This study's aims are: 1) To use the Delphi method to determine the level of consensus among hospital pharmacists (HPs) as regards the factors involved in the current approach to patients with atopic dermatitis (AD); 2) To identify potential areas for improvement in hospital pharmacy in terms of dealing with patients with severe AD; and 3) To contribute to adequate pharmaceutical care for patients with AD by drawing up recommendations.
MethodsA two-round Delphi survey with participation from HPs from all over Spain. Three theme-based blocks were set out: 1) AD; 2) Management of patients with severe AD in the Hospital Pharmacy setting; and 3) Unmet needs (pathology, patient, treatment and management).
ResultsThe 42 HPs participating reached a consensus in recognising the impact of severe AD on the patients suffering from it, the need to encourage adherence and the recommendations to use scales that take into account the patient's quality of life and indicators of the patient's experience. It has also been demonstrated that it is worthwhile evaluating the results in real clinical practice in consensus with other specialists from the multidisciplinary team. Finally, it is advisable to use drugs that have demonstrated long-term effectiveness and safety for patients with severe AD, given the disease's chronic nature.
ConclusionsThis Delphi consensus highlights the impact of severe AD on patients, the importance of a multidisciplinary and holistic approach, in which HP play a major role. It also highlights the importance of increased access to new drugs to improve health outcomes.
Los objetivos de este estudio son: 1) Determinar, mediante el método Delphi, el grado de consenso existente entre los farmacéuticos de hospital (FH) en cuanto a los factores que intervienen en el abordaje actual de los pacientes con dermatitis atópica (DA); 2) Identificar posibles áreas de mejora en la farmacia hospitalaria en cuanto al abordaje de los pacientes con DA grave; y 3) Contribuir a una adecuada atención farmacéutica a los pacientes con DA mediante la elaboración de recomendaciones.
MétodoUna encuesta Delphi con participación de FHs de toda España. Se establecieron tres bloques temáticos: 1) DA; 2) Manejo de pacientes con DA grave desde Farmacia Hospitalaria; y 3) Necesidades no cubiertas (patología, paciente, tratamiento y manejo).
ResultadosLos 42 FHs participantes llegaron a un consenso en el reconocimiento del impacto de la DA grave en los pacientes, la necesidad de fomentar la adherencia y las recomendaciones de utilizar escalas que tengan en cuenta la calidad de vida del paciente e indicadores de la experiencia. También se muestra la conveniencia de evaluar los resultados en la práctica clínica real en consenso con otros especialistas del equipo multidisciplinar. Por último, es aconsejable utilizar fármacos que hayan demostrado eficacia y seguridad a largo plazo para los pacientes con DA grave, dado el carácter crónico de la enfermedad.
ConclusionesEste consenso Delphi pone de manifiesto el impacto de la DA grave en los pacientes, la importancia del abordaje multidisciplinar y holístico, en el que el FH juega un papel de gran importancia. También se resalta la importancia de un mayor acceso a nuevos fármacos que permitan mejorar resultados en salud.
Atopic dermatitis (AD) is an inflammatory, chronic and relapsing skin disease. It typically involves an increase in type 2 immune responses, an impaired skin barrier, and greater colonisation by Staphylococcus aureus1,2. It has characteristic symptoms such as erythema, oedema, xerosis, erosions, excoriations, exudation, crusting/scabs and lichenification, which vary depending on the patient's age and the lesions' level of chronicity3. In patients with moderate-to-severe AD, skin lesions can cover a large area and be accompanied by intense, persistent itching, with a significant impact on the patient's quality of life, disturbed sleep, anxiety or depression1,4,5. It is often associated with other type 2 inflammatory diseases like asthma, allergic rhinitis or food allergies, which increase the burden of the disease even more6. AD is one of the most common skin diseases, with an estimated prevalence of 1–10% in adults and 10–20% in children. In Spain, the prevalence of AD is estimated at 7.2% in the adult population, with moderate–severe forms accounting for 41–69% of patients7.
The aim of AD treatment is to reduce the symptoms, prevent flare-ups, minimise the risks in the treatment, and control the illness in the long term6. The introduction of new drugs in treating AD implies a change in the therapeutic strategy for these patients. However, these kinds of treatment increase the complexity in overall management of the patient and make it especially important optimise a multidisciplinary approach. Given this context, the hospital pharmacist (HP) takes a prominent role in the team. Since 2014, the Spanish Society of Hospital Pharmacy (SEFH in Spanish) has been working hard to meet outpatients' needs with initiatives such as the MAPEX project (Strategic Map of Outpatient Pharmaceutical Care)8. To define the strategic map of pharmaceutical care for patients with AD, it is necessary to identify the actions at the macro, meso and micro levels to be developed by HP, and the ideal framework that favours and values their contribution. This strategy will help HP to develop their role, facing the challenges posed by the present and future needs of this type of patients. Thus, as a starting point, this project was created with the aim of analysing and agreeing upon pharmaceutical care of patients with AD, as well as identify needs and laying down recommendations.
