Standard treatment of metastatic colorectal cancer includes oxaliplatin and 5-fluorouracil in continuous infusion. Although FOLFOX-6 is the reference combination, it is aggressive and has high toxicity. Variants such as the TTD regimen, which does not include folinic acid or 5-fluorouracil bolus, are used. This study evaluates the toxicity of FOLFOX-6 and TTD in first line treatment for metastatic colorectal cancer and its effectiveness.
MethodRetrospective observational study with patients who started treatment with FOLFOX-6 and TTD, for 3 years. Demographic and clinical data were collected (age, sex, chronic pathologies, molecular profile, laterality, Eastern Cooperative Oncology Group classification, and stage), as well as treatment variables (previous adjuvant chemotherapy, intentionality, number of cycles, duration, and pharmacogenetic aspects) and toxicity. Objective response rate and progression-free survival were calculated.
ResultsThe study included 71 patients, 35 treated with FOLFOX-6, and 36 with TTD. Both groups showed similar overall toxicity profiles. FOLFOX-6 had a higher incidence of neutropenia (46% vs 8%; P < .01) and mucositis (51% vs 22%; P < .013). In addition, there were more treatment delays (40% vs 11%; P < .05) and 5-fluorouracil dose reductions (22% vs 14%; P < .05) in the FOLFOX-6 group. Deaths due to toxicity were only recorded in the FOLFOX-6 group. Effectiveness was similar in both groups.
ConclusionsThe TTD regimen could be a beneficial first-line option for metastatic colorectal cancer, with lower toxicity and effectiveness comparable to FOLFOX-6. It is a safe alternative for elderly or frail patients, suitable for reduced-dose 5-fluorouracil regimen with oxaliplatin.
El tratamiento estándar del cáncer colorrectal metastásico incluye oxaliplatino y 5-fluorouracilo en infusión continua. Aunque FOLFOX-6 es la combinación de referencia, es agresivo y tiene alta toxicidad. Se utilizan variantes como el esquema TTD, que no incluye ácido folínico ni bolo de 5-fluorouracilo. Este estudio evalúa la toxicidad de FOLFOX-6 y TTD en primera línea de tratamiento para cáncer colorrectal metastásico y su efectividad.
MétodoEstudio observacional retrospectivo con pacientes que comenzaron tratamiento con FOLFOX-6 y TTD, durante tres años. Se recopilaron datos demográficos y clínicos (edad, sexo, patologías crónicas, perfil molecular, lateralidad, clasificación ECOG y estadio), además de variables de tratamiento (quimioterapia adyuvante previa, intencionalidad, número de ciclos, duración y aspectos farmacogenéticos) y toxicidad. Se calcularon la tasa de respuesta objetiva y la supervivencia libre de progresión.
ResultadosEl estudio incluyó 71 pacientes, 35 tratados con FOLFOX-6 y 36 con TTD. Ambos grupos mostraron perfiles de toxicidad global similares. FOLFOX-6 presentó una mayor incidencia de neutropenia (46% vs 8%;p < 0,01) y mucositis (51% vs 22%;p < 0,013). Además, hubo más retrasos en el tratamiento (40% vs 11%; p < 0,05) y reducciones de dosis de 5-fluorouracilo (22% vs 14%;p < 0,05) en el grupo FOLFOX-6. Solo se registraron muertes por toxicidad en el grupo FOLFOX-6. La efectividad fue similar en ambos grupos.
ConclusionesEl esquema TTD es una opción beneficiosa en primera línea para cáncer colorrectal metastásico, con menor toxicidad y efectividad comparable a FOLFOX-6. Podría ser una alternativa segura para pacientes ancianos o frágiles, adecuados para esquemas de 5-fluorouracilo a dosis reducidas con oxaliplatino.
Oxaliplatin combined with continuous infusion of 5-fluorouracil (5-FU) with or without a monoclonal antibody is a standard first-line treatment for metastatic colorectal cancer (mCRC).1,2
FOLFOX (folinic acid + fluorouracil + oxaliplatin) is a combination regimen of oxaliplatin and 5-FU, administered as a bolus or continuous infusion, along with folinic acid. Folinic acid is used to biochemically modulate 5-FU, enhancing its cytotoxic activity. Administration of 5-FU by continuous infusion also increases the efficacy of bolus 5-FU by extending its half-life through prolonged infusion time. The combination of the 2 strategies increases the efficacy of 5-FU.3
There are different FOLFOX regimens, which differ in the way their components are dosed and administered.4 FOLFOX-6 is the current reference for the first-line treatment for mCRC.2 The regimen consists of oxaliplatin 85 mg/m2 administered on day 1, with a bolus of 5-FU 400 mg/m2, modulated with folinic acid 400 mg/m2, followed by 5-FU 2400 mg/m2 administered as a 48-h continuous infusion, repeated every 2 weeks.
