies, studies written in languages other than English or Spanish, or studies published in non-indexed journals.
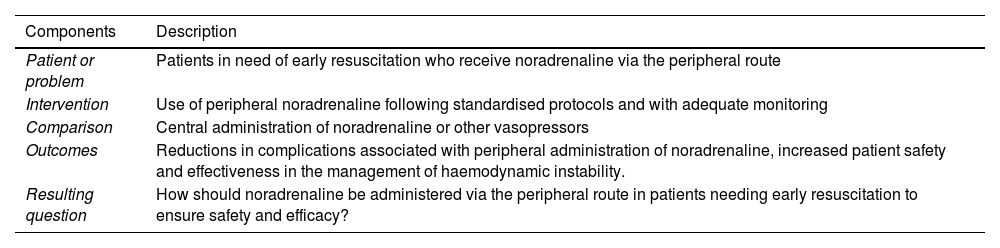
The PICO framework was used to design a coherent literature search strategy. The resulting research question was: “How should noradrenaline be administered via the peripheral route in patients needing early resuscitation to ensure safety and efficacy?” Table 2 shows a breakdown of the process.
Research question in PICO format.
| Components | Description |
|---|---|
| Patient or problem | Patients in need of early resuscitation who receive noradrenaline via the peripheral route |
| Intervention | Use of peripheral noradrenaline following standardised protocols and with adequate monitoring |
| Comparison | Central administration of noradrenaline or other vasopressors |
| Outcomes | Reductions in complications associated with peripheral administration of noradrenaline, increased patient safety and effectiveness in the management of haemodynamic instability. |
| Resulting question | How should noradrenaline be administered via the peripheral route in patients needing early resuscitation to ensure safety and efficacy? |
Abbreviation: PICO, Population/Problem, Intervention, Comparison, Outcome.
Three reviewers independently assessed all titles and abstracts based on the inclusion criteria. If abstracts met the criteria, the full texts were read and analysed. Differences in opinion were resolved by discussion and consensus or consultation with another reviewer. To ensure the quality of the analysis, we only included studies published in indexed journals and those detailing the essential data defined by Dartois et al.19 These essential data include patient characteristics, dosage and route of administration, number of observations, model selection, structural model, inter-individual variability, error modelling, estimation methods, and software used.
This review was conducted following the PRISMA criteria, which aims to optimise the quality of systematic reviews.