Based on the above mentioned, this study's aims are: 1) To use the Delphi method to determine the level of consensus among HPs as regards the factors involved in the current approach to patients with AD; 2) To identify potential areas for improvement in hospital pharmacy in terms of dealing with patients with severe AD; and 3) To contribute to adequate pharmaceutical care for patients with AD by drawing up recommendations.
MethodStructure of the studyIn the approach to AD, the role of HP has been limited, resulting in the paucity of information available on the subject. Therefore, the Delphi method was considered appropriate to organise and structure an expert discussion, aiming to determine the level of consensus among HPs.
A two-round cross-sectional Delphi survey was conducted over ten months (between March and December 2021) with HPs from all over Spain taking part. The Spanish Society of Hospital Pharmacy (SEFH) supported the study.
Scientific committee and panel of expertsIn the early phases, the scientific committee was created with two coordinators and an advisory committee of four members, all of whom are specialists in hospital pharmacy with proven experience and interest in AD. Delphi coordinators belong to the SEFH Working Group on Immune-mediated Inflammatory Diseases and actively participated in the development of the MAPEX project. The members of the advisory committee were selected for their experience in the management of immune-mediated inflammatory diseases and the management of complex patients in general. They were identified as national opinion leaders and key publications. The scientific committee was tasked with analysing the main factors involved in the current approach to patients with AD, preparing the questionnaire, interpreting the results, and critically reviewing the final report.
Delphi panel members were pharmacists, with expert knowledge and experience in the management of patients with AD. They were identified as responsible of AD external patients and invited via professional networks. In total, 50 regional experts were invited to complete Delphi to ensure a minimum of 40 panel members took part. Having a regional perspective on the panel may increase the representativity. The surveys were sent via email, the most common way of distributing Delphi questionnaires.
ProcedureThe study's coordinators identified the areas of uncertainty that should form the basis for the questionnaire's structure. For the questionnaire development, it was taken into account that there was no previous approach to the management of AD from Hospital Pharmacy. Three theme-based blocks were set out: 1) AD; 2) Handling of patients with severe AD through the Hospital Pharmacy (initial visit, follow-up visit; referral; treatment and management); and 3) Detection of unmet needs (pathology, patient, medication therapy management).
The scientific committee members drafted the items to be included in the study's questionnaire. It was then posted in a microsite that the participants accessed via a web link with a user password.
The panel of participants scored each statement on a nine-point Likert scale. The level of agreement was classified as 1–3 (disagree), 4–6 (neither agree nor disagree), or 7–9 (agree). Hence, the higher the score, the higher the level of agreement. In order to evaluate the concordance between the participants' responses to each question, values were calculated with data between 1–3, 4–6, and 7–9, estimating how many replies for the item were in the tercile containing the median. A question with a concordant answer was taken into consideration when at least two thirds of the replies were within that range.
After the first round, the questions that did not reach a consensus in replies went on to the second round, reformulating the ones whose wording could be improved or clarify. When the statistical analysis of the results was available, the advisory committee met to discuss and define the conclusions from the study.
Statistical analysis and interpretation of resultsThe mean values (and standard deviation) were calculated, as well as the median and interquartile range (p25–p75) for each of the questionnaire's items using the Statistical Analysis System (SAS 9.4). The level of significance was measured with the Kolmogorov–Smirnov goodness-of-fit test for distribution.
The criteria included “unanimity” when 100% of participants agreed on the same Likert scale category, “consensus” when there was agreement among ≥80% of participants, “majority” when there was agreement among ≥66% of participants, and “disagreement” when there was agreement among <66% of the participants. For the purposes of this analysis, the ‘unanimity’ and ‘consensus’ groups were considered all together as consensus.
In order to issue the recommendations, the items for which consensus was reached were taken into account.
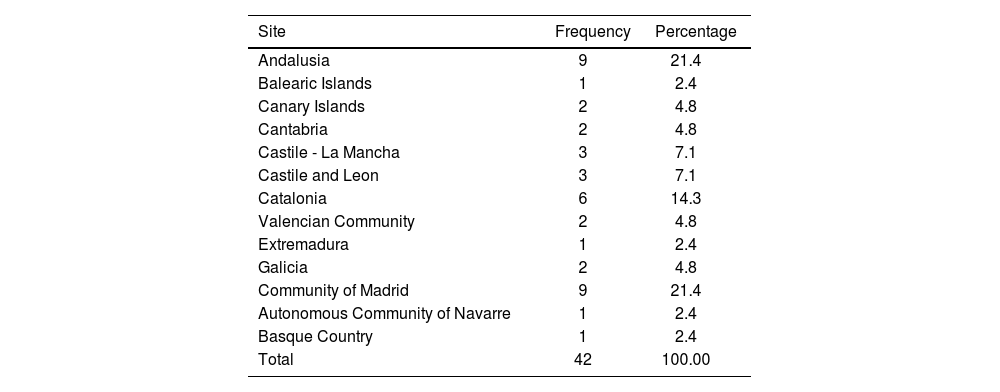
ResultsA total of 42 HPs invited to participate in the study accepted and completed both Delphi rounds. Table 1 shows the distribution of the participating hospitals by Autonomous Communities (regions of Spain). In terms of types of hospital, 24 of them were tertiary hospitals, 15 secondary and 3 primary.