Although the addition of folinic acid and the combination of bolus and continuous infusion 5-FU have increased the efficacy of the FOLFOX regimen, these changes have also increased its toxicity.5 It is an intensive treatment limited by its main toxic effects: peripheral sensory neuropathy, neutropenia, and diarrhea. Sensory neuropathy is an adverse reaction associated with oxaliplatin, while neutropenia and diarrhea are mainly related to 5-FU.6 The MOSAIC study found that the FOLFOX regimen was associated with neutropenia and grade 3/4 diarrhea (41% and 11%, respectively).7 Similar results have been reported in other phase III studies of first-line treatment for mCRC.8–10
Several studies have explored combinations of oxaliplatin with different routes of 5-FU administration to minimize the toxicity of the regimen without compromising its efficacy. In this context, the Spanish Group for the Treatment of Digestive Tumors has conducted extensive research into combination regimens that eliminate the 5-FU bolus and/or modulation with folinic acid.11 One reported regimen, known as TTD, consists of oxaliplatin 85 mg/m2, administered on day 1, followed by 5-FU at a dose of 2500 mg/m2 administered as a 48-h continuous infusion every 2 weeks. With this regimen, neutropenia and grade 3/4 diarrhea were 16% and 11%, respectively.11 Another study by the same group using other doses of 5-FU without bolus or modulation reported grade 3/4 neutropenia and diarrhea in 11% and 24% of patients, respectively.12 Although these regimens are now used in clinical practice, supporting studies are limited and provide low levels of clinical evidence.11–14
Given these findings, new insights could be gained by conducting a comparative toxicity study of the FOLFOX-6 and TTD regimens.
The primary objective of this study was to evaluate the toxicity of the FOLFOX-6 and TTD regimens, with or without a monoclonal antibody, as first-line treatments for mCRC under real-world conditions. The secondary objective was to evaluate the effectiveness of both treatment regimens.
MethodA single-center, observational, retrospective study conducted as part of the change in the oncological treatment protocol for mCRC at our hospital. It was approved by the Andalusian Biomedical Research Ethics Coordinating Committee, with code FAB-OXA-2023-01 protocol v.3.0 (approval date: June 27, 2023).
The study included all patients with mCRC who initiated first-line treatment with FOLFOX-6 or TTD regimens, with or without a monoclonal antibody (bevacizumab, cetuximab, or panitumumab), between July 2019 and December 2022, with a minimum follow-up of 6 months. The study excluded patients enrolled in clinical trials, those with other active malignancies, and patients who began palliative treatment following progression during adjuvant XELOX (capecitabine + oxaliplatin) due to associated cumulative toxicity.
Data were collected from digital medical records (DIRAYA) and oncology pharmacy software (Oncogest).
The following demographic and clinical variables were collected: age, sex, primary tumor location, molecular profile, colorectal cancer laterality, Eastern Cooperative Oncology Group (ECOG) classification, and stage according to the TNM (8th edition) system (M1a: single metastatic site or organ; M1b: multiple metastatic sites or organs; M1c: peritoneal metastases).
Treatment-related variables were also collected, including treatment intent (neoadjuvant or palliative), pharmacogenetic aspects (dihydropyrimidine dehydrogenase [DPD] enzyme phenotyping for 5-FU dosing), number of cycles administered, and treatment duration.
The primary endpoint was treatment toxicity, with severity graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0, identifying both differential and common toxicities between the 2 treatment regimens. Data were also collected on cycle delays, 5-FU dose reductions, treatment discontinuations due to toxicity, and the use of colony-stimulating factors.
Treatment effectiveness was assessed as a secondary variable. The Response Evaluation Criteria in Solid Tumors criteria were used to assess the objective response rate (percentage of patients achieving complete or partial response) and disease control rate (percentage of patients achieving complete response, partial response, or stable disease). Progression-free survival was calculated as the time from initiation of treatment of metastatic disease according to the study schedules, to radiological progression (determined by CT scan), clinical or analytical progression, and/or death. Cases were censored at the time of dropout (therapeutic break or treatment abandonment), discontinuation (primarily due to toxicity or progression leading to initiation of another treatment line), or loss to follow-up (e.g., transfer to other communities).
All statistical analyses were performed using SPSS (IBM, Chicago). A descriptive analysis was conducted using measures of central tendency and dispersion for quantitative variables and frequency distributions for qualitative variables. The incidence of toxicity-related variables was analyzed based on the treatment regimen and differences were assessed using the chi-square test. Comparisons between quantitative variables were conducted using the Student’s t-test. Survival outcomes were estimated using the Kaplan–Meier method. A P-value of <.05 was used as a cut-off for statistical significance.
