The objectives are to know the opinion of neurologists and hospital pharmacists on those aspects still under debate regarding the role of anti-Calcitonin Gene-related Peptide monoclonal antibodies in the preventive treatment of migraine. To identify those controversies that still exist. To propose agreed recommendations for improvement of care. And to promote access of clinicians and patients to these new treatments in the prevention of migraine with biological drugs, in order to improve patient care and follow-up.
MethodologyRecommendations for the use of biological drugs in the prevention of migraine were identified and evaluated through the Delphi consensus methodology, proposing 88 statements grouped into 3 themes: a clinical module that deals with the management of biological treatments in migraine; a patient module that discusses patient education and adherence improvement strategies; and a coordination module that includes statements related to strategies to improve joint work between the two groups. The 9-point Likert ordinal scale was used to score these recommendations and, subsequently, the data was statistically analysed through different metrics.
ResultsAfter both rounds of voting, consensus was reached in agreement on 71 of the 88 statements (80.7%), leaving 1 statement (1.1%) with consensus in disagreement and 16 remaining as indeterminate (18.2%).
ConclusionsThe high degree of consensus indicates that the opinion of neurologists and hospital pharmacists on the role of anti-Calcitonin Gene-related Peptide monoclonal antibodies in the preventive treatment of migraine is very similar and allows identifying those controversies that still exist, to improve the care and follow-up of patients with migraine.
Los objetivos son conocer la opinión de neurólogos y farmacéuticos hospitalarios sobre aquellos aspectos aún en debate respecto al papel de los anticuerpos monoclonales anti-péptido relacionado con el gel de la calcitonina en el tratamiento preventivo de la migraña. Identificar aquellas controversias aún existentes. Proponer recomendaciones consensuadas de mejora asistencial. Y promover el acceso de los clínicos y los pacientes a estos nuevos tratamientos en la prevención de la migraña con fármacos biológicos, a fin de mejorar la atención y seguimiento del paciente.
MétodosSe identificaron y valoraron recomendaciones para la utilización de fármacos biológicos en la prevención de la migraña a través de la metodología de consenso Delphi proponiendo 88 aseveraciones agrupadas en tres temas: un módulo de clínica que trata sobre el manejo de los tratamientos biológicos en la migraña; un módulo de pacientes que trata sobre las estrategias de educación al paciente y mejora de la adhesión; y un módulo de coordinación que incluye las aseveraciones relacionadas con las estrategias para mejorar el trabajo conjunto entre los dos colectivos. Se empleó la escala ordinal de Likert de 9 puntos para puntuar dichas recomendaciones y, posteriormente, los datos se analizaron estadísticamente a través de diferentes métricas.
ResultadosTras las dos rondas de consulta, se alcanzó consenso en el acuerdo en 71 aseveraciones (80,7%) y consenso en el desacuerdo en una de ellas (1,1%), quedando como indeterminadas 16 aseveraciones (18,2%) de las 88 debatidas.
ConclusionesEl alto grado de consenso indica que la opinión de neurólogos y farmacéuticos hospitalarios sobre el papel de los anticuerpos monoclonales anti-péptido relacionado con el gen de la calcitonina en el tratamiento preventivo de la migraña es muy similar y permite identificar aquellas controversias aún existentes, para mejorar la atención y seguimiento del paciente con migraña.
Los resultados del consenso para el tratamiento de la prevención de la migraña entre las especialidades de neurología y farmacia hospitalaria, indican un entorno general de sinergia entre neurólogos y farmacéuticos hospitalarios.
A nivel de práctica clínica, los resultados confirman como opción terapéutica para pacientes con migraña refractaria, el uso de fármacos con diferentes mecanismos de acción de forma simultánea, como ocurre en el uso de AMC con oros fármacos biológicos. Además, los resultados del consenso indican que se debe valorar la retirada temporal tras un año de tratamiento cuando el paciente alcanza una buena respuesta, haciendo seguimiento por si fuera necesario reinstaurarlo.
Referente a la relación médico-paciente, los resultados del consenso indican que una correcta educación sanitaria sobre el manejo y conservación del AMC, así como informar al paciente de los posibles efectos adversos y/o complicaciones, y estableciendo las visitas de seguimiento, mejoran la calidad de vida de los pacientes y la adherencia al tratamiento.
IntroductionThe low effectiveness and tolerability of current oral preventive treatments for migraine has prompted the search for new therapeutic strategies, leading to the development of monoclonal antibodies (MCAs) that specifically target the calcitonin gene-related peptide (CGRP)—which is of relevance in the pathophysiology of migraine—or its receptor (anti-CGRP MCAs)1.
Clinical trials and real-world studies have demonstrated the safety and effectiveness of anti-CGRP MCAs. They do not cross the blood–brain barrier, thus avoiding adverse events in the central nervous system, there is no risk of drug interactions compared to traditional preventive treatments as they are not metabolised in the liver, they initiate their response within a few weeks of starting treatment, and they have excellent tolerability. Their effectiveness has also been demonstrated in episodic and chronic migraine as measured using objective and subjective parameters2.