Autonomous communities (Spanish regions) participating in the Delphi study.
| Site | Frequency | Percentage |
|---|---|---|
| Andalusia | 9 | 21.4 |
| Balearic Islands | 1 | 2.4 |
| Canary Islands | 2 | 4.8 |
| Cantabria | 2 | 4.8 |
| Castile - La Mancha | 3 | 7.1 |
| Castile and Leon | 3 | 7.1 |
| Catalonia | 6 | 14.3 |
| Valencian Community | 2 | 4.8 |
| Extremadura | 1 | 2.4 |
| Galicia | 2 | 4.8 |
| Community of Madrid | 9 | 21.4 |
| Autonomous Community of Navarre | 1 | 2.4 |
| Basque Country | 1 | 2.4 |
| Total | 42 | 100.00 |
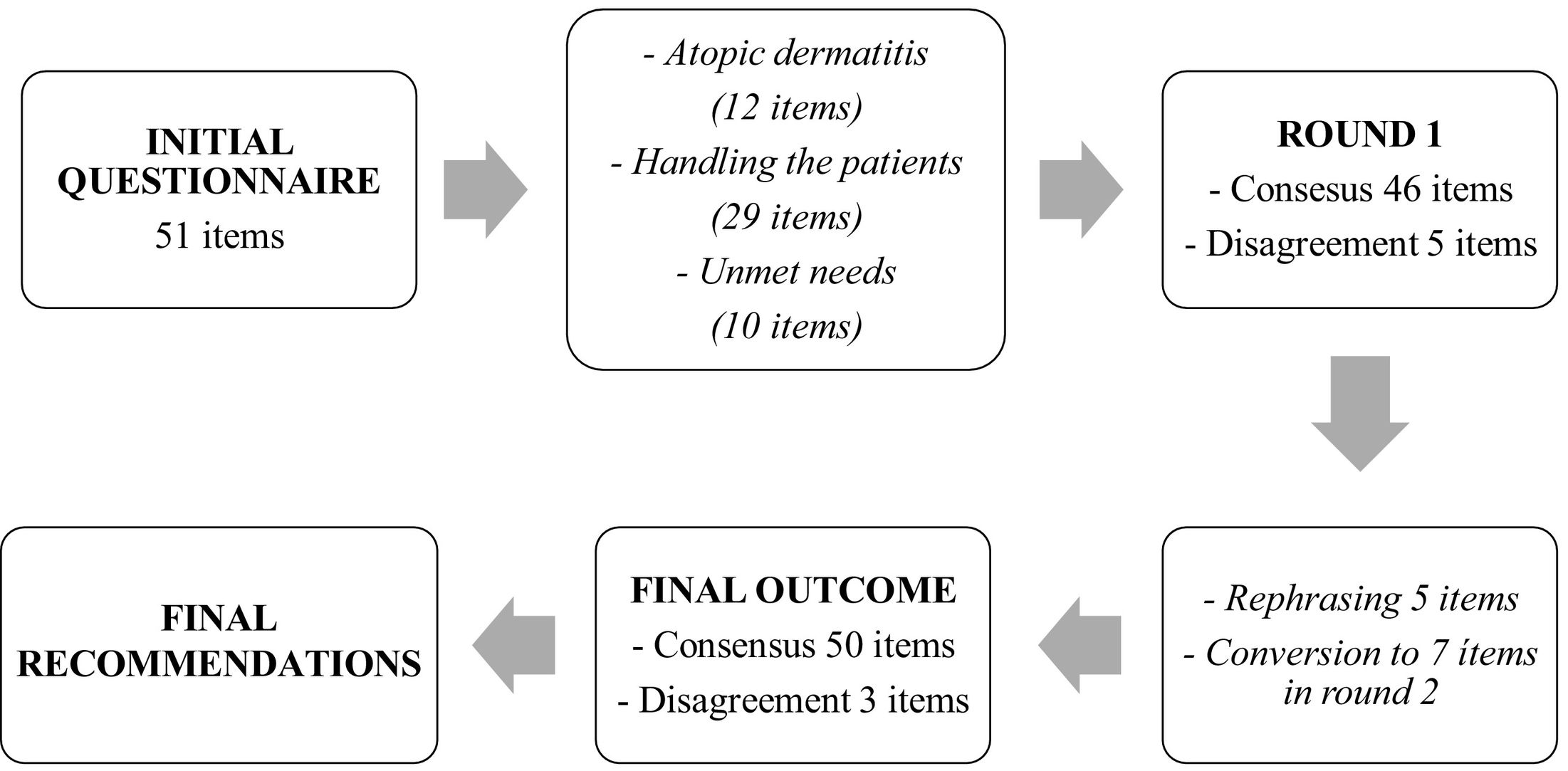
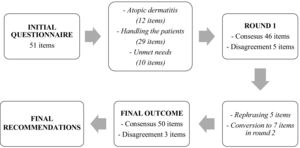
The initial questionnaire contained 51 statements divided among the three aforementioned blocks (with 12, 29 and 10 statements respectively) (Fig. 1). Table 2 gives the results from the study, including the results where consensus was obtained in the first round and those in the second.