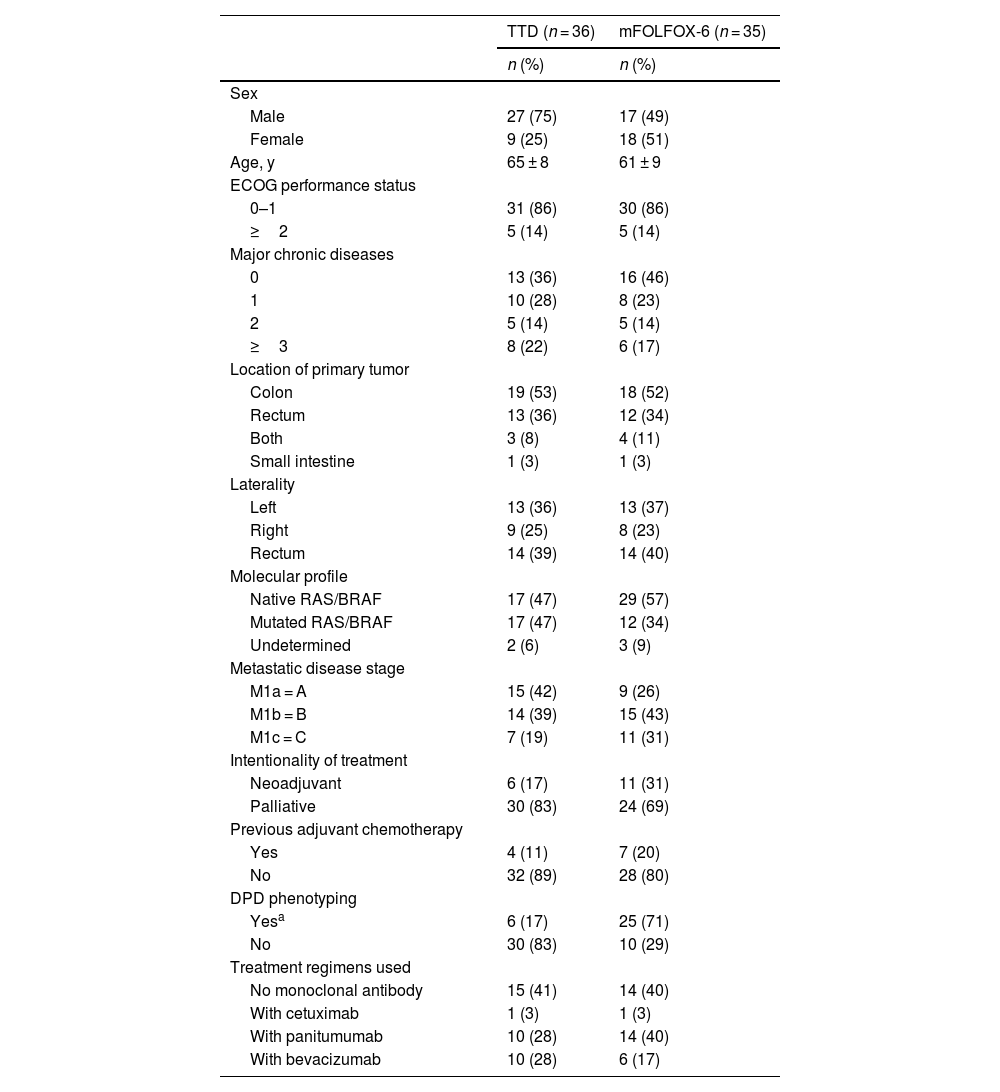
ResultsA total of 71 patients were included; 35 in the FOLFOX-6 and 36 in the TTD treatment groups. Table 1 shows demographic and clinical characteristics.
Demographic and clinical characteristics of patientsa.
| TTD (n = 36) | mFOLFOX-6 (n = 35) | |
|---|---|---|
| n (%) | n (%) | |
| Sex | ||
| Male | 27 (75) | 17 (49) |
| Female | 9 (25) | 18 (51) |
| Age, y | 65 ± 8 | 61 ± 9 |
| ECOG performance status | ||
| 0–1 | 31 (86) | 30 (86) |
| ≥2 | 5 (14) | 5 (14) |
| Major chronic diseases | ||
| 0 | 13 (36) | 16 (46) |
| 1 | 10 (28) | 8 (23) |
| 2 | 5 (14) | 5 (14) |
| ≥3 | 8 (22) | 6 (17) |
| Location of primary tumor | ||
| Colon | 19 (53) | 18 (52) |
| Rectum | 13 (36) | 12 (34) |
| Both | 3 (8) | 4 (11) |
| Small intestine | 1 (3) | 1 (3) |
| Laterality | ||
| Left | 13 (36) | 13 (37) |
| Right | 9 (25) | 8 (23) |
| Rectum | 14 (39) | 14 (40) |
| Molecular profile | ||
| Native RAS/BRAF | 17 (47) | 29 (57) |
| Mutated RAS/BRAF | 17 (47) | 12 (34) |
| Undetermined | 2 (6) | 3 (9) |
| Metastatic disease stage | ||
| M1a = A | 15 (42) | 9 (26) |
| M1b = B | 14 (39) | 15 (43) |
| M1c = C | 7 (19) | 11 (31) |
| Intentionality of treatment | ||
| Neoadjuvant | 6 (17) | 11 (31) |
| Palliative | 30 (83) | 24 (69) |
| Previous adjuvant chemotherapy | ||
| Yes | 4 (11) | 7 (20) |
| No | 32 (89) | 28 (80) |
| DPD phenotyping | ||
| Yesa | 6 (17) | 25 (71) |
| No | 30 (83) | 10 (29) |
| Treatment regimens used | ||
| No monoclonal antibody | 15 (41) | 14 (40) |
| With cetuximab | 1 (3) | 1 (3) |
| With panitumumab | 10 (28) | 14 (40) |
| With bevacizumab | 10 (28) | 6 (17) |
DPD, dihydropyrimidine dehydrogenase; ECOG, Eastern Cooperative Oncology Group; M1a, single site; M1b, 2 or more sites; M1c, peritoneal carcinomatosis; mFOLFOX-6, folinic acid + fluorouracil infusion and bolus + oxaliplatin regimen; TTD, fluorouracil infusion + oxaliplatin regimen.
