Mycetoma is a type of pulmonary aspergillosis comprising a mass caused by fungal infection, which grows in lung cavities. The clinical practice guidelines recommend voriconazole and itraconazole as first-line treatment and posaconazole, isavuconazole, or amphotericin-B as alternatives.1,2
Voriconazole can be administered orally and intravenously. In both cases, a loading dose is given to rapidly obtain therapeutic plasma concentrations.2
Pharmacokinetic monitoring of voriconazole is justified by high interindividual variability in plasma concentrations at standard doses, its narrow therapeutic range, nonlinear pharmacokinetics due to its saturable metabolism, high risk of interactions with drugs metabolized through cytochrome-P450, and the presence in the population of genetic polymorphisms of the isoforms responsible for its metabolism (CYP3A4, CYP2C9, CYP2C19). Of these, CYP2C19 is responsible for most of the reactions.3
Case descriptionWe present the case of a female patient (32 years of age, height 153 cm, weight 45 kg), who was diagnosed with vasculitis and under follow-up by the pneumology department for a cavitary lesion in the upper lobe of the right lung. She attended the emergency department of a tertiary level hospital due to the worsening of her usual cough and the appearance of hemotysis without fever or other accompanying symptoms. On arrival, it was noted that the patient had diffuse expiratory wheezing and was admitted to the pulmononary department.
On the second day of admission, it was confirmed by bronchoscopy that the purulent secretions were coming from the right bronchus-B2. Computed angiotomography of the chest showed that the cavitary lesion was smaller than that shown by bronchoscopy, hypertrophy of the right bronchial and right lateral thoracic arteries, and probable hypertrophy of the pulmonary arteries. On the fourth day of admission, galactomannan antigen was detected in a bronchoalveolar lavage sample. Finally, a diagnostic was made of invasive pulmonary aspergillosis and progressing vasculitis, for which treatment was started with voriconazole. The pharmacy service was asked to conduct pharmacokinetic monitoring.