Although there are few reports on anaphylactic reactions, anti-CGRP MCAs are contraindicated in patients with hypersensitivity to the active ingredient; furthermore, their long-term safety remains unknown in patients with a history of cardiovascular disease or at cardiovascular risk, and in patients more than 65 years. As a precautionary measure, they should not be used in pregnant women3,4.
The following anti-CGRP MCAs have demonstrated effectiveness in chronic and episodic migraine: eptinezumab (not yet marketed in Spain), galcanezumab, fremanezumab, and erenumab5–8. These drugs are reimbursed by the Spanish public healthcare service for the preventive treatment of patients with high-frequency episodic migraine (8–14 migraine days/month) and in patients with chronic migraine after the failure of 3 preventive treatments (including botulinum toxin in patients with chronic migraine) used at the appropriate doses and for a minimum of 3 months.
Consensus among multidisciplinary teams on the treatment of migraine patients could facilitate progress in the management of these drugs and achieve the best health outcomes.
Thus, this consensus document had the following objectives: to determine the opinion of neurologists and hospital pharmacists on those aspects still under debate regarding the role of anti-CGRP MCAs in the treatment of migraine; to identify remaining controversies; to propose consensus recommendations for the improvement of care; and to promote access by clinicians and patients to these new treatments for the prevention of migraine with biological drugs in order to improve patient care and follow-up.
MethodsFirstly, a scientific committee (SC) was set up comprising 4 hospital pharmacists and 4 neurologists with expertise in migraine. The SC reviewed the most recent literature on migraine treatment and proposed 88 statements grouped into 3 modules: clinical aspects in the management of biological treatments; strategies for patient education and improved adherence; and coordination to improve workflow.
The SC then selected an initial panel of 62 neurologists and hospital pharmacists with recognised experience in the management and follow-up of migraine patients; however, due to professional commitments on the part of 3 of the panellists, the second round included 59 participants.
Technical and methodological support was provided by the technical-scientific team of the Luzán 5 Health Consulting Research Unit.
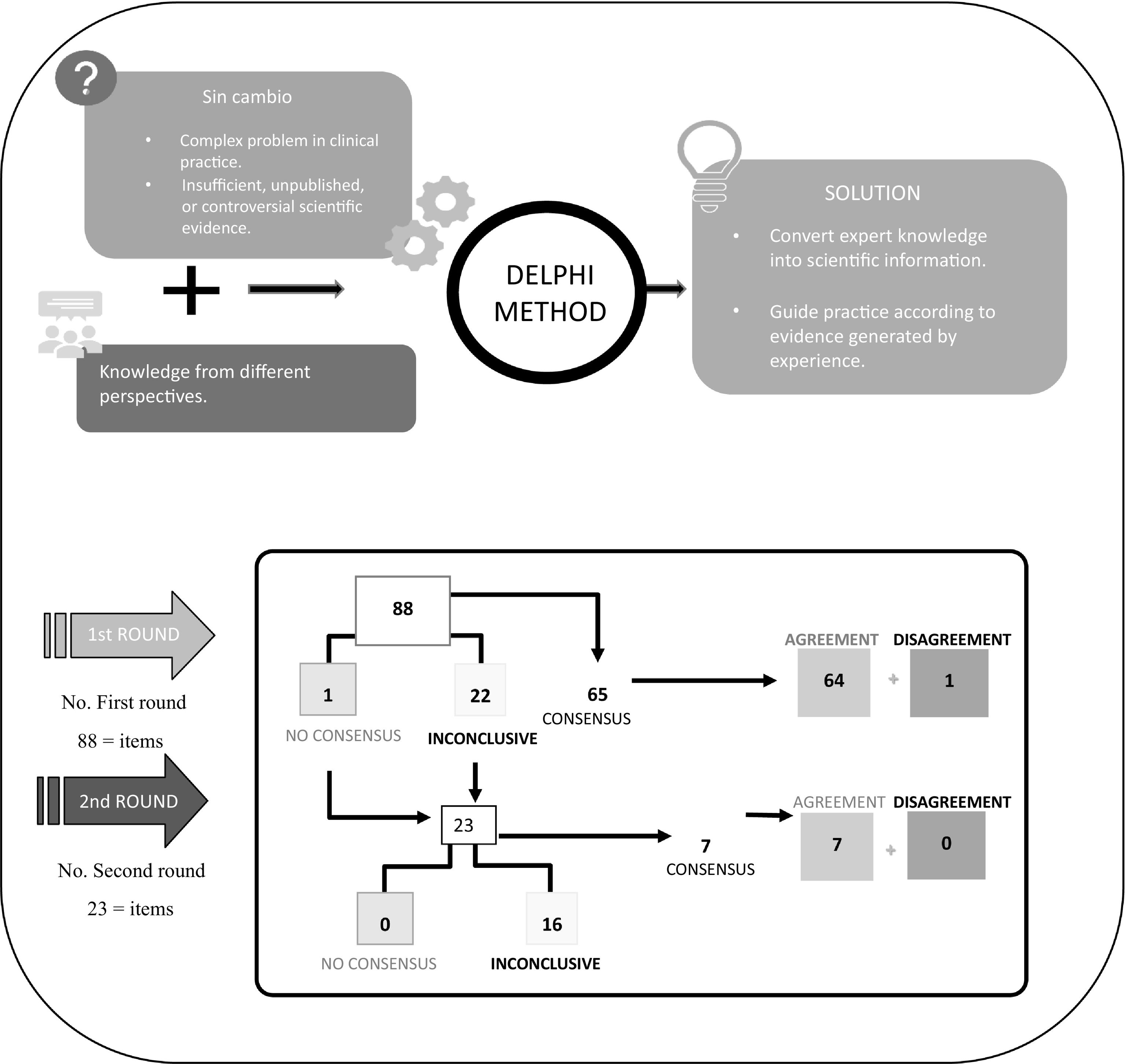
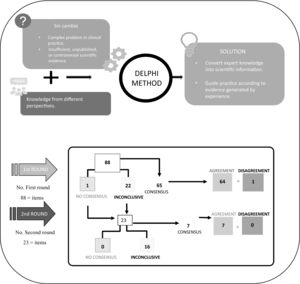
The Delphi method was used to identify consensus or otherwise on the proposed statements. This method is a structured communication technique which, through the guidance and supervision of the coordinators and without the need for physical presence, allows a large group of experts to obtain a solution to a complex problem in clinical practice or one with little evidence9–11.