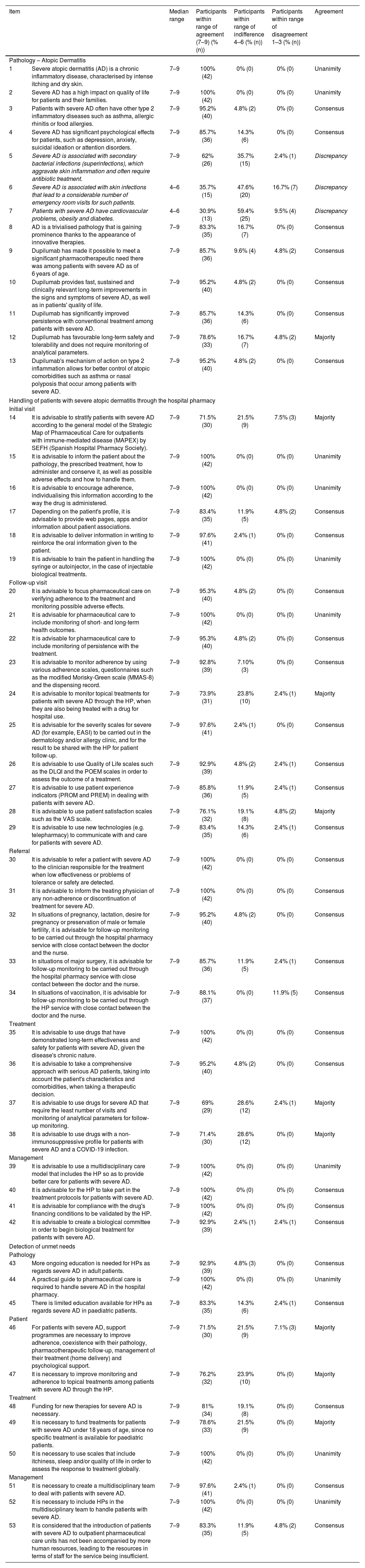
Final results from the Delphi study. Statements that did not reach consensus by the end of the study are indicated in italics.
| Item | Median range | Participants within range of agreement (7–9) (% (n)) | Participants within range of indifference 4–6 (% (n)) | Participants within range of disagreement 1–3 (% (n)) | Agreement | |
|---|---|---|---|---|---|---|
| Pathology – Atopic Dermatitis | ||||||
| 1 | Severe atopic dermatitis (AD) is a chronic inflammatory disease, characterised by intense itching and dry skin. | 7–9 | 100% (42) | 0% (0) | 0% (0) | Unanimity |
| 2 | Severe AD has a high impact on quality of life for patients and their families. | 7–9 | 100% (42) | 0% (0) | 0% (0) | Unanimity |
| 3 | Patients with severe AD often have other type 2 inflammatory diseases such as asthma, allergic rhinitis or food allergies. | 7–9 | 95.2% (40) | 4.8% (2) | 0% (0) | Consensus |
| 4 | Severe AD has significant psychological effects for patients, such as depression, anxiety, suicidal ideation or attention disorders. | 7–9 | 85.7% (36) | 14.3% (6) | 0% (0) | Consensus |
| 5 | Severe AD is associated with secondary bacterial infections (superinfections), which aggravate skin inflammation and often require antibiotic treatment. | 7–9 | 62% (26) | 35.7% (15) | 2.4% (1) | Discrepancy |
| 6 | Severe AD is associated with skin infections that lead to a considerable number of emergency room visits for such patients. | 4–6 | 35.7% (15) | 47.6% (20) | 16.7% (7) | Discrepancy |
| 7 | Patients with severe AD have cardiovascular problems, obesity and diabetes. | 4–6 | 30.9% (13) | 59.4% (25) | 9.5% (4) | Discrepancy |
| 8 | AD is a trivialised pathology that is gaining prominence thanks to the appearance of innovative therapies. | 7–9 | 83.3% (35) | 16.7% (7) | 0% (0) | Consensus |
| 9 | Dupilumab has made it possible to meet a significant pharmacotherapeutic need there was among patients with severe AD as of 6 years of age. | 7–9 | 85.7% (36) | 9.6% (4) | 4.8% (2) | Consensus |
| 10 | Dupilumab provides fast, sustained and clinically relevant long-term improvements in the signs and symptoms of severe AD, as well as in patients' quality of life. | 7–9 | 95.2% (40) | 4.8% (2) | 0% (0) | Consensus |
| 11 | Dupilumab has significantly improved persistence with conventional treatment among patients with severe AD. | 7–9 | 85.7% (36) | 14.3% (6) | 0% (0) | Consensus |
| 12 | Dupilumab has favourable long-term safety and tolerability and does not require monitoring of analytical parameters. | 7–9 | 78.6% (33) | 16.7% (7) | 4.8% (2) | Majority |
| 13 | Dupilumab's mechanism of action on type 2 inflammation allows for better control of atopic comorbidities such as asthma or nasal polyposis that occur among patients with severe AD. | 7–9 | 95.2% (40) | 4.8% (2) | 0% (0) | Consensus |
| Handling of patients with severe atopic dermatitis through the hospital pharmacy | ||||||
| Initial visit | ||||||
| 14 | It is advisable to stratify patients with severe AD according to the general model of the Strategic Map of Pharmaceutical Care for outpatients with immune-mediated disease (MAPEX) by SEFH (Spanish Hospital Pharmacy Society). | 7–9 | 71.5% (30) | 21.5% (9) | 7.5% (3) | Majority |
| 15 | It is advisable to inform the patient about the pathology, the prescribed treatment, how to administer and conserve it, as well as possible adverse effects and how to handle them. | 7–9 | 100% (42) | 0% (0) | 0% (0) | Unanimity |
| 16 | It is advisable to encourage adherence, individualising this information according to the way the drug is administered. | 7–9 | 100% (42) | 0% (0) | 0% (0) | Unanimity |
| 17 | Depending on the patient's profile, it is advisable to provide web pages, apps and/or information about patient associations. | 7–9 | 83.4% (35) | 11.9% (5) | 4.8% (2) | Consensus |
| 18 | It is advisable to deliver information in writing to reinforce the oral information given to the patient. | 7–9 | 97.6% (41) | 2.4% (1) | 0% (0) | Consensus |
| 19 | It is advisable to train the patient in handling the syringe or autoinjector, in the case of injectable biological treatments. | 7–9 | 100% (42) | 0% (0) | 0% (0) | Unanimity |