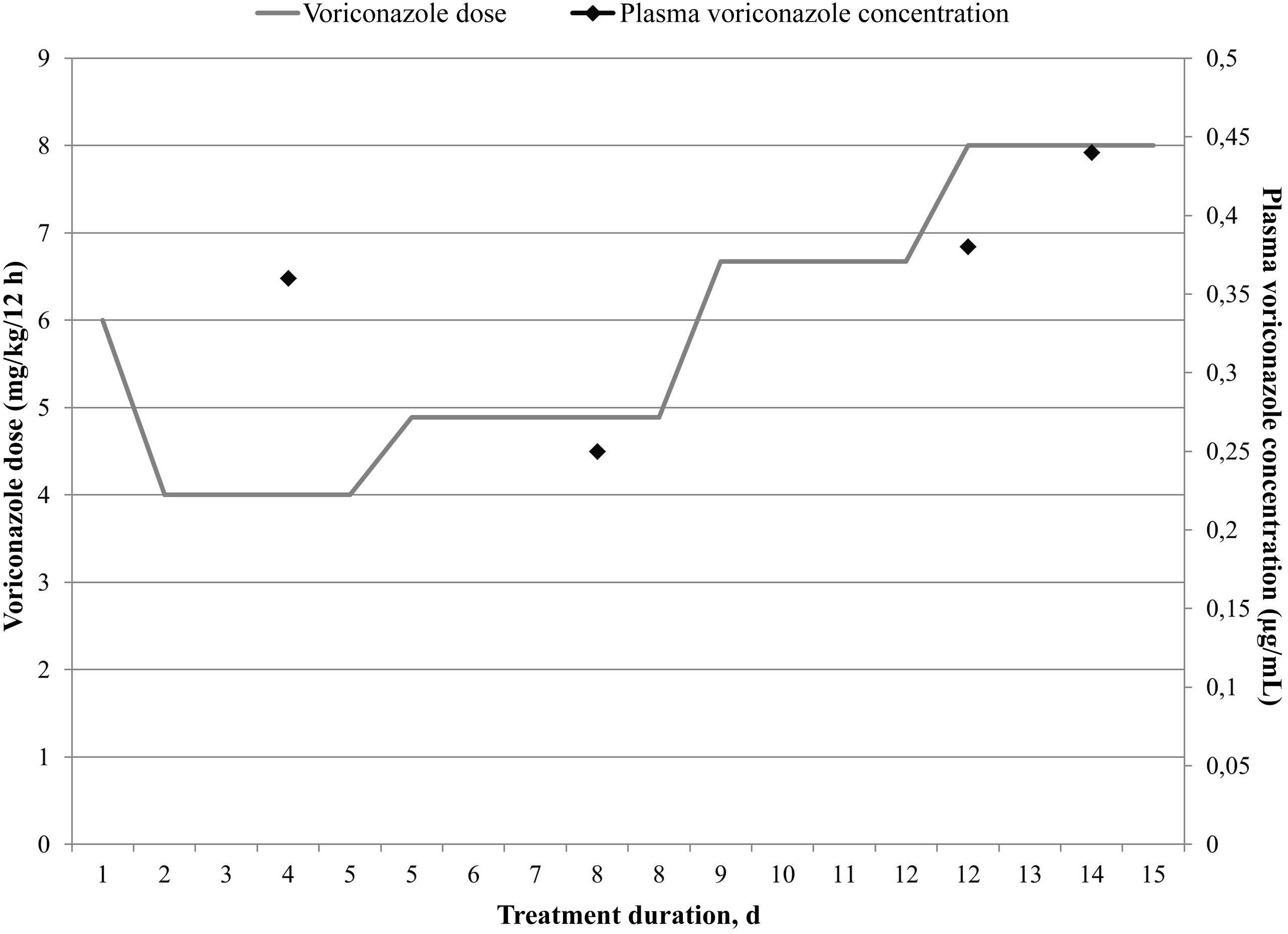
Treatment with voriconazole was initiated intravenously with a loading dose of 6 mg/kg/12 h on the first day, followed by maintenance doses of 4 mg/kg/12 h on the second day onward. Pharmacokinetic monitoring was performed on the basis of trough concentrations measured 48 h after the first dose (target range: 1–5 μg/mL).4 Homogeneous enzyme immunoassay (Cobas-Integra-400; Roche) showed that the first concentration measured was less than 1 μg/mL (i.e. subtherapeutic). Monitoring was performed by means of a population pharmacokinetic model using Abbottbase-Pharmacokinetic-System (PKS) software.5 Based on the estimations, it was decided to increase the dose to 4.89 mg/kg/12 h (+22.25%), maintain the dosing interval, and—given the clinical severity—to monitor after 3 days. Therapeutic concentrations were estimated at this time point. Once again, the concentration was below the target concentration, so the dose was increased to 6.67 mg/kg/12 h (+36.40%). However, the concentration remained subtherapeutic, and so the dose was increased to 8 mg/kg/12 h (+19.94%), which is twice the recommended dose.1,2 The drug was monitored for 2 weeks, during which period the patient's weight did not change. Fig. 1 shows how the doses and concentrations evolved over time.
Several hypotheses were proposed to explain the difficulties encountered in reaching therapeutic concentrations. Firstly, pre-analytical and analytical incidents were ruled out, and the results were confirmed by liquid chromatography–tandem mass spectrometry (LC–MS/MS) at the reference laboratory. The patient's treatment was also reviewed to rule out concomitant treatment with CYP2C19 inducers, which could accelerate voriconazole metabolism.
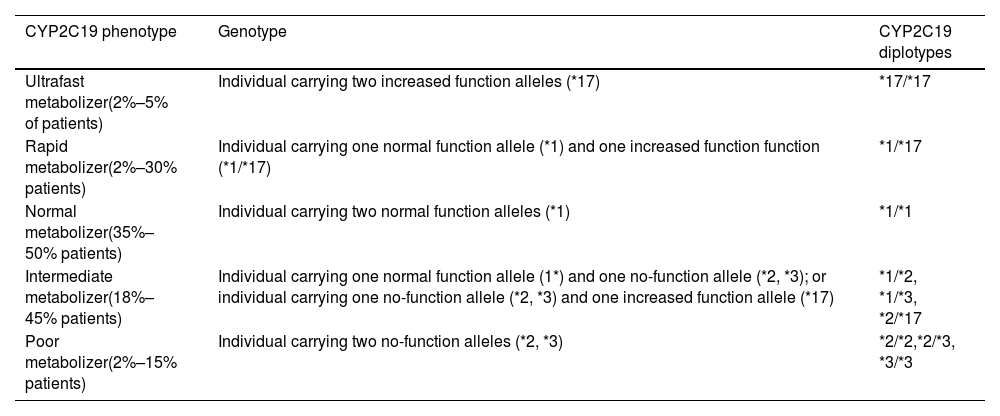
At the same time, the possibility was raised that the patient might have a rapid or ultrarapid voriconazole-metabolizing phenotype, and so CYP2C19 genotyping was requested from the genetics unit of the hospital's clinical analysis service. Primers were designed to detect the most frequent CYP2C19 allelic variants in the European population6 (Table 1).