Based on this method, the panellists were sent a questionnaire on which they could show their degree of agreement with the statements on a single 9-point Likert-type ordinal scale structured into 3 groups according to the level of agreement-disagreement. A score of 1–3 represented no consensus, 4–6 represented inconclusive, and 7–9 represented consensus. Statements without sufficient consensus were subjected to a second round. The results were obtained over a period of 4 months and all communication was conducted via email and the Delphi platform.
Finally, the SC met to discuss and analyse the results. They defined each statement as follows: there was consensus when two-thirds or more (≥66.66%) scored within the ranges 1–3 or 7–9; no consensus when one-third or more (≥33.33%) scored within the 1–3 range and another third in the 7–9 range; and inconclusive when the median score was within the 4–6 range12.
ResultsAs shown in Fig. 1, after two rounds of consultation, consensus of agreement was reached on 71 out of 88 statements (80.7%) and consensus of disagreement on one statement (1.1%); 16 statements (18.2%) remained inconclusive. Tables 1, 2, and 3 show the scores and degree of agreement by module.
Clinical module.
| Median (IQR) | % agreement | % disagreement | |
|---|---|---|---|
| MCAs should be considered eligible for reimbursement in patients who experience episodic or chronic migraine who have failed at least 3 previous preventive treatments (one of which being botulinum toxin in the case of chronic migraine)a with levels of evidence from 1 to 2a | 9 (1) | 95.2 | 1.6 |
| Lack of tolerance to one of the oral preventive drugs with levels of evidence of 1–2 can be considered as treatment failurea | 8 (2) | 79.0 | 8.1 |
| Contraindication to one of the oral preventive drugs with levels of evidence 1–2 can be considered as treatment failureb | 7 (5) | 51.7 | 31.7 |
| Two cycles of botulinum toxin, at least one of which must include doses of 195 U, may be sufficient criteria for non-responsea | 8 (3) | 67.8 | 9.7 |
| In episodic migraine, pre-assessment should include an impact or disability scale (HIT-6 or MIDAS) demonstrating moderate–severe disabilitya | 8 (2) | 85.5 | 4.8 |
| Prior to initiation of treatment, patients should provide a diary showing the number of days of headache and migraine, the use of symptomatic medication, or the need to visit the emergency rooma | 8 (1) | 91.9 | 1.6 |
| Despite the similarities and differences between the currently available MCAs, all of them should be available in each centre in order for experience to be gained in the use of each MCA in clinical practicea | 8 (5) | 67.7 | 24.2 |
| In the absence of data, no MCAs should be used in pregnant womena | 9 (2) | 80.6 | 6.5 |
| There is no contraindication to the use of MCAs in immunocompromised patientsa | 8 (2) | 83.9 | 1.6 |
| There are no contraindications to the use of MCAs in patients with other chronic diseasesa | 7 (2) | 67.7 | 11.3 |
| Concomitant use of MCAs with other biologics should be considered on a case-by-case basisa | 8 (2) | 91.9 | 1.6 |
| MCAs are not indicated in patients with a history of stroke or ischaemic heart disease, especially if this is recenta | 8 (4) | 72.6 | 12.9 |
| MCAs should be used with caution in patients with vascular risk factors or Raynaud syndromea | 8 (2) | 79.0 | 0.0 |
| MCAs should be used on a case-by-case basis in patients of more 65 years because of age-related increased cardiovascular riskb | 7 (2) | 58.3 | 13.3 |
| MCAs should be used on a case-by-case basis in patients with active oncohematological disease or severe systemic diseasea | 7 (2) | 73.3 | 1.7 |
| MCAs should be used with caution in patients with a confirmed diagnosis of migraine with onset after the age of 55 yearsb | 5 (2) | 20.0 | 26.7 |