| Follow-up visit | ||||||
| 20 | It is advisable to focus pharmaceutical care on verifying adherence to the treatment and monitoring possible adverse effects. | 7–9 | 95.3% (40) | 4.8% (2) | 0% (0) | Consensus |
| 21 | It is advisable for pharmaceutical care to include monitoring of short- and long-term health outcomes. | 7–9 | 100% (42) | 0% (0) | 0% (0) | Unanimity |
| 22 | It is advisable for pharmaceutical care to include monitoring of persistence with the treatment. | 7–9 | 95.3% (40) | 4.8% (2) | 0% (0) | Consensus |
| 23 | It is advisable to monitor adherence by using various adherence scales, questionnaires such as the modified Morisky-Green scale (MMAS-8) and the dispensing record. | 7–9 | 92.8% (39) | 7.10% (3) | 0% (0) | Consensus |
| 24 | It is advisable to monitor topical treatments for patients with severe AD through the HP, when they are also being treated with a drug for hospital use. | 7–9 | 73.9% (31) | 23.8% (10) | 2.4% (1) | Majority |
| 25 | It is advisable for the severity scales for severe AD (for example, EASI) to be carried out in the dermatology and/or allergy clinic, and for the result to be shared with the HP for patient follow-up. | 7–9 | 97.6% (41) | 2.4% (1) | 0% (0) | Consensus |
| 26 | It is advisable to use Quality of Life scales such as the DLQI and the POEM scales in order to assess the outcome of a treatment. | 7–9 | 92.9% (39) | 4.8% (2) | 2.4% (1) | Consensus |
| 27 | It is advisable to use patient experience indicators (PROM and PREM) in dealing with patients with severe AD. | 7–9 | 85.8% (36) | 11.9% (5) | 2.4% (1) | Consensus |
| 28 | It is advisable to use patient satisfaction scales such as the VAS scale. | 7–9 | 76.1% (32) | 19.1% (8) | 4.8% (2) | Majority |
| 29 | It is advisable to use new technologies (e.g. telepharmacy) to communicate with and care for patients with severe AD. | 7–9 | 83.4% (35) | 14.3% (6) | 2.4% (1) | Consensus |
| Referral | ||||||
| 30 | It is advisable to refer a patient with severe AD to the clinician responsible for the treatment when low effectiveness or problems of tolerance or safety are detected. | 7–9 | 100% (42) | 0% (0) | 0% (0) | Consensus |
| 31 | It is advisable to inform the treating physician of any non-adherence or discontinuation of treatment for severe AD. | 7–9 | 100% (42) | 0% (0) | 0% (0) | Consensus |
| 32 | In situations of pregnancy, lactation, desire for pregnancy or preservation of male or female fertility, it is advisable for follow-up monitoring to be carried out through the hospital pharmacy service with close contact between the doctor and the nurse. | 7–9 | 95.2% (40) | 4.8% (2) | 0% (0) | Consensus |
| 33 | In situations of major surgery, it is advisable for follow-up monitoring to be carried out through the hospital pharmacy service with close contact between the doctor and the nurse. | 7–9 | 85.7% (36) | 11.9% (5) | 2.4% (1) | Consensus |
| 34 | In situations of vaccination, it is advisable for follow-up monitoring to be carried out through the HP service with close contact between the doctor and the nurse. | 7–9 | 88.1% (37) | 0% (0) | 11.9% (5) | Consensus |
| Treatment | ||||||
| 35 | It is advisable to use drugs that have demonstrated long-term effectiveness and safety for patients with severe AD, given the disease's chronic nature. | 7–9 | 100% (42) | 0% (0) | 0% (0) | Consensus |
| 36 | It is advisable to take a comprehensive approach with serious AD patients, taking into account the patient's characteristics and comorbidities, when taking a therapeutic decision. | 7–9 | 95.2% (40) | 4.8% (2) | 0% (0) | Consensus |
| 37 | It is advisable to use drugs for severe AD that require the least number of visits and monitoring of analytical parameters for follow-up monitoring. | 7–9 | 69% (29) | 28.6% (12) | 2.4% (1) | Majority |
| 38 | It is advisable to use drugs with a non-immunosuppressive profile for patients with severe AD and a COVID-19 infection. | 7–9 | 71.4% (30) | 28.6% (12) | 0% (0) | Majority |
| Management | ||||||