CYP2C19 phenotype/genotype relationship.8
| CYP2C19 phenotype | Genotype | CYP2C19 diplotypes |
|---|---|---|
| Ultrafast metabolizer(2%–5% of patients) | Individual carrying two increased function alleles (*17) | *17/*17 |
| Rapid metabolizer(2%–30% patients) | Individual carrying one normal function allele (*1) and one increased function function (*1/*17) | *1/*17 |
| Normal metabolizer(35%–50% patients) | Individual carrying two normal function alleles (*1) | *1/*1 |
| Intermediate metabolizer(18%–45% patients) | Individual carrying one normal function allele (1*) and one no-function allele (*2, *3); or individual carrying one no-function allele (*2, *3) and one increased function allele (*17) | *1/*2, *1/*3, *2/*17 |
| Poor metabolizer(2%–15% patients) | Individual carrying two no-function alleles (*2, *3) | *2/*2,*2/*3, *3/*3 |
Abbreviation: CYP2C19, cytochrome P450 2C19.
As it proved impossible to achieve therapeutic concentrations with voriconazole, and pending the results of the genetic study, it was decided to switch to isavuconazole, which has fewer interactions and is metabolized by a metabolic pathway that does not involve CYP2C19.6,7
The results of the genetic study showed that the patient had the *1/*17 genotype. According to the Clinical Pharmacogenetics Implementation Consortium (CPIC) consensus guidelines6 for CYP2C19 and voriconazole, the patient's phenotype identified her as a rapid metabolizer, because she has one allele with normal function (*1) and one with increased function (*17) (Table 1).
DiscussionIn rapid metabolizers the probability of achieving therapeutic concentrations with standard doses of voriconazole is subject to wide variations. In these cases, it is recommended to substitute voriconazole for another antifungal drug with CYP2C19-independent metabolism. Although the percentage of rapid metabolizers in the population is not negligible (2%–30% of Caucasians), genotyping is rare in practice and few cases have been described in the literature.
Previous works have recommended genotyping and the use of an alternative antifungal in patients who maintain subtherapeutic concentrations despite 2 dose adjustments by pharmacokinetic monitoring.4,8
Therapeutic monitoring of triazoles such as voriconazole, itraconazole, and posaconazole may improve efficacy, detect therapeutic failures attributable to subtherapeutic concentrations, and minimize the risk of toxicity.2
Isavuconazole has a favorable pharmacokinetic and safety profile, yet there are few studies on the relationship between its plasma concentrations and efficacy and safety. Its plasma concentrations appear to be subject to less variability than those of voriconazole and it has a better safety profile with a lower incidence of hepatotoxicity.9 However, although it may not be necessary to monitor short-course isavuconazole,10 there is a lack of information on this issue in relation to courses longer than 2 months.
ConclusionsCYP2C19 genotyping can predict therapeutic responses to voriconazole, but it is not currently recommended as a routine activity and does not provide immediate results. Nevertheless, this test was the one that confirmed the need to change the antifungal therapy, which had been determined early through pharmacokinetic monitoring.
Ethical considerationsThis study has not been previously published, nor is it under review in any other journal.
The instructions for manuscript submission and ethical responsibilities have been taken into account (all the authors meet the requirements for authorship and all declare that they have no conflicts of interest).
Declaration of authorshipAll named authors contributed to the preparation of the article regarding the conception and design of the clinical case described. Jorge Esquivel Negrín was responsible for writing the article. Enrique Tévar Alonso, Ruth López Travieso, Jesús Rodríguez González, Javier Merino Alonso, and Andrea Santos Fagundo undertook a critical review of the article and offered relevant intellectual contributions. All six approved the final version of the work for its publication. Data collection was conducted by Jorge Esquivel Negrín, Enrique Tévar Alfonso, Ruth López Travieso, and Jesús Rodríguez González.
FundingNone declared.
Contribution to the scientific literatureA clinical case report of the pharmacokinetic and pharmacogenetic study of voriconazole during therapeutic follow up.
These studies are justified as part of voriconazole post-treatment follow-up protocols.