| In the event of treatment failure, wait for 5 half-lives as a washout period in patients who had been receiving an MCA before initiating treatment with another MCAb | 3 (3) | 11.7 | 61.7 |
| In the event of treatment failure, it is safe in clinical practice to immediately switch to an MCA without a washout periodb | 7 (2) | 60.0 | 18.3 |
| In the event of a serious adverse event, it is advisable, based on pharmacokinetics, to wait for 5 half-lives as a washout period in patients who had been receiving a MCA before initiating treatment with another MCAb | 7 (2) | 60.0 | 8.3 |
| In the event of a serious adverse event, in clinical practice, wait for at least 3 half-lives as a washout period before switching to another MCAb | 6 (3) | 38.3 | 16.7 |
| Following treatment with MCAs, a waiting period of least 3 months should elapse before effectiveness is assesseda | 9 (1) | 88.7 | 3.2 |
| The most relevant parameter in assessing effectiveness is the decrease in the number of migraine days per montha | 7 (2) | 72.6 | 9.7 |
| A patient should be considered to be responsive if the decrease in the number of migraine days per month is more than 50% in episodic migraine and 30% in chronic migrainea | 7 (2) | 74.2 | 8.1 |
| In the absence of a 50% decrease, continuation of treatment should be considered if there is a significant decrease in pain intensitya | 8 (1) | 87.1 | 1.6 |
| In the absence of a 50% decrease, continuation of treatment should be considered if there is a significant decrease in the need for symptomatic treatmenta | 8 (1) | 75.8 | 4.8 |
| In the absence of a 50% decrease, continuation of treatment should be considered if there are objective criteria of improvementa | 7 (0) | 81.7 | 6.7 |
| In the absence of a 50% decrease, continuation of treatment should be considered if there is a significant increase in response to symptomatic treatmenta | 7 (0) | 80.0 | 3.3 |
| In the absence of a 50% decrease, continuation of treatment should be considered if there is significant improvement on impact or disability scalesa | 7 (1) | 77.4 | 3.2 |
| Prior to the initiation of MCA therapy, oral preventive drugs or botulinum toxin do not need to be discontinued in patients with an initial partial response to thema | 7 (2) | 74.2 | 12.9 |
| The fact that patients do not respond to a specific MCA does not mean that they will not respond to another, regardless of whether they target receptors or ligandsa | 8 (3) | 72.6 | 9.7 |
| It is advisable to try a different MCA if there is a lack of response to the first onea | 8 (3) | 74.2 | 8.1 |
| In patients with a good response to an MCA, temporary discontinuation of the drug after 1 year should be considereda | 8 (3) | 69.4 | 12.9 |
| In patients with loss of response to MCA, drug discontinuation after 3 months will be consideredb | 6 (3) | 36.7 | 20.0 |
| Patients in whom MCA has been discontinued due to good response should be followed up in case another period of treatment is neededa | 9 (1) | 93.6 | 3.2 |
| Women should wait for 5 half-lives without receiving any MCA before trying to become pregnanta | 7 (2) | 71.7 | 3.3 |
| Serum MCA levels should be monitored and matched to the inter-individual characteristics of patients in order to individualise future dosesb | 5 (4) | 30.0 | 26.7 |
| It is advisable to monitor antidrug antibody levels if there is a lack of drug effectivenessb | 5 (2) | 15.0 | 28.3 |
| In the absence of studies, MCA should be temporarily discontinued in patients with COVID-19b | 2 (3) | 11.7 | 65.0 |
| It is advisable that a period of 5–7 days should elapse between a dose of MCA and a dose of any COVID-19b vaccineb | 7 (3) | 58.3 | 10.0 |
MCA, monoclonal antibodies; HIT-6, Headache Impact Test-6; MIDAS, Migraine Disability Assessment Scale; IQR, interquartile range.
Patient module.
