To assess the use of resources and the costs associated with following up patients infected with the human immunodeficiency virus after discontinuation of an antiretroviral treatment and initiation of a new one due to a lack of effectiveness or unacceptable toxicity, as compared to the costs involved in the routine follow-up of patients on antiretroviral treatment, from the Spanish National Health System perspective.
MethodThe use of resources (clinical tests, medical visits, and hospital pharmacy visits) associated with following three profiles of patients infected with the human immunodeficiency virus (stable ones, those discontinuing an existing antiretroviral treatment and being switched to a new one due to a lack of effectiveness, and those discontinuing an existing antiretroviral treatment and being switched to a new one due to unacceptable toxicity) was identified, based on clinical practice guidelines and the findings of a multidisciplinary expert panel (n = 5). The experts agreed on the main adverse events leading to discontinuation, classifying them into gastrointestinal, renal, osseous, musculoskeletal, dermatological, hepatic, lipid profile-related, neuropsychiatric and sexual alterations. Unit costs were identified from official healthcare costs databases. The cost (€, 2020) of following up each patient profile was estimated, excluding the cost of the antiretroviral treatment itself, with a time horizon of two years.
ResultsThe per-patient cost of following up stable patients over two years was estimated at €4,148 (tests: €2,293; visits: €1,855). Patient follow-up after discontinuation of an existing antiretroviral treatment and initiation of a different one due to a lack of effectiveness was estimated at €5,434 (tests: €2,777; visits: €2,657). The cost of follow-up after discontinuation of an existing regimen and initiation of a new one due to unacceptable toxicity varied according to the adverse event prompting the switch, ranging from €4,690 for lipid profile dysregulation, to €5,304, for musculoskeletal alterations. In this patient profile, the cost of tests ranged from €2,403 to €3,017, and that of visits from €2,287 to €2,842.
ConclusionsThe cost associated with following up of patients infected with the human immunodeficiency virus after discontinuation of an existing antiretroviral regimen and initiation of a new one is higher than that of routine follow-up, without taking the cost of drugs into account. The treatment discontinuation rate is a relevant factor when selecting the most appropriate therapy for each patient.
Estimar el uso de recursos y costes asociados al seguimiento de pacientes con infección por el virus de la inmunodeficiencia humana tras discontinuación del tratamiento antirretroviral actual debido a falta de efectividad o toxicidad inaceptable y cambio a un nuevo tratamiento antirretroviral, comparado con el seguimiento habitual de los pacientes con tratamiento antirretroviral, desde la perspectiva del Sistema Nacional de Salud español.
MétodoSe identificó el uso de recursos (pruebas clínicas, visitas médicas, visitas a la farmacia hospitalaria) asociado al seguimiento de pacientes con infección por el virus de la inmunodeficiencia humana en tres perfiles de pacientes (estable, discontinuación y cambio por falta de efectividad, discontinuación y cambio por toxicidad inaceptable), a partir de las guías de práctica clínica y un panel de expertos multidisciplinar (n = 5). Los expertos consensuaron los principales eventos adversos que conducían a la discontinuación, agrupándolos en: alteraciones gastrointestinales, renales, óseas, musculoesqueléticas, dermatológicas, hepáticas y del perfil lipídico, trastornos neuropsiquiátricos y sexuales. Los costes unitarios se identificaron a partir de bases de datos oficiales de costes sanitarios y de la literatura. Se estimó el coste (€, 2020) del seguimiento en cada perfil de paciente, sin incluir el coste derivado del tratamiento antirretroviral, en un horizonte temporal de dos años.
ResultadosEl coste por paciente a dos años se estimó en 4.148 € (pruebas: 2.293 €; visitas: 1.855 €) para el seguimiento del paciente estable. El seguimiento del paciente tras discontinuación por falta de efectividad y cambio de tratamiento antirretroviral se estimó en 5.434 € (pruebas: 2.777 €; visitas: 2.657 €). El coste del seguimiento tras la discontinuación por toxicidad inaceptable y cambio de tratamiento antirretroviral varió en función del evento adverso que motivó el cambio, oscilando entre 4.690 € para las alteraciones del perfil lipídico, y 5.304 € para las alteraciones musculoesqueléticas. En este perfil de pacientes, las pruebas variaron entre 2.403 € y 3.017 € y las visitas entre 2.287 € y 2.842 €.