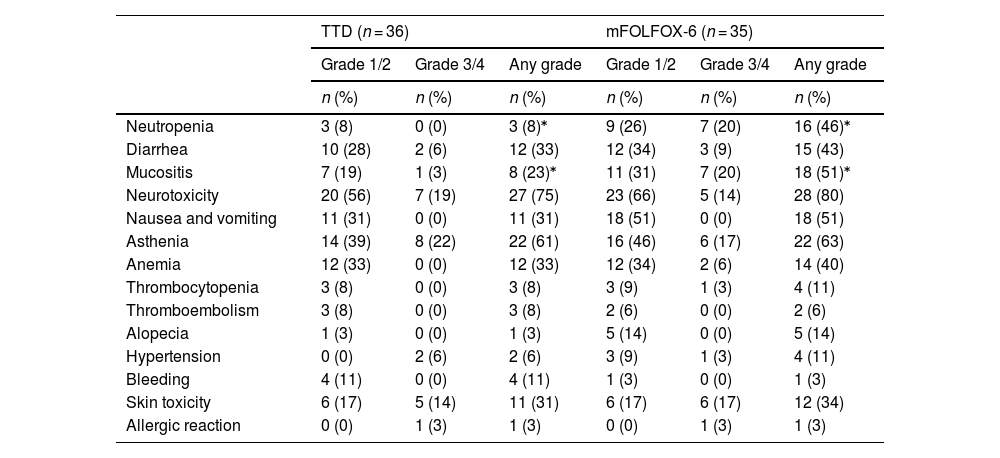
Table 2 shows the frequency of adverse events by the highest grade observed. Some type of toxicity was experienced by all patients in the FOLFOX-6 group and by 94% in the TTD group. Overall, the most common adverse reactions associated with FOLFOX-6 were neurotoxicity, asthenia, mucositis, neutropenia, diarrhea, nausea, anemia, and skin toxicity. The most frequent adverse reactions associated with the TTD regimen were, in order, neurotoxicity, asthenia, diarrhea, anemia, skin toxicity, nausea, and mucositis, with very few cases of neutropenia. The FOLFOX-6 group experienced higher rates of neutropenia (46% vs 8%; P < .01) and mucositis (51% vs. 22%; P < .013). No granulocyte colony-stimulating factors were required, and no febrile neutropenia was documented.
Maximum toxicity episodes per patient.⁎
| TTD (n = 36) | mFOLFOX-6 (n = 35) | |||||
|---|---|---|---|---|---|---|
| Grade 1/2 | Grade 3/4 | Any grade | Grade 1/2 | Grade 3/4 | Any grade | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Neutropenia | 3 (8) | 0 (0) | 3 (8)⁎ | 9 (26) | 7 (20) | 16 (46)⁎ |
| Diarrhea | 10 (28) | 2 (6) | 12 (33) | 12 (34) | 3 (9) | 15 (43) |
| Mucositis | 7 (19) | 1 (3) | 8 (23)⁎ | 11 (31) | 7 (20) | 18 (51)⁎ |
| Neurotoxicity | 20 (56) | 7 (19) | 27 (75) | 23 (66) | 5 (14) | 28 (80) |
| Nausea and vomiting | 11 (31) | 0 (0) | 11 (31) | 18 (51) | 0 (0) | 18 (51) |
| Asthenia | 14 (39) | 8 (22) | 22 (61) | 16 (46) | 6 (17) | 22 (63) |
| Anemia | 12 (33) | 0 (0) | 12 (33) | 12 (34) | 2 (6) | 14 (40) |
| Thrombocytopenia | 3 (8) | 0 (0) | 3 (8) | 3 (9) | 1 (3) | 4 (11) |
| Thromboembolism | 3 (8) | 0 (0) | 3 (8) | 2 (6) | 0 (0) | 2 (6) |
| Alopecia | 1 (3) | 0 (0) | 1 (3) | 5 (14) | 0 (0) | 5 (14) |
| Hypertension | 0 (0) | 2 (6) | 2 (6) | 3 (9) | 1 (3) | 4 (11) |
| Bleeding | 4 (11) | 0 (0) | 4 (11) | 1 (3) | 0 (0) | 1 (3) |
| Skin toxicity | 6 (17) | 5 (14) | 11 (31) | 6 (17) | 6 (17) | 12 (34) |
| Allergic reaction | 0 (0) | 1 (3) | 1 (3) | 0 (0) | 1 (3) | 1 (3) |
mFOLFOX-6, folinic acid + fluorouracil + oxaliplatin infusion and bolus regimen; TTD, fluorouracil + oxaliplatin infusion regimen.