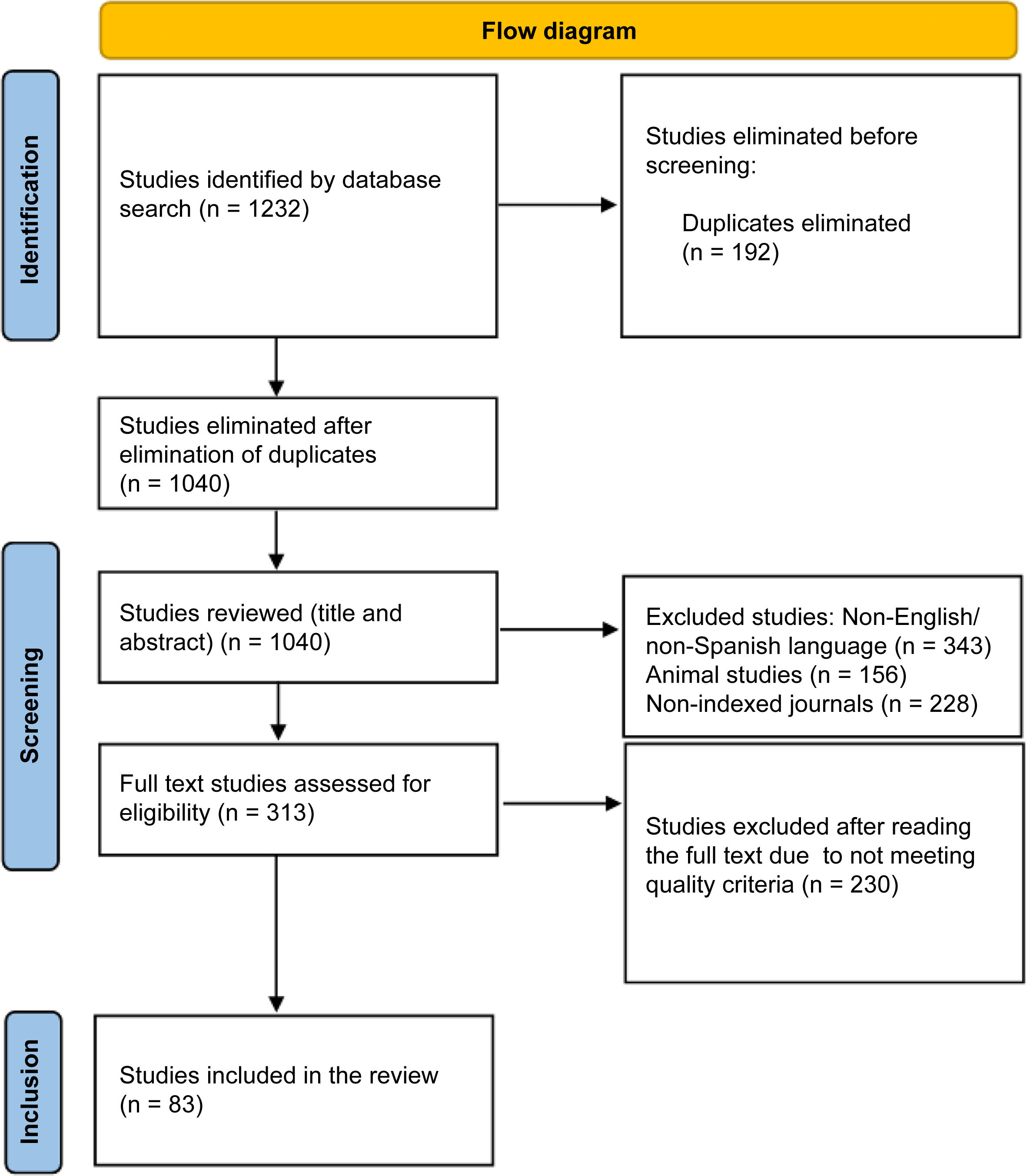
ResultsThe electronic search identified 1040 unique articles in the databases.
After an initial review, 83 articles were selected for analysis. Of these, 20 focused on medical protocols established by various health institutions worldwide.
Fig. 1 shows the PRISMA flowchart of the literature search and article selection.
The rest of this section presents a set of questions pertaining to pNA.
Can NA be administered peripherally? Yes, but it is typically administered via the central venous route for safety, given the risk of extravasation (inadvertent leakage of the drug into surrounding tissues).5
Recently, the peripheral administration of various vasopressor agents has been emerging in medical practice—mainly during perioperative anaesthesia— in situations involving complex insertion or contraindications to central catheter placement, during the establishment of central catheters, in the setting of developing countries, and/or due to patient preference (Table 1).
Is it safe to use pNA in perioperative medicine? In Spain, reports on the use of pNA are anecdotal, in contrast to northern Europe, particularly the Netherlands.20 A retrospective study estimated that the risk of extravasation at 1–8 cases per 10 000 patients. All cases were mild and did not require medical and/or surgical treatment. The study included 14 385 surgical patients who required pNA during general anaesthesia in Amsterdam and Utrecht between 2012 and 2016.5 Fu et al.21 recently showed that pNA infused at very low concentrations (8 μg/mL) at 0.08 μg/kg/min was highly effective in controlling arterial hypotension during subarachnoid anaesthesia for caesarean delivery with no differences in serious adverse effects.
Studies conducted in the Netherlands support its safety in the perioperative period for several reasons, including patient “hypervigilance” in the surgical setting, dilution of NA, and low infusion rates.