| Median (IQR) | % agreement | % disagreement | |
|---|---|---|---|
| Proper patient education and involvement facilitates better management of their diseasea | 9 (0) | 100.0 | 0.0 |
| Patient education starts with raising their awareness of the relevance of lifestyle and hygiene measuresa | 9 (0) | 98.4 | 0.0 |
| Measures should be established prior to the introduction of an MCAa | 9 (1) | 85.5 | 4.8 |
| As a measure prior to the administration of an MCA, patients should be educated on appropriate lifestyles, such as physical exercise, quality of sleep, and dieta | 9 (1) | 85.5 | 4.8 |
| Prior to the administration of an MCA, appropriate and individualised symptomatic treatment should be implementeda | 9 (1) | 90.3 | 6.5 |
| Appropriate oral prophylactic drugs should be used before the administration of an MCAa | 9 (1) | 98.4 | 1.6 |
| Patient education and training programmes improve treatment successa | 9 (1) | 95.2 | 0.0 |
| Patient education should be offered by multidisciplinary teams from neurology, nursing, and hospital pharmacy servicesa | 9 (0) | 95.2 | 0.0 |
| Treatment success can be improved if patients are made aware of the expected results, such that they can identify a possible lack of effectivenessa | 9 (1) | 98.4 | 0.0 |
| Treatment success can be improved by educating patients on how to follow instructions for administrationa | 9 (0) | 98.4 | 0.0 |
| Treatment success can be improved by training in MCA storagea | 9 (0) | 96.8 | 0.0 |
| Treatment success can be improved by providing patients with information on possible adverse effects and/or complications in order to avoid non-adherence to treatmenta | 9 (0) | 98.4 | 0.0 |
| Treatment success can be improved by providing patients with tools for identifying non-adherencea | 9 (2) | 95.2 | 1.6 |
| Treatment success can be improved by providing timely and specialised professional contact in the case of any questions, problems, or unforeseen eventsa | 9 (0) | 100.0 | 0.0 |
| Treatment success can be improved by patients keeping a headache diary in which they can record medicines taken and the appearance of symptomsa | 9 (0) | 100.0 | 0.0 |
| Follow-up visits should be conducted 3 months after starting treatmenta | 9 (0) | 98.4 | 1.6 |
| At the first follow-up visit, the effectiveness and safety of treatment should be assesseda | 9 (0) | 98.4 | 0.0 |
| At the first follow-up visit, the correct mode of treatment administration should be ensureda | 9 (1) | 98.4 | 0.0 |
| At all follow-up visits, the occurrence of adverse effects and their correct management should be revieweda | 9 (0) | 98.4 | 0.0 |
| It is advisable, for treatment success, to hold group sessions for training and the exchange of experiences, problems, or concernsa | 7 (2) | 71.7 | 1.7 |
| The inclusion of the patients' opinions on disease management strengthens the doctor–patient relationshipa | 9 (1) | 95.2 | 0.0 |
| The inclusion of the patients' opinions on disease management facilitates adherence to treatment and, therefore, therapeutic successa | 9 (1) | 98.4 | 0.0 |
| Inclusion of patient feedback on disease management is useful for assessing treatment effectivenessa | 9 (1) | 95.2 | 0.0 |
| Patient feedback is key to deciding whether preventive treatment should be continued throughout follow-upa | 8 (2) | 88.7 | 1.6 |
| Drug effectiveness during follow-up should be assessed using objective parameters: diary, scales (HIT-6 and MIDAS), quality of life, and the patients' opinionsa | 9 (1) | 98.4 | 0.0 |
MCA, monoclonal antibodies; HIT-6, Headache Impact Test-6; MIDAS, Migraine Disability Assessment Scale; IQR, interquartile range.
Coordination module.
| Median (IQR) | % agreement | % disagreement | |
|---|---|---|---|
| There is a need to improve coordination between hospital pharmacy and neurology services from a clinical point of viewa | 9 (1) | 93.5 | 0.0 |
| Collaboration between hospital pharmacy and neurology services has a positive impact on the care of migraine patientsa | 9 (0) | 95.2 | 0.0 |
| The appointment of interlocutors with specific training in migraine and the rational use of medication would improve collaboration between hospital pharmacy and neurology servicesa | 9 (2) | 80.6 | 3.2 |
| It is essential for both groups to agree with patients on the definition of an action procedurea | 9 (1) | 93.6 | 0.0 |
| The development of joint training activities is essential to improve coordination between the 2 groupsa | 9 (1) | 91.9 | 1.6 |
| The pharmacy training programme should include specific rotations in neurology servicesa | 8 (3) | 70.0 | 8.3 |
| The neurology training programme should include specific rotations in pharmacy servicesb | 5 (2) | 21.7 | 23.3 |
| Migraine patients should be provided with health education by neurology servicesb | 5 (3) | 41.7 | 23.3 |
| Migraine patients should be provided with health education by hospital pharmacy servicesb | 4 (3) | 5.0 | 46.7 |
| Hospital pharmacy and neurology services in cooperation should design and create information material related to migraine and treatment with MCAsa | 8 (2) | 90.3 | 1.6 |
| Coordination between the 2 disciplines should involve all healthcare professionals involved in the care of migraine patientsa | 9 (1) | 91.9 | 0.0 |
| The creation of a combined neurology–hospital pharmacy consultation would facilitate coordination between the 2 groupsa | 8 (2) | 75.8 | 3.2 |
| Migraine patients should be made aware of the existence of a multidisciplinary team involved in their carea | 9 (1) | 93.5 | 0.0 |
| The neurologist requesting treatment must be the neurologist responsible for the patienta | 9 (1) | 91.9 | 1.6 |
| MCAs for the treatment of migraine should be prescribed by neurologists with expertise in headachesa | 9 (1) | 87.1 | 3.2 |
| The validation and dispensing of MCA treatment for migraine should be the responsibility of pharmacists with specific training in migrainea | 8 (3) | 72.6 | 1.6 |
| In complex patients, any aspect related to the indication, validation, dispensing, and administration of treatment should be jointly agreed by the neurology and hospital pharmacy servicesa | 8 (2) | 87.1 | 1.6 |
| Follow-up of patients receiving MCA treatment is the responsibility of the neurology servicea | 9 (4) | 71.7 | 16.7 |
| Follow-up of patients receiving MCA treatment is the responsibility of the hospital pharmacy serviceb | 4 (5) | 26.7 | 48.3 |
| Consensus on response criteria must be reached to avoid hindering the continuation of treatmenta | 9 (1) | 95.2 | 0.0 |
| The decision on whether to continue treatment beyond 1 year should be shared by the neurology and hospital pharmacy servicesa | 7 (3) | 67.7 | 14.5 |
| It is relevant to include clinical and pharmacological variables in the real-world records of patients receiving MCA treatmenta | 9 (1) | 93.5 | 0.0 |
| The final choice of treatment is the responsibility of neurologistsa | 8 (2) | 75.8 | 9.7 |
| The final choice of treatment is the responsibility of hospital pharmacistsc | 2 (3) | 9.7 | 72.6 |
MCA, monoclonal antibodies; IQR, interquartile range.