Skin toxicity was more prevalent in patients treated with panitumumab or cetuximab, reaching 93% (43% grade 3/4) in the panitumumab/cetuximab-FOLFOX-6 group and 90% (50% grade 3/4) in the panitumumab/cetuximab-TTD group.
Adverse drug reactions resulted in discontinuation of treatment regimens in 11% (4) of patients in each group. The most common adverse events in FOLFOX-6 and TTD were asthenia (3 and 4 patients, respectively), neurotoxicity (3 and 3 patients), mucositis (3 and 1 patients), diarrhea (1 and 3 patients), neutropenia (1 and 0 patients), and anemia (1 and 1 patient). Toxicity-related deaths occurred exclusively in patients treated with the FOLFOX-6 regimen, with 3 deaths due to mucositis and myelotoxicity (neutropenia and anemia). These deaths occurred in 2 patients with ECOG 2 and 1 patient with ECOG 1.
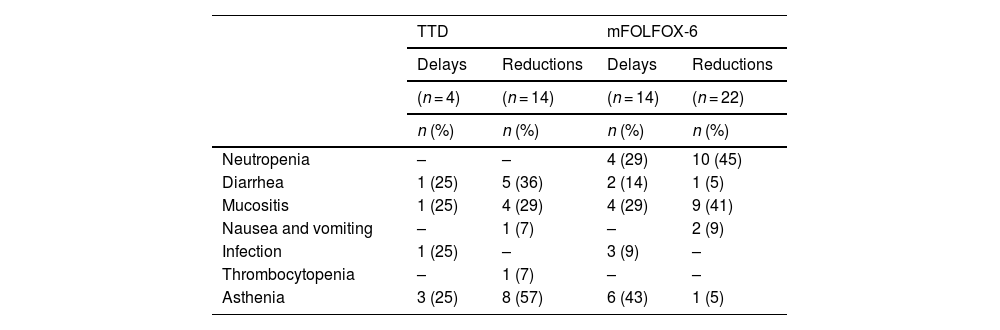
Patients treated with the FOLFOX-6 regimen experienced significantly more treatment delays due to toxicity (40% vs 11%; P < .05) and 5-FU dose reductions (22% vs 14%; P < .05). Table 3 shows the most common adverse reactions, with neutropenia, mucositis, and asthenia in FOLFOX-6-treated patients, and asthenia, diarrhea, and mucositis in TTD-treated patients. A total of 11 (31%) and 22 (61%) patients had no delays or dose reductions of 5-FU during their treatment with FOLFOX-6 and TTD, respectively (P < .05).
Adverse reactions leading to 5-fluorouracil cycle delays and dose reductions.a
| TTD | mFOLFOX-6 | |||
|---|---|---|---|---|
| Delays | Reductions | Delays | Reductions | |
| (n = 4) | (n = 14) | (n = 14) | (n = 22) | |
| n (%) | n (%) | n (%) | n (%) | |
| Neutropenia | – | – | 4 (29) | 10 (45) |
| Diarrhea | 1 (25) | 5 (36) | 2 (14) | 1 (5) |
| Mucositis | 1 (25) | 4 (29) | 4 (29) | 9 (41) |
| Nausea and vomiting | – | 1 (7) | – | 2 (9) |
| Infection | 1 (25) | – | 3 (9) | – |
| Thrombocytopenia | – | 1 (7) | – | – |
| Asthenia | 3 (25) | 8 (57) | 6 (43) | 1 (5) |
mFOLFOX-6, folinic acid + fluorouracil + oxaliplatin infusion and bolus regimen; TTD, fluorouracil + oxaliplatin infusion regimen.
During the study period, the FOLFOX-6 group received a total of 426 cycles, and the TTD group received a total of 450 cycles (mean: 12 and 13 cycles per patient in each group, respectively). Regarding treatment intent for metastatic disease, all patients in the TTD group receiving neoadjuvant therapy completed the planned cycles, whereas 1 patient (9%) in the FOLFOX-6 group discontinued neoadjuvant therapy due to toxicity.