Can pNA be used in paediatric patients? The literature on its paediatric use is very limited compared to that of adults, but studies have reported its use without obvious complications. Intraosseous use has also been described.22,23
Charbel et al.23 conducted a retrospective study of 37 paediatric patients, finding a high use of distal sites, higher concentration infusions (between 40 and 245 μg/mL), and high infusion rates of up to 0.4 μg/kg/min (0.9–2 mL/h). However, as in adults, the infusion time was short (maximum: 270 min) and there was only 1 case of mild extravasation, requiring no treatment.
Is it important to standardise the dose, concentration, and duration of infusion of vasoactive drugs? There is little information on this issue, but it seems advisable to use standard concentrations in all clinical areas to reduce the risk of error. Concentrations should always be lower for peripheral use than for central catheter use, and are never interchangeable (Table 1). Peripheral NA is typically administered at concentrations between 16 and 32 μg/mL due to concerns about extravasation and tissue necrosis.24 The rate of complications associated with pNA is dose-dependent (12% vs 2%) if the doses are less than 0.13 μg/kg/min and the concentrations are less than 22.3 μg/mL.25 Studies conducted in the Netherlands, based on extensive experience in the field of anaesthesia, suggest 20 μg/mL as a standard dilution, with an infusion rate of 0.01 and 0.1 μg/kg/min at an administered volume of approximately 2–15 mL/h.5
Such low concentrations are necessary because of increased local vasoconstriction caused by drug infusion together with associated tissue hypoperfusion, which can lead to local tissue damage over time.4
Infusion should always be performed using electronic pumps with sensitive alarms to facilitate early detection of flow obstruction.2
There is a correlation between the duration of infusion and the risk of extravasation. Infusion time should be determined by local protocols and determined on a case-by-case basis (Table 1), as there are no high quality studies on this issue. A recent review by Loubani and Green showed that drug administration times ranged from 1 to 528 h (mean: 24 h).4 Cárdenas et al.26 conducted an observational study and reported reduced complications with the use of ultrasound for venous cannulation, use of 20G or 18G catheters in the antecubital fossa, and less than 72 h of infusion. Cape et al.27 showed that cases of extravasation tend to occur early (2.43 h), highlighting the need for close monitoring, especially in cases with thin peripheral catheters and high infusion rates.
Can any diluent be used? No. Several hospitals recommend using 0.9% saline as the standard diluent for pNA following a report of a case of severe hyponatraemia and seizures secondary to pNA at 4 μg/mL in 5% dextrose.24 The policy of these hospitals is to discourage the use of such low concentrations because they are associated with excessive crystalloid load.
How important is catheter size and location? There is considerable variation in the literature regarding the importance of catheter size.28,29 The cases shown in Table 1 indicate that catheter size does not influence the occurrence of adverse events. However, there is broad consensus on the importance of placing large-calibre catheters (>4 mm) in the antecubital fossa and above the popliteal fossa.11 Groetzinger et al.30 described an extravasation rate similar to that reported by other authors, despite the fact that up to 44% of cannulations were performed under ultrasound guidance.
Is peripheral administration associated with a low risk of adverse effects? The following adverse effects have been associated with pNA: extravasation, thrombophlebitis, cellulitis, tissue necrosis, limb ischaemia, and gangrene.31,32 Not all vasopressors have the same effect when administered peripherally; for example, NA causes the most ischaemia when administered distally, but this is not the case for adrenaline or subcutaneous phenylephrine.