This module comprised 39 statements of which 27 were agreed by consensus and 12 were left inconclusive (Table 1).
Regarding treatment initiation and patient assessment, a consensus of over 85% was reached that the criteria for selecting the patients eligible for funding for MCA treatment were correct, that prior assessment should include an impact or disability scale, and that prior to treatment initiation, patients should provide a diary showing the number of days of headache and migraine, the use of symptomatic medication, or the need to visit the emergency department.
A consensus of between 66% and 85% was reached on assessing the lack of tolerability to oral preventive treatment as a criterion for treatment failure, and that 2 cycles of botulinum toxin, at least one of which must include doses of 195 U, could be criteria for non-response. A consensus was also reached that prior to the initiation of MCA treatment, oral preventive drugs or botulinum toxin do not need to be initially discontinued in patients with a partial response to them, and that it was relevant that each centre should have all MCAs available in order to gain experience in the use all of them.
However, the following issues were left inconclusive: contraindications to one of the oral preventive drugs could be considered as treatment failure, the need to monitoring MCA levels and antidrug antibody levels in the absence of effectiveness, and the use of MCAs with caution in patients with a confirmed diagnosis of migraine onset after the age of 55 years.
Regarding treatment duration and response, there was a consensus of more than 85% that the concomitant use of MCAs with other biologics should be considered on a case-by-case basis; there was also a consensus of between 66% and 85% that the most relevant parameter in assessing effectiveness was the reduction in the number of days with migraine, and that a patient should be considered to be responsive if the decrease in the number of migraine days per month is more than 50% in episodic migraine and 30% in chronic migraine. Consensus was also reached on following: patients may respond to an MCA regardless of not having responded to a previous one, and a different MCA may be used when there has been no response to a previous one. Regarding safety, the following statements were assessed as inconclusive: in the event of treatment failure, immediately switch to another MCA without a washout period; in the event of treatment failure, wait for 5 half-lives as a washout period in patients who had been receiving an MCA before switching another MCA; in the case of a serious adverse event, wait for 5 half-lives as a washout period in patients who had been receiving a MCA before switching to another MCA; and in the case of a serious adverse event, wait for at least 3 half-lives as a washout period before switching to another MCA.
Regarding continuation of treatment, there was a consensus of more than 85% on the need to wait for at least 3 months before assessing effectiveness of treatment, to implement follow-up after discontinuation in case treatment needs to be restarted, and, in the absence of a 50% response rate, to continue treatment if there is a significant decrease in pain intensity.
There was consensus of between 66% and 85% on the need to continue treatment in the absence of a 50% response rate if there is a significant decrease in the need for symptomatic treatment, if there are objective criteria for improvement, if there is a significant increase in response to symptomatic treatment, and if there is a significant improvement on impact or disability scales.
Consensus was also reached that temporary discontinuation of the drug after 1 year should be considered in patients with a good response rate, and that women of childbearing age should wait at least 5 half-lives without receiving any MCA before they try to become pregnant.
However, the following statements were left as inconclusive: in the absence of studies, MCA should be temporarily discontinued in patients with COVID-19, and drug discontinuation after 3 months should be considered in patients with loss of response to MCA.
Regarding treatment in special situations, a consensus of between 66% and 85% was reached on the indication not to use MCAs in pregnant women or in patients with stroke or ischaemic heart disease, to use them with caution in patients with cardiovascular risk factors or Raynaud's syndrome, and to use them on a case-by-case basis in patients with active oncohematological disease, severe systemic disease, or compromised immune function.
Statements remained inconclusive regarding the following issues: the use of MCAs in patients of more than 65 years because of age-related increased cardiovascular risk; patients with a confirmed diagnosis of migraine and onset after the age of 55 years; and the recommendation to allow at least 5–7 days to elapse between a dose of MCA and a dose of COVID-19 vaccine.
Patient moduleTable 2 shows the 25 statements in this module. A consensus of between 66% and 85% was reached on recommendations to hold group sessions, training sessions, and sessions for the exchange of experiences, problems, or concerns.
Regarding patient education strategies, a consensus of more than 98% was reached on the following statements: proper patient education and involvement facilitates a better approach to the disease; patient education should begin with their being made aware of the relevance of lifestyle and hygiene measures; appropriate oral prophylactic drugs should be used before starting on an MCA; and it is useful for patients to keep headache diaries to record medications taken and the appearance of symptoms. Regarding patient adherence, consensus was also reached on the usefulness of making patients aware of the expected results, educating them on how to follow instructions for administration, providing them with information on possible adverse effects and/or complications, and providing timely and specialised professional contact.