ConclusionesEl coste asociado al seguimiento del paciente con infección por el virus de la inmunodeficiencia humana tras discontinuación y cambio a un nuevo tratamiento antirretroviral es mayor comparado con el seguimiento habitual, sin tener en cuenta el coste farmacológico. La tasa de discontinuación del tratamiento antirretroviral es un factor relevante a la hora de seleccionar la terapia más adecuada para cada paciente.
A total of 56,758 new cases of infection with the human immunodeficiency virus (HIV) have been reported in Spain since 2003. Annual rates of new diagnoses per 100,000 inhabitants fell from 12.81 in 2008 to 5.94 in 2019, a reduction associated with the use of antiretroviral therapy (ART)1. According to a recently published study, the implementation of ART in Spain required an investment of €6,185 billion from its introduction in 1987 to 2018. Moreover, the cost of ART in 2019 was estimated at €677 million, which represents 2.86% of the total spend of the National Health System on pharmaceutical and medicinal products2. Taking into account the pharmaceutical cost, the cost of the adverse events resulting from treatment and that of resistance studies and of the required HLA B*5710 tests, the total cost of ART typically ranges between €6,788 and €10,649 per patient3.
Implementation of ART has decreased morbidity and mortality as well as HIV infection transmission rates, making it possible for patients to enjoy a life expectancy similar to that of the general population3. According to the recommendations of the AIDS Study Group (GeSIDA) of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) and to the Spanish National AIDS Plan (PNS)4, ART should be administered to every HIV patient. Prior to initiation of ART, it is essential to individually determine which agents should be part the initial regimen given their different efficacy, toxicity, resistance, tropism, interactions, etc., profiles. In addition, the selected regimen should be adapted to the patient’s lifestyle and comorbidities, with a particular focus on promoting adherence4. Currently, the most often recommended initial regimens are aimed at reducing plasma viral load to less than 50 copies/ml and usually comprise a combination of two (double therapy) or three (triple therapy) drugs. The preferred recommended initial triple therapies are as follows: bictegravir/emtricitabine (FTC)/tenofovir alafenamide (TAF); dolutegravir (DTG)/abacavir/lamivudine (3TC); DTG + FTC/TAF; and raltegravir (400 mg twice a day or 1,200 mg once a day) + FTC/TAF. The only double therapy currently recommended is a combination of DTG/3TC (an integrase inhibitor [INI] and a nucleoside/tide reverse transcriptase inhibitor [NRTI]). However, this therapy is associated with a series of restrictions as it is not recommended for patients with a baseline CD4+ count below 200/µL and it cannot be used in subjects with chronic hepatitis B virus (HBV) infection4.
Once the patient has been started on a given ART regimen, there are several reasons why it may be necessary to switch them to a different one, including lack of efficacy, poor tolerability, toxicity, comorbidities, drug-drug interactions, the need to reduce the number of daily doses or tablets, dietary requirements, pregnancy, and the cost of ART itself5. Although most switches are motivated by a desire to simplify the therapy, factors such as toxicity or therapeutic failure are also frequent reasons for switching ART therapies in Spain6,7.
In this regard, with a view to comparing the real world results of discontinuing INI-based triple therapy as compared with DTG and/or boosted protease inhibitors (bPIs) double therapy in Spain, an analysis was carried out of patients in the VACH group (a prospective cohort comprising over 14,833 HIV patients followed up at 23 Spanish hospitals)8. Results showed a higher risk of all-cause discontinuation, as well as a higher risk of discontinuation caused by virologic failure in patients treated with double therapy. Along the same lines, another subanalysis of the same cohort that included patients switched to DTG-based double therapy or to INI-based triple therapy concluded that the risk of discontinuation of double therapy due to treatment failure was 2.3 times higher than that associated with triple therapy9.
In an environment of limited resources, judicious management of the resources allocated to ART is paramount4. A few pharmacoeconomic studies carried out in Spain3,10 have evaluated the cost-effectiveness of the different therapies and the economic impact of the optimization of ART recommended by GeSIDA. However, to the best of our knowledge no studies have focused on the use of resources and the healthcare costs associated with discontinuation of an existing ART regimen and switching to a different one. Such an analysis would provide for more effective decision-making.