The available medical evidence shows that the incidence of these adverse effects is relatively low (2%–5%) and appears to be associated with prolonged administration and distal sites.10 Most adverse effects occur after more than 6 h of peripheral vasopressor infusion, although one case of tissue damage within 5 min of initiating peripheral administration of phenylephrine has been reported.33 Cases of skin necrosis have been described with the use of extremely low doses of NA (4 μg/mL).34 The occurrence of intraprocedural complications is lower than in critically ill patients (0.035% vs 4%), likely due to the absence of endothelial damage and a more robust peripheral venous network.10,21
A systematic review and meta-analysis found that in most cases complications were minor, predominantly involving local erythema and thrombophlebitis, and did not require major interventions.35 Extravasation during surgery is extremely rare, and none of the reported cases involved NA. However, it has been associated with the peripheral administration of dopamine (6 cases), calcium (5 cases), phenylephrine (2 cases), adrenaline (1 case), and dobutamine (1 case).2
A recent systematic review involving more than 1300 patients found that this clinical practice carries an extremely low risk.7
Nonetheless, it is important to closely monitor for development of this complication.
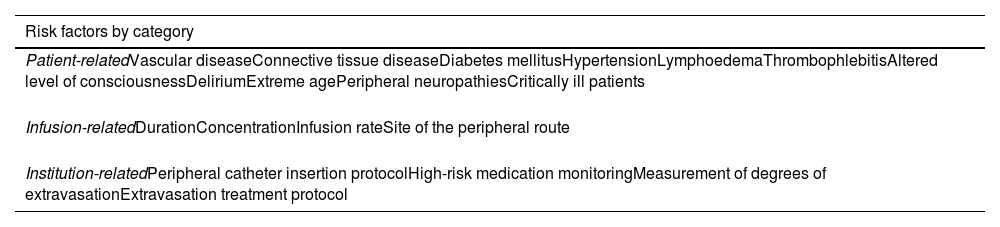
What are the risk factors for extravasation? Peripheral NA may cause adverse effects related to extravasation. Table 3 shows factors that have been associated with the potential for extravasation. Some factors, such as catheter placement site, infusion time, pNA concentration and dose, can be controlled and should be the focus of future research. Various studies have reported mean times to extravasation of 21–35 h.10,11,36
Risk factors for extravasation.
| Risk factors by category |
|---|
| Patient-relatedVascular diseaseConnective tissue diseaseDiabetes mellitusHypertensionLymphoedemaThrombophlebitisAltered level of consciousnessDeliriumExtreme agePeripheral neuropathiesCritically ill patients |
| Infusion-relatedDurationConcentrationInfusion rateSite of the peripheral route |
| Institution-relatedPeripheral catheter insertion protocolHigh-risk medication monitoringMeasurement of degrees of extravasationExtravasation treatment protocol |
Can protocols be designed to reduce the potential for complications? Yes, it is essential that local protocols are developed to monitor peripheral vasopressor administration. A meta-analysis of the incidence of complications of peripheral vasopressor infusion found an association between the presence of safety guideline controls and a significantly lower incidence of adverse events.35 Furthermore, a recent study found an association between the implementation of a pNA protocol and a reduction in the need for central venous catheterisation by up to 30%. A key measure is to train staff to recognise the early signs and symptoms of possible extravasation. The venous catheter should have a minimum size of 20G and should be placed as proximally as possible, while avoiding flexion sites in awake patients. Proper cannulation should be checked by aspirating blood and administering a direct bolus of 5–10 mL of saline.25
Local protocols should specify the maximum rate, duration of vasopressor administration, and review intervals needed to monitor peripheral intravenous infusion in various clinical contexts (see Table 4). The recommended pNA protocol from the Virginia Commonwealth University Health System Emergency Department37 is shown in Table 4. In addition, local protocols should also specify the concentration of NA in the preparations, include the measurement of blood pressure in the lower limbs or contralateral to the infusion site, and allow for checking vein and catheter size by ultrasound. It seems inadvisable to use combinations of vasopressors, although previous studies have reported the use of NA with adrenaline and NA with vasopressin without an increased rate of complications.38,39
Virginia Commonwealth University Health System Protocol.