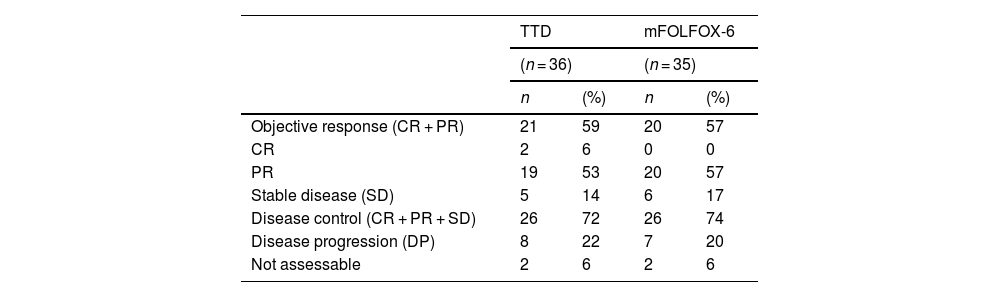
EffectivenessThe objective response rate was 57% in the FOLFOX-6 group and 59% in the TTD group, with no significant difference between the 2 groups (Fisher exact test, P = .705). Disease control was also similar in both groups. Response could not be measured in 4 patients; 2 in the FOLFOX-6 group and 2 in the TTD group (Table 4).
Response to treatment.
| TTD | mFOLFOX-6 | |||
|---|---|---|---|---|
| (n = 36) | (n = 35) | |||
| n | (%) | n | (%) | |
| Objective response (CR + PR) | 21 | 59 | 20 | 57 |
| CR | 2 | 6 | 0 | 0 |
| PR | 19 | 53 | 20 | 57 |
| Stable disease (SD) | 5 | 14 | 6 | 17 |
| Disease control (CR + PR + SD) | 26 | 72 | 26 | 74 |
| Disease progression (DP) | 8 | 22 | 7 | 20 |
| Not assessable | 2 | 6 | 2 | 6 |
SD, stable disease; CR, complete response; PR, partial response; DP, disease progression.
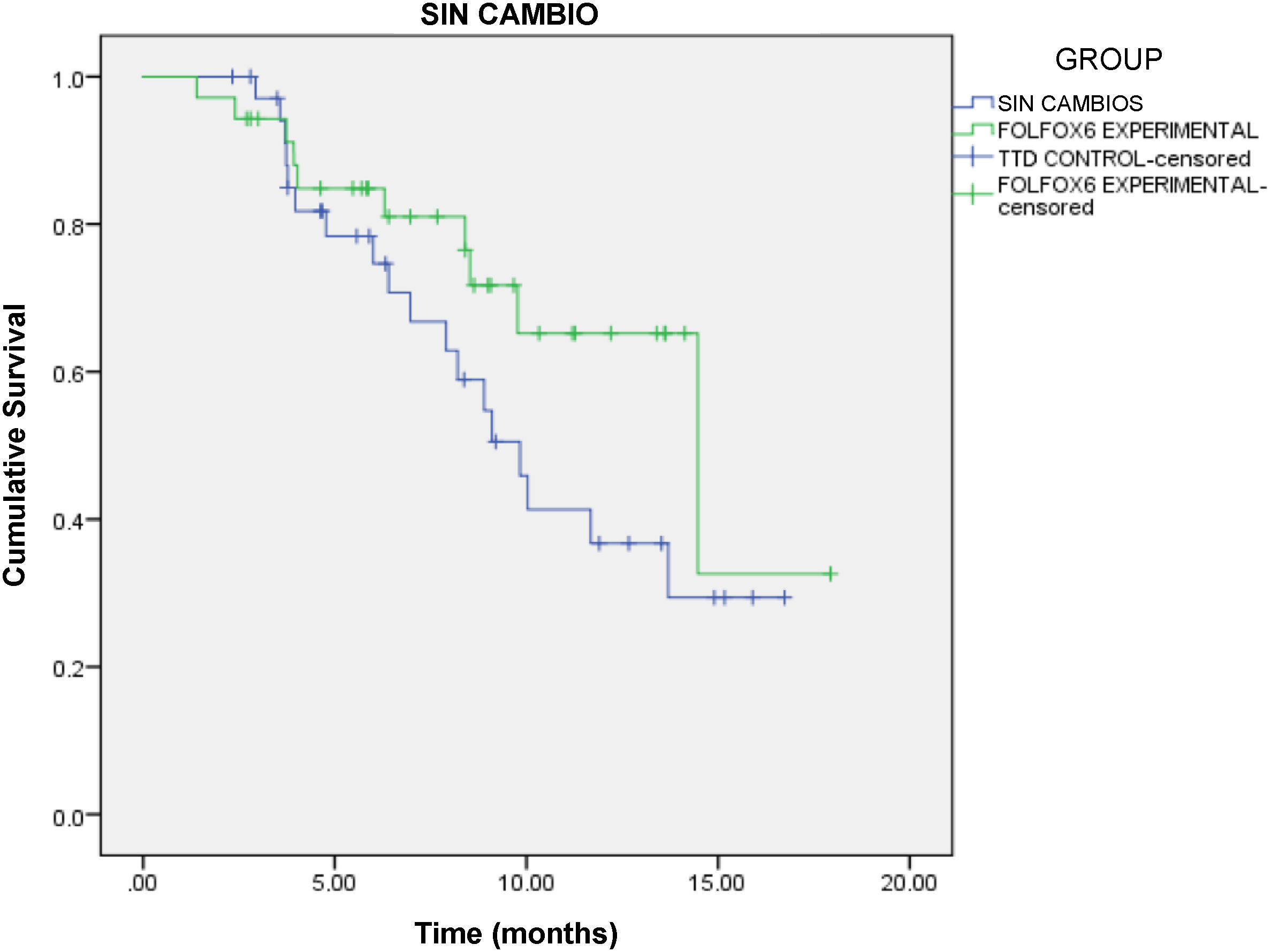
At study completion, 10 patients and 7 patients were still receiving active treatment with FOLFOX-6 and TTD, respectively. Progression-free survival was 8.4 months (95% confidence interval [CI]: 6.7–9.5 months) in the FOLFOX-6 group and 6.7 months (95% CI: 6.4–9.3 months) in the TTD group, with no significant difference between the 2 groups (HR = 0.567, 95% CI: 0.261–1.231, P = .146, log-rank test) (Fig. 1).