A high degree of consensus was also reached on follow-up visits 3 months after starting treatment involving the initial assessment of effectiveness and safety at the first visit using objective parameters (diaries, scales, and quality of life), ensuring the correct mode of treatment administration, and reviewing the occurrence of adverse effects and their correct management at all follow-up visits.
Finally, consensus was reached on the need to include the patients' opinions on the management of the disease in order to facilitate adherence and, therefore, therapeutic success.
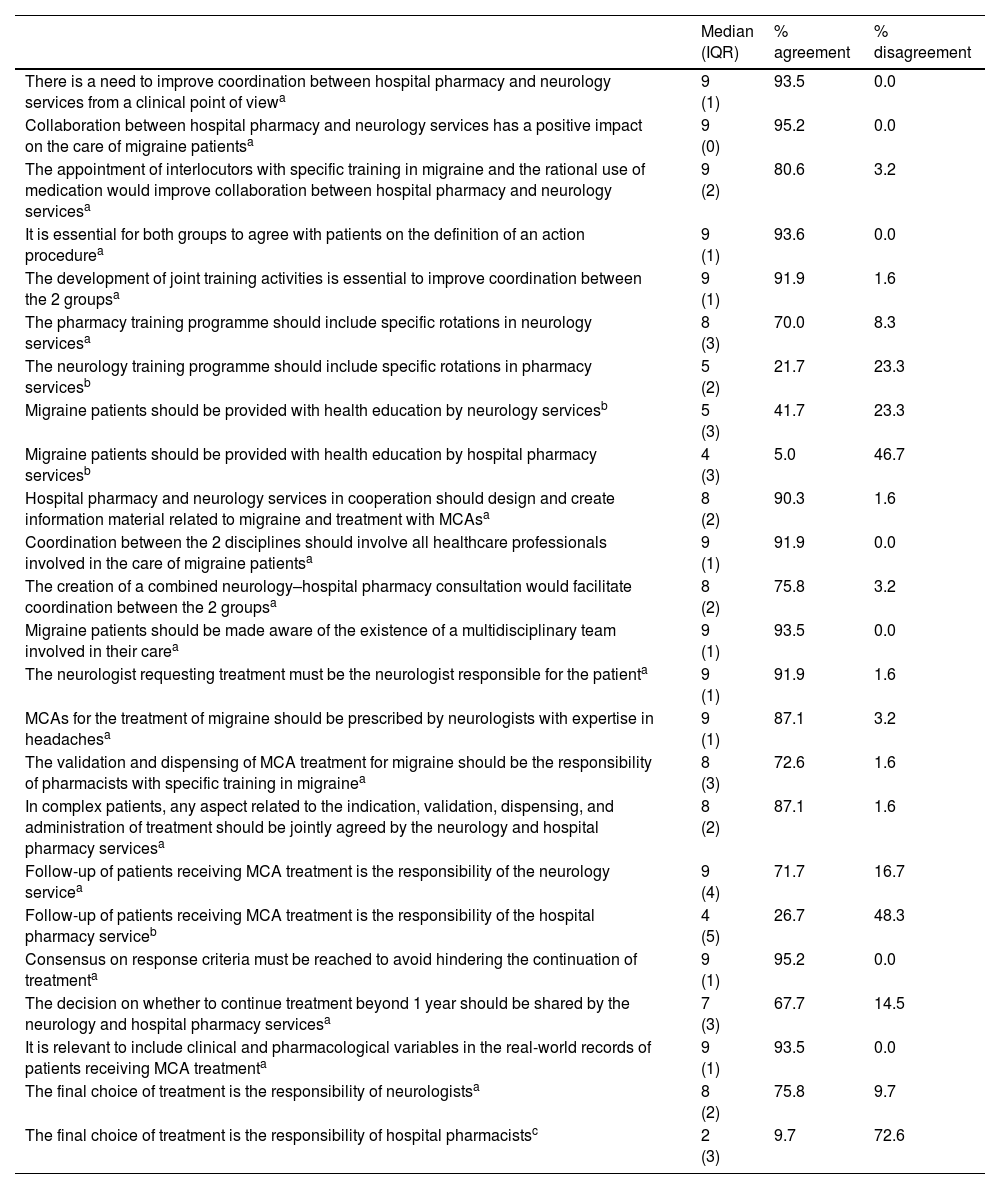
Coordination moduleThis module comprised 24 statements related to strategies for improving joint work: 19 reached consensus in agreement, one in disagreement, and four remained inconclusive.
A consensus in agreement was reached of more than 85% on the need to improve coordination between hospital pharmacy and neurology from a clinical point of view, given its positive impact on patient care, on the need to define an agreed action procedure, and on developing joint training activities. Agreement was also reached that both services in coordination should design and create informational material, that such coordination should involve all healthcare professionals involved in patient care, and that patients should be made aware of the existence of the multidisciplinary team.
It was also agreed that neurologists with expertise in headaches should be responsible for patients and for requesting treatment. A consensus was also reached that the neurology and hospital pharmacy services should in coordination agree on any aspect related to the indication, validation, dispensing, and administration of treatment, and on response criteria to avoid hindering the continuation of treatment and the real-world records of patient follow-up.