| Maximum NA dose, 20 μg/minPlace central catheter as early as possible to reduce risk of extravasationPeripheral catheter in the antecubital fossae or external jugular veinCatheter gauge must be 18G or 20GRun blood aspiration test using 5–10 mL saline bolusHourly monitoring of peripheral catheter by nursing staffMonitor for signs/symptoms of extravasation:Skin pallorAcrocyanosisSwellingDermal sloughingMaximum infusion duration, 4 hNot applicable in cases of cardiac arrestIn case of suspected extravasation: stop the infusion and consider restarting by another route (in a different limb)Treatment according to protocol (Table 5)Other lines: topical nitroglycerine 2% or subcutaneous terbutaline |
How can NA extravasation be treated? Table 5 provides a protocol for the management of extravasation.
Extravasation treatment with vasoactive drugs.
| Stop infusion of the vasopressor drugAspirate 4–5 mL, peripherally if possibleApply warm gauzeRemove venous cannula and apply compressive bandageElevate the affected limbFirst line of treatment: subcutaneous phentolamineOther lines: topical nitroglycerine 2% or subcutaneous terbutalineApply analgesics if requiredAssessment by plastic surgery serviceNotification of medical incident to appropriate agency |
| Treatment protocol at the Resuscitation Unit of the NeuroTrauma Hospital of Granada (Spain) for extravasation caused by peripheral vasopressors. |
Phentolamine is the first line of treatment. It is an α-adrenergic antagonist that promotes vasodilatation, increases capillary flow, and is considered effective during the first 12 h post-extravasation.40 Phentolamine is not currently available in Spanish hospitals; however, its availability is necessary for the safe administration of pNA. It is available as a foreign medication at a cost of €603 for 5 ampoules.40 The preparation involves reconstituting 5 mg of phentolamine with 5 mL of saline to obtain a final concentration of 1 mg/mL. The maximum dose is 50 mg and intravenous use should be avoided. Up to 5 injections of 0.5 mL of this solution are administered into the area of extravasation using 25G or 27G needles.30
Alternatives include topical nitroglycerine 2% and subcutaneous terbutaline, although the latter is neither marketed in Spain nor available as a foreign medication.41 Nitroglycerine 2% is available as a fine powder from Fagron at a cost of €110.38 (100 g), which can be used to prepare the topical master formula. The classic formula involves adding 10 g of 2% nitroglycerine to 80 g of white petroleum jelly and adding 10 g of liquid paraffin under agitation.42
The present study has some limitations. Our review was restricted to articles in English and Spanish, which may have led to the exclusion of relevant information published in other languages. The study is also limited by the heterogeneity of the reviewed studies, as they were conducted in various settings and involved patients with different characteristics. There is also a risk of bias due to the quality of the original articles.
ConclusionsWe believe that pNA is a viable option for early resuscitation in both adult and paediatric populations. It is associated with a more rapid resolution of haemodynamic instability, which in turn has been associated with a reduction in morbidity and mortality. However, safe protocols for pNA need to be established to reduce the complications associated with this route. Current medical evidence suggests that there is an association between complication rates and patients' baseline conditions, comorbidities, NA concentration, and infusion time.
We therefore believe that large prospective multicentre trials should be conducted to identify and resolve the many areas of uncertainty in the peripheral vasopressor infusion.
Contribution to the scientific literatureTo date, no systematic review of pNA has been published in the scientific literature. Although central administration is the preferred route, there are occasions when peripheral administration is needed. This appears to reduce the time required to treat and control haemodynamic instability, which in turn is associated with a reduction in morbidity and mortality.
Therefore, there is a need for guidelines to facilitate the use of this route and reduce associated complications. This article could be very useful in clinical settings, as it summarises the current information on pNA to ensure correct use and patient safety.
FundingNone declared.
CRediT authorship contribution statementF. Dámaso Fernández-Ginés: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. María T. Gómez Sánchez: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Marina Sánchez Valera: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Beatriz Tauste Hernández: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Marta Garrido Ortiz: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Manuel Cortiñas-Sáenz: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.