The main purpose of this study is to quantify the use of resources and calculate the costs associated with following up HIV patients following discontinuation of an ART regimen and initiation of a new one due to lack of effectiveness or unacceptable toxicity (i.e., an adverse event whose severity outweighs the benefits of treatment), as compared with the usual follow-up of patients on ART, from the perspective of the Spanish National Health System.
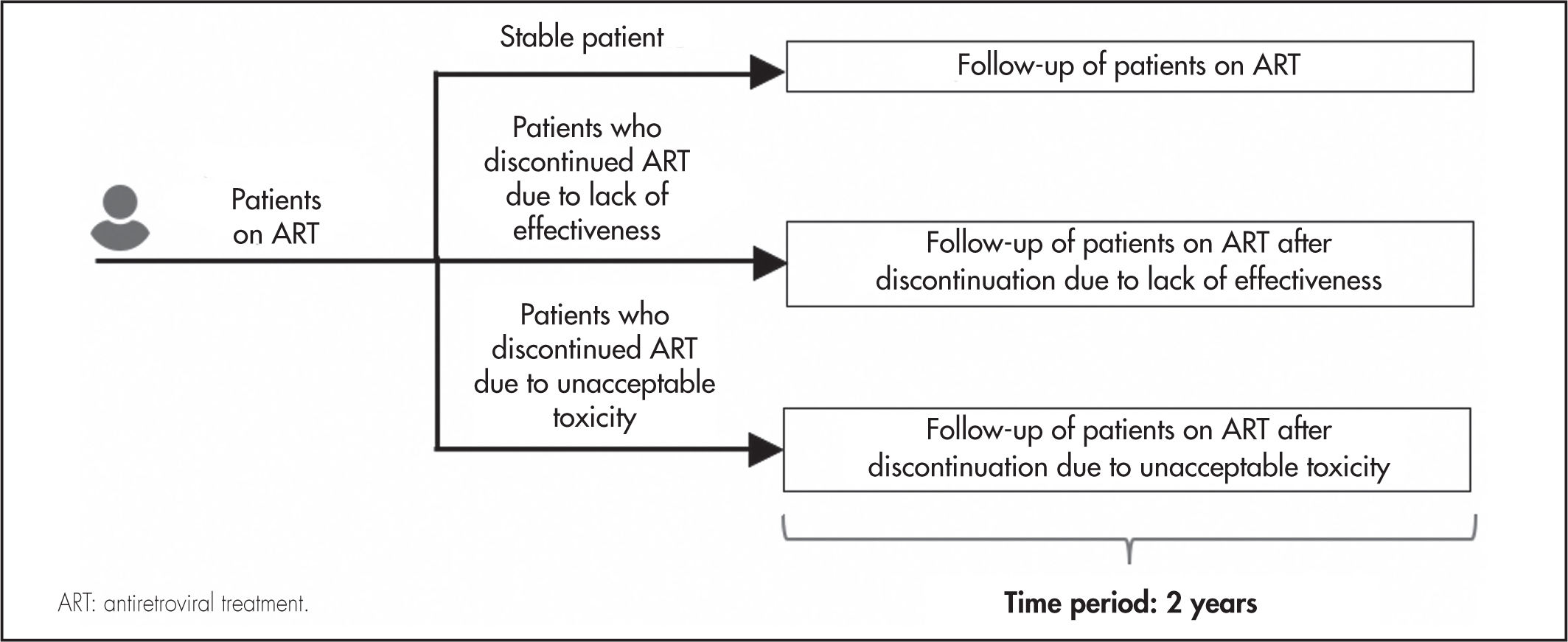
MethodsDrawing on the existing literature, a multidisciplinary expert panel comprising three clinicians specializing in infectious diseases and two hospital pharmacists carried out an estimation of the consumption of resources associated with following up a stable patient and switching their ART regimen as a result of treatment discontinuation. The analysis was based on three hypothetical profiles of HIV patients on ART (Figure 1): stable patient; patient who discontinues their treatment due to lack of effectiveness and is switched to a new ART; and patient who discontinues their treatment due to unacceptable toxicity and is switched to a different ART. Use of resources was calculated over a two-year period from discontinuation.