| 39 | It is advisable to use a multidisciplinary care model that includes the HP so as to provide better care for patients with severe AD. | 7–9 | 100% (42) | 0% (0) | 0% (0) | Unanimity |
| 40 | It is advisable for the HP to take part in the treatment protocols for patients with severe AD. | 7–9 | 100% (42) | 0% (0) | 0% (0) | Consensus |
| 41 | It is advisable for compliance with the drug's financing conditions to be validated by the HP. | 7–9 | 100% (42) | 0% (0) | 0% (0) | Consensus |
| 42 | It is advisable to create a biological committee in order to begin biological treatment for patients with severe AD. | 7–9 | 92.9% (39) | 2.4% (1) | 2.4% (1) | Consensus |
| Detection of unmet needs | ||||||
| Pathology | ||||||
| 43 | More ongoing education is needed for HPs as regards severe AD in adult patients. | 7–9 | 92.9% (39) | 4.8% (3) | 0% (0) | Consensus |
| 44 | A practical guide to pharmaceutical care is required to handle severe AD in the hospital pharmacy. | 7–9 | 100% (42) | 0% (0) | 0% (0) | Unanimity |
| 45 | There is limited education available for HPs as regards severe AD in paediatric patients. | 7–9 | 83.3% (35) | 14.3% (6) | 2.4% (1) | Consensus |
| Patient | ||||||
| 46 | For patients with severe AD, support programmes are necessary to improve adherence, coexistence with their pathology, pharmacotherapeutic follow-up, management of their treatment (home delivery) and psychological support. | 7–9 | 71.5% (30) | 21.5% (9) | 7.1% (3) | Majority |
| 47 | It is necessary to improve monitoring and adherence to topical treatments among patients with severe AD through the HP. | 7–9 | 76.2% (32) | 23.9% (10) | 0% (0) | Majority |
| Treatment | ||||||
| 48 | Funding for new therapies for severe AD is necessary. | 7–9 | 81% (34) | 19.1% (8) | 0% (0) | Consensus |
| 49 | It is necessary to fund treatments for patients with severe AD under 18 years of age, since no specific treatment is available for paediatric patients. | 7–9 | 78.6% (33) | 21.5% (9) | 0% (0) | Majority |
| 50 | It is necessary to use scales that include itchiness, sleep and/or quality of life in order to assess the response to treatment globally. | 7–9 | 100% (42) | 0% (0) | 0% (0) | Unanimity |
| Management | ||||||
| 51 | It is necessary to create a multidisciplinary team to deal with patients with severe AD. | 7–9 | 97.6% (41) | 2.4% (1) | 0% (0) | Consensus |
| 52 | It is necessary to include HPs in the multidisciplinary team to handle patients with severe AD. | 7–9 | 100% (42) | 0% (0) | 0% (0) | Unanimity |
| 53 | It is considered that the introduction of patients with severe AD to outpatient pharmaceutical care units has not been accompanied by more human resources, leading to the resources in terms of staff for the service being insufficient. | 7–9 | 83.3% (35) | 11.9% (5) | 4.8% (2) | Consensus |
AD: atopic dermatitis; HP: hospital pharmacy; PROMS: Patient Reported Outcome Measures; PREMs: Patient Reported Experience Measures.
In the first round, discrepancy was reached for 5 questions, which went on to the second round, dividing them into 7 to make them more understandable, so that the final results came from 53 statements. After the two Delphi rounds, consensus was reached on 41 of the 53 items (77.4%). There were nine items in which the majority agreed (17.0%), while there was a discrepancy in three items (5.7%).
DiscussionThe aim of the Delphi is to obtain an opinion, level of agreement, or consensus on a current topic or concern from among a group of experts in Hospital Pharmacy. This is an iterative and anonymous process with controlled feedback and analysis of the results widely used in health sciences. Although the Delphi methodology has been applied in some previous studies of AD in clinicians9,10, no previous studies comprising a consensus of hospital pharmacists have been published on AD using the Delphi technique. This Delphi consensus gives a real-life clinical perspective on handling severe AD from the HP's point of view and provides recommendations on the different aspects included in dealing with patients with severe AD. Furthermore, unmet needs are revealed that could improve the patients' care through the hospital pharmacy.