DiscussionThe study found that both regimens had similar overall toxicity profiles. However, a distinction must be made between the common toxicity profile—driven by shared drugs such as monoclonal antibodies (panitumumab, cetuximab, and bevacizumab) and oxaliplatin—and the differential toxicity, which is related to the mode of 5-FU administration, whether as a continuous infusion, in combination with a bolus, or with folinic acid.
Both FOLFOX-6 and TTD regimens were predominantly used in combination with monoclonal antibodies, and the frequency of associated adverse events was very similar in both groups. Vascular endothelial growth factor inhibitors (bevacizumab) cause vascular toxicity, mainly manifested by hypertension and thromboembolic disease. Epidermal growth factor receptor inhibitors (cetuximab and panitumumab) also cause thromboembolism, although the main adverse reaction is skin toxicity. Its most common form is acneiform rash, with an incidence of 60%–80% (5%–20%; grade 3/4).15 The PRIME study assessed the efficacy and safety of panitumumab combined with continuous infusion 5-FU, folinic acid, and oxaliplatin (FOLFOX-4) as first-line treatment for mCRC. It found that the incidence of any grade of skin toxicity was 96%. We also observed this reaction in 34% of patients in the FOLFOX-6 group and 31% in the TTD group. These percentages are much lower than those in the PRIME study. Patients treated with panitumumab had similar results, with an overall incidence of skin toxicity of approximately 90% in both groups. These figures are also comparable to those observed in the PEAK study, which evaluated the efficacy and safety of FOLFOX-6 in combination with a monoclonal antibody in the first-line treatment for mCRC.16
The toxicological profile of oxaliplatin was also very similar in both groups. Oxaliplatin-induced neuropathy presents in 2 patterns: acute peripheral neuropathy, affecting over 85% of patients, and late-onset peripheral neuropathy, which occurs in 10%–20% of patients. The latter increases in severity with cumulative doses, persists between chemotherapy cycles, is dose-limiting, can worsen even after treatment cessation, and is partially reversible.17 The safety results of the MOSAIC study on FOLFOX-4 showed that 92% of patients developed peripheral neuropathy, with 12% classified as grade 3/4.7 We observed peripheral neuropathy in 80% of the FOLFOX-6 group and 75% of the TTD group, with grade 3 neuropathy in 14% of the FOLFOX-6 group and 19% of the TTD group. These results are also similar to those observed in several phase III studies of first-line treatment for mCRC, with or without a monoclonal antibody.8,10,11,13,14
Other common reactions, although less frequent and with similar incidence in both groups, were nausea and vomiting, asthenia, anemia, thrombocytopenia, thromboembolism, and alopecia.
Differential toxicity in the 2 treatment regimens were neutropenia and gastrointestinal reactions, mainly mucositis and diarrhea. These reactions are related to the administration of 5-FU, which has method- and dose-dependent toxicity. Bolus combined with continuous infusion regimens have a higher incidence of myelosuppression and mucositis than continuous infusion alone, leading to a higher incidence of diarrhea, a risk that is further increased with the addition of folinic acid.18 The incidence of diarrhea and mucositis in the TTD regimen was comparable to that reported by Díaz Rubio et al.13 The regimen used, without bolus 5-FU or folinic acid modulation, was associated with lower rates of diarrhea and mucositis than the FOLFOX-6 regimen, whose toxicity profile was more consistent with studies of bolus 5-FU combined with folinic acid modulation regimens.7–9,14
Neutropenia was the most significant reaction with differences in toxicity, affecting 46% of patients (20% grade 3/4) in the FOLFOX-6 group compared to only 8% (no grade 3/4 cases) in the TTD group. Together with mucositis, and diarrhea, it caused the majority of treatment discontinuations due to toxicity in both groups. It was also present in the only 2 patients in the study who died as a result of toxicity associated with the FOLFOX-6 regimen. Although the neutropenia rates were lower than those observed in other studies, the higher incidence of neutropenia in the FOLFOX-6 regimen was close to that seen in studies of bolus 5-FU combined with folinic acid modulation, as was the case with diarrhea and mucositis.7–9,14 The incidence of grade 3/4 neutropenia with the TTD regimen was even lower than that reported by Díaz Rubio et al. This was likely due to differences in the continuous 5-FU administration schedule, with weekly dosing in their study compared to the fortnightly schedule in ours.13
Adherence to the treatment plan was poorer in patients receiving FOLFOX-6, as reflected in higher rates of 5-FU dose reductions and treatment delays, which were attributed to the differences in toxicity between the 2 regimens. This aspect is particularly significant in neoadjuvant treatments, in preparation for either initial surgery or salvage surgery for metastases, typically liver or lung.