There was unanimous consensus that the final choice of treatment is not the responsibility of hospital pharmacists.
The following statements remained inconclusive: patient follow-up is the responsibility of the hospital pharmacy service; patient health education should be undertaken by the hospital pharmacy or neurology service; and the neurology training programme should include specific rotations in pharmacy services.
DiscussionThe Delphi technique is a consensus method enabling a panel of experts to make decisions in situations of uncertainty; their opinions can assist in generating ideas or guidelines, developing recommendations, or defining indicators or clinically significant interactions12–16.
Although expert consensus should be considered at the lower level in the evidence pyramid, its strength lies both in the inputs available to the experts (systematic reviews, experiments, personal experience, and qualitative studies) and in the methods used to reach consensus16. The high degree of consensus reached, which was over 80%, together with initiatives such as the publication of the White Paper on migraine in Spain, or the NEXOS Neurology multidisciplinary conference, show a general environment of synergy between neurologists and hospital pharmacists and represents a starting point for establishing a practical basis for their implementation in real practice, recording the real-world results of MCA treatment, or including pharmacists in headache units17,18.
During the above-mentioned conference, it became clear that the available oral preventive drugs are associated with an insufficient response, whereas anti-CGRP MCAs have high selectivity, that access to the new drugs and education in their administration should be facilitated, and that the creation of a working group between the two services for a better approach to migraine patients should be encouraged.
The Spanish Society of Neurology (SEN) Clinical Practice Manual on Headache recommends the simultaneous use of drugs with different mechanisms of action as a therapeutic option for patients with refractory migraine19; the results of our study are in line with this recommendation. Specifically, the concomitant use of MCAs with other biological drugs should be considered on a case-by-case basis, the discontinuation of preventive treatment before initiating MCA treatment is not needed when there is a partial response, a different MCA should be used when no response has been obtained with the first one given that up to 30% of initially non-responsive patients may benefit from a change in an MCA drug, and at least 3 months should elapse before assessing its effectiveness by evaluating the reduction in the number of days with migraine, but without taking into account its intensity, as some studies have done20.
The study by Briceño-Casado et al.21 compared the effectiveness and safety of three anti-CGRP MCAs. They found that the drugs could be considered equivalent therapeutic alternatives and that their use would facilitate the sustainability of healthcare systems through price competition.
Consensus was reached on temporary discontinuation after 1 year of treatment in patients with a good response, with follow-up in case another period of treatment is needed, and in patients without a reduction in migraine days in the range of 30%–50% after 12 weeks, thus following recommendations for rational drug use22.
Adherence is the basis of treatment success, which can be improved by making patients aware of the expected results, educating them in how to follow instructions for administration and the management of MCA storage, informing them possible adverse effects and/or complications, providing them with tools for identifying non-adherence, providing timely and specialised professional contact, and encouraging the use of a headache diary, since health education improves the quality of life of patients23. A study conducted in Spain found that around 30% of patients with migraine receiving prophylaxis maintained their treatment at 6 months and 12 months24. It is also essential to conduct follow-up visits, which include assessing effectiveness and safety using objective parameters, ensuring the correct mode of administration, and reviewing the occurrence of adverse events and their correct management. These aspects require tools for documenting pharmaceutical care, such as the Pharmacists' Care of Migraineurs Scale (PCMS), which was proposed by Skomo25,26.
The White Paper on Migraine in Spain identifies hospital pharmacy as an involved agent, especially in relation to the validation of the prescription and dispensing of anti-CGRP monoclonal antibodies17.
The role of the Pharmacy and Therapeutics Committee in the selection of MCAs included in the Pharmacotherapeutic Guidelines could affect the availability of all MCAs and the choice of treatment, and could have influenced the apparent disagreement over the responsibility of hospital pharmacists in the final choice of treatment.
Contribution to the scientific literatureThe results of consensus between neurology and hospital pharmacy on the preventative treatment of migraine are suggestive of a general environment of synergy between neurologists and hospital pharmacists.
At the clinical practice level, the results confirm the simultaneous use of drugs with different mechanisms of action as a therapeutic option for patients with refractory migraine, such as the use of monoclonal antibodies (MCAs) along with other biological drugs. The results of the consensus also suggest that temporary discontinuation should be considered after 1 year of treatment in patients with good response, but with follow-up in case another period of treatment is needed.
Regarding the doctor–patient relationship, the results of the consensus also suggest that the patients' quality of life and adherence to treatment are improved by providing correct health education on the management and maintenance of MCAs, making patients aware of possible adverse effects and/or complications, and establishing follow-up visits.
Statement of authorshipThe findings, comments, and conclusions of this work are those of the authors and are therefore the sole responsibility of the authors. This article may mention therapeutic options that refer to indications, dosages, and/or forms of administration of products that are not currently authorised in Spain. We emphasise that any drug mentioned must be used in accordance with the technical data sheet currently in force in Spain.
FundingNone declared.
The authors would like to thank Luzán 5 Health Consulting S.A. for their support during the preparation of this manuscript.