This study highlights that severe AD patients' quality of life is clearly impaired. According to a report published by the European Federation of Allergy and Airways Diseases Patients' Associations (EFA), 45% of the participants showed severe symptoms and recognised that AD influences their daily life, as well as their social and sexual relationships. In addition, 13% of patients stated that they had missed more than 11 days a year from work or school11. Also, patients with severe AD often have other type 2 inflammatory diseases such as asthma, allergic rhinitis, or food allergies11,12.
On the other hand, it is striking to see the high percentage of experts who appear as indifferent to the statements related to non-T2 comorbidities as infections, resulting in “non-consensus” for this item. Despite this, the majority (62%) agree that severe AD is associated with secondary bacterial infections (superinfections), which aggravate skin inflammation and often require antibiotic treatment.
Regarding AD, the need for continuous training and the creation of a pharmaceutical care guide has been highlighted since, as shown in the high participation in this study, it is a pathology that is growing in importance and interest among HPs.
As for treating severe AD, the participants in this study reached a high consensus in considering that dupilumab has covered the significant pharmacotherapeutic need there was among patients with severe AD, which is logical if one takes into account that since cyclosporine was approved over 20 years ago, no new systemic treatment had been approved for treating severe AD. Furthermore, it is the first treatment that has enabled long-term use, thanks to its safe profile, critically with chronic disease, and also the first approved treatment for patients ≥6 years old. The participants agreed by consensus that dupilumab provides significantly fast, sustained and clinically relevant long-term improvements in severe AD patients, as well as in quality of life, and improves persistence compared to conventional treatment with immunosuppressants. This consensus is supported by published evidence that dupilumab treatment is effective and well tolerated, with rapid onset of action on signs, symptoms and quality of life in patients with moderate to severe AD13. The new treatments for severe AD recently approved in Spain have introduced new mechanisms for action and they must demonstrate their effectiveness and safety in real life.
This study also achieved a broad consensus in recommending the use of patients' experience indicators in managing severe AD. Evaluation instruments such as PREMs and PROMs (patient-reported outcome measures and patient-reported care experience measures, respectively) are increasingly being promoted as a way of enabling clinicians to efficiently evaluate and respond to aspects of health that are relevant to patients and their caregivers13–15. Major studies suggest that the information provided by PREMs and PROMs can improve communication with the patient, raise awareness of issues that might otherwise go unidentified, and improve care plans and multidisciplinary collaboration14. In fact, successful cases regarding AD have been described in other countries16.
Nonetheless, barriers have been identified to full integration of these techniques in practice, such as a lack of training in applying them or difficulty in interpreting the data17. In order to boost their use, it is essential for these instruments to be seamlessly integrated into clinical practice and for the information to be summarised in easily understandable reports on priority areas for patients and their environment14,18,19.
The HP must be given greater relevance and contribute to the management of patients with severe AD, from participating in defining treatment protocols and the patient's training so as to achieve maximum adherence, to optimise medication therapy management and evaluating the health outcomes. In recent years, the culture of measuring health outcomes has been growing in Spain. For example, the Valtermed registry gets information from different innovative pharmaceutical products recently on the market20.
This study has shown that the commonly used scales do not take a holistic view of the impact of AD on patients' lives. The SCORAD scale (Scoring Atopic Dermatitis) measures the extent of the affected areas, the severity of the lesions and the subjective symptoms, whereas the EASI (Eczema Area and Severity Index) does not include subjective symptoms3,21. The participants in this study unanimously stated that scales and measurements of the impact of AD on patients that take into account symptoms such as itching, sleep and quality of life should be agreed upon in order to globally assess the response to treatment. For example, nearly 93% of the participants recommended using Quality of Life scales such as the DLQI and the POEM scale.
Collaboration between HPs and other specialists remains essential. It is crucial to create multidisciplinary teams that include the hospital pharmacist in order to achieve comprehensive, coordinated and sustainable care from all health professionals. The HPs not only take part in dispensing medication; they also have a relevant role in setting out treatment protocols, educating the patient and monitoring the adherence, compliance, effectiveness and safety of the prescribed treatment22, helping to improve patients' clinical response22.
Additionally, it should be noted that this consensus has several strong points, such as the use of the Delphi method and participation from a panel of AD experts. Since it involves a consensus, it has been possible to include more topics than would normally be dealt with in a systematic review or guidelines, which are generally based on a strict method that restricts the scope of the research. Nevertheless, consensus also has its limitations. Not all statements reached 100% agreement. Furthermore, although these recommendations show experts' points of view, they are not universal; the patient's individual characteristics and convenience should always be taken into account before choosing the type of treatment.
Finally, the creation of a clinical practice guideline for the pharmaceutical care could help to optimise therapeutic medication management and health outcomes in severe AD, as well as the development of new observational studies to determine its impact on patients' clinical outcomes and quality of life.
We are confident that this analysis helps to improve the care and quality of life for patients with severe AD from the perspective of the HPs who care for them in the multidisciplinary team.