The effectiveness of the 2 regimens was similar. Progression-free survival was similar that seen in other studies combining oxaliplatin and 5-FU as first-line treatment for mCRC, with or without biological agents.8–11,13,14
The demographic characteristics of the study population are consistent with the epidemiological data on patients with mCRC.19 There were significantly more female patients in the FOLFOX-6 group. Recent studies have shown that women are at significantly higher risk of the more common adverse events.20–22 This study did investigate the effect of sex on the occurrence of chemotherapy-associated adverse events.
In addition, DPD enzyme testing was more common in the FOLFOX-6 group, as this was not a routine test in CRC treatment protocols until 2020. The majority of patients in the TTD group were diagnosed and treated prior to this date. However, it is unlikely that this factor influenced the differences in results, as the polymorphism affects less than 5% of the general population and, if anything, would have contributed to a lower incidence of toxicity in the FOLFOX-6 group.23
The main limitations of this study include its retrospective and observational design, its single-center scope, small sample size, as well as potential bias due to missing data in medical records, which may have contributed to an underestimation of the incidence of toxicity (information bias). However, as this study reflects routine clinical practice, its results can be extrapolated to other hospital settings.
In summary, the TTD regimen can be considered safer than the current standard FOLFOX-6 regimen, while maintaining comparable effectiveness. The European Society for Medical Oncology classifies mCRC patients as “fit” and “unfit” based on tumor characteristics and associated comorbidities. Elderly patients can be included in either treatment group, and age alone should not be a barrier to receiving the benefits of all active and available drugs. The combination of reduced doses of fluoropyrimidines and oxaliplatin is a recommended option for the first-line treatment of mCRC in elderly “unfit” patients.1 Sáez-López et al. take a similar stance in a review of surgical and chemotherapeutic treatments for mCRC in elderly patients. They provide standardized and/or individualized guidelines, particularly when deterioration is due to the oncological disease rather than pre-existing comorbidities.23
In conclusion, the TTD regimen appears to be a beneficial option for the first-line treatment of mCRC, offering reduced toxicity compared to FOLFOX-6 while maintaining equivalent effectiveness. It could be offered as a safer alternative for elderly, frail patients who are candidates for a reduced-dose 5-FU regimen combined with oxaliplatin, with or without biologics. Further studies comparing the safety and efficacy of both regimens are needed to provide more robust results.
Contribution to the scientific literatureThe results of this study provide insight into the extent to which the change in the oncological treatment protocol for mCRC at our hospital has contributed to improving patient health outcomes.
We found that omitting colony-stimulating factors is justified on safety grounds. Although these agents can be used to manage neutropenia, their use may also increase the risk of thrombocytopenia in patients with frequent active bleeding. In addition, the adverse effects of colony-stimulating factors, such as flu-like syndrome, and the dosing challenges that arise within the bi-weekly chemotherapy schedule should also be taken into account.
The study results suggest that a new care protocol could improve patient health outcomes, as they demonstrate that such an approach can achieve similar efficacy with lower toxicity than those reported in pivotal trials. This study provides key information to guide clinical decision-making by providing a comprehensive comparison of the safety and efficacy of different regimens in routine clinical practice. Such evidence is essential to optimize the therapeutic management of mCRC.
Ethical responsibilitiesProject authorized by the Andalusian Biomedical Research Ethics Coordinating Committee, with code FAB-OXA-2023-01 protocol v.3.0 (approval date: June 27, 2023).
FundingThis study was supported by a grant from the Andalusian Foundation of Hospital Pharmacy.
CRediT authorship contribution statementMaría Teresa Garrido Martínez: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. María Rodríguez Jorge: Writing – review & editing, Supervision, Investigation, Data curation. Ignacio García Giménez: Writing – review & editing, Visualization, Validation, Supervision, Investigation, Data curation. María Isabel Guzmán Ramos: Writing – review & editing, Supervision, Data curation. Salvador Grutzmancher Sáiz: Writing – review & editing, Supervision. Victoria Aviñó Tarazona: Writing – review & editing, Supervision.
To the Andalusian Foundation of Hospital Pharmacy.