A consensus has been reached in recognising the impact of severe AD on the patients suffering from it, as well as on the need to use scales that take into account the patient's quality of life (as well as relevant symptoms), and indicators of the patient's experience. It has also been demonstrated that it is worthwhile evaluating the results in real clinical practice in consensus with other specialists from the multidisciplinary team. Furthermore, access to new drugs must be improved so that action may be taken as soon as possible in the patients' therapeutic programme. It is acknowledged that dupilumab has meant a breakthrough for patients with severe AD. The creation of a practical guideline is recommended for pharmaceutical care to manage AD. Finally, this Delphi consensus has highlighted the key role the HP must play within the multidisciplinary team caring for patients with severe AD.
Impact statements- •
This Delphi consensus has highlighted the key role that hospital pharmacists must play within the multidisciplinary team caring for patients with severe atopic dermatitis.
- •
Scales should be used that consider the patient's quality of life (as well as relevant symptoms like itchiness), in addition to indicators of their experience (like sleep, or anxiety/depression).
- •
It is worthwhile evaluating the results in real clinical practice in consensus with other specialists from the multidisciplinary team.
- •
It is advisable to use drugs that have demonstrated long-term effectiveness and safety for patients with severe AD, given the disease's chronic nature.
- •
Finally, access to new drugs must be improved so that action may be taken as soon as possible in the course of the patient's therapy.
This project has been funded by Sanofi. The sponsor took no part in designing, implementing, interpreting or writing the document.
The authors would like to thank the experts who have participated as panellists: Emilio Molina Cuadrado, Torrecárdenas University Hospital, Almería. Ramón Morillo Verdugo, Virgen de Valme University Hospital, Cádiz. Mercedes Romero Alonso, Infanta Elena Local Hospital, Huelva. Juan Jerez, H. Jaén University Hospital, Jaén. Andrés Sánchez Ruiz, H. Alto Guadalquivir, Jaen. María Espinosa Bosch, Regional University Hospital of Málaga, Málaga. Pablo Quintero García, Virgen del Rocío University Hospital, Seville. María Sáez-Torres De Vicente, Hospital of Osuna, Seville. Vicente Merino Bohórquez, Virgen Macarena University Hospital, Seville. Ángel Antonio Castelo Luque, Llerena Zafra Public Hospital Complex, Badajoz. Ana Gómez Lobón, Son Espases University Hospital, Palma, Majorca. Inmaculada Plasencia García, Nuestra Señora de Candelaria University Hospital, Santa Cruz de Tenerife. Elisenda Dolz Bubi, H. Universitario Insular de Gran Canaria, Las Palmas de Gran Canaria. Julia Sánchez Gundin, H. Universitario Marqués de Valdecilla, Santander. Aitziber Illaro Uranga, H. Universitario Marqués de Valdecilla, Santander. Elena González Colominas, H. del Mar, Barcelona. Carlos Seguí Solanes, H. de Granollers, Barcelona. Glòria Cardona Peitx, H. Universitario Germans Trias i Pujol, Barcelona. Azhara Sánchez Ulayar, H. Mataró, Barcelona. Neus Pagès Puigdemont, H. de la Santa Creu i Sant Pau, Barcelona. Pau Riera Armengol, H. de la Santa Creu i Sant Pau, Barcelona. Amparo Flor García, H. Virgen de la Luz, Cuenca. María Blanco Crespo, H. Universitario Guadalajara, Guadalajara. Ana Domínguez Barahona, Complejo Hospitalario de Toledo, Toledo. Magdalena Güemes García, H. Universitario de Burgos, Burgos. Roberto Santos Del Prado, H. General Río Carrión, Palencia. Jesús María Prada Lobato, H. Universitario Río Hortega, Valladolid. Nieves Valcarce Pardeiro, H. Arquitecto Marcide, A Coruña. Ana Belén Veiga Villaverde, H. Provincial de Pontevedra, Pontevedra. Cristina García Yubero, H. Universitario Infanta Sofía, Madrid. María Fernández-Pacheco, H. Universitario Príncipe de Asturias, Madrid. Ángel Luis Salcedo Mingorranz, H. Universitario Severo Ochoa, Madrid. Mª Ángeles Campos, H. Universitario del Henares, Madrid. Miguel Ángel Rodríguez Cabezas, H. Clínico San Carlos, Madrid. Blanca Rodríguez Vargas, H. Universitario Infanta Cristina, Madrid. Ana Acuña Vega, H. General de la Defensa Gómez Ulla, Madrid. Irene Cañamares Orbis, H. Universitario Infanta Leonor, Madrid. Belén Menchen Viso, H. Universitario Puerta de Hierro, Madrid. Amaya Arrondo Velasco, Complejo Hospitalario de Navarra, Pamplona. Mila Álvarez Lavin, H. de Basurto, Bilbao. Francisco José Rodríguez Lucena, H. Vega Baja, Alicante. Emilio Monte Boquet, H. Universitario y Politécnico la Fe, Valencia. The authors also thank CLOVER SGM for providing methodology advice during the study and for the help in writing the manuscript and editorial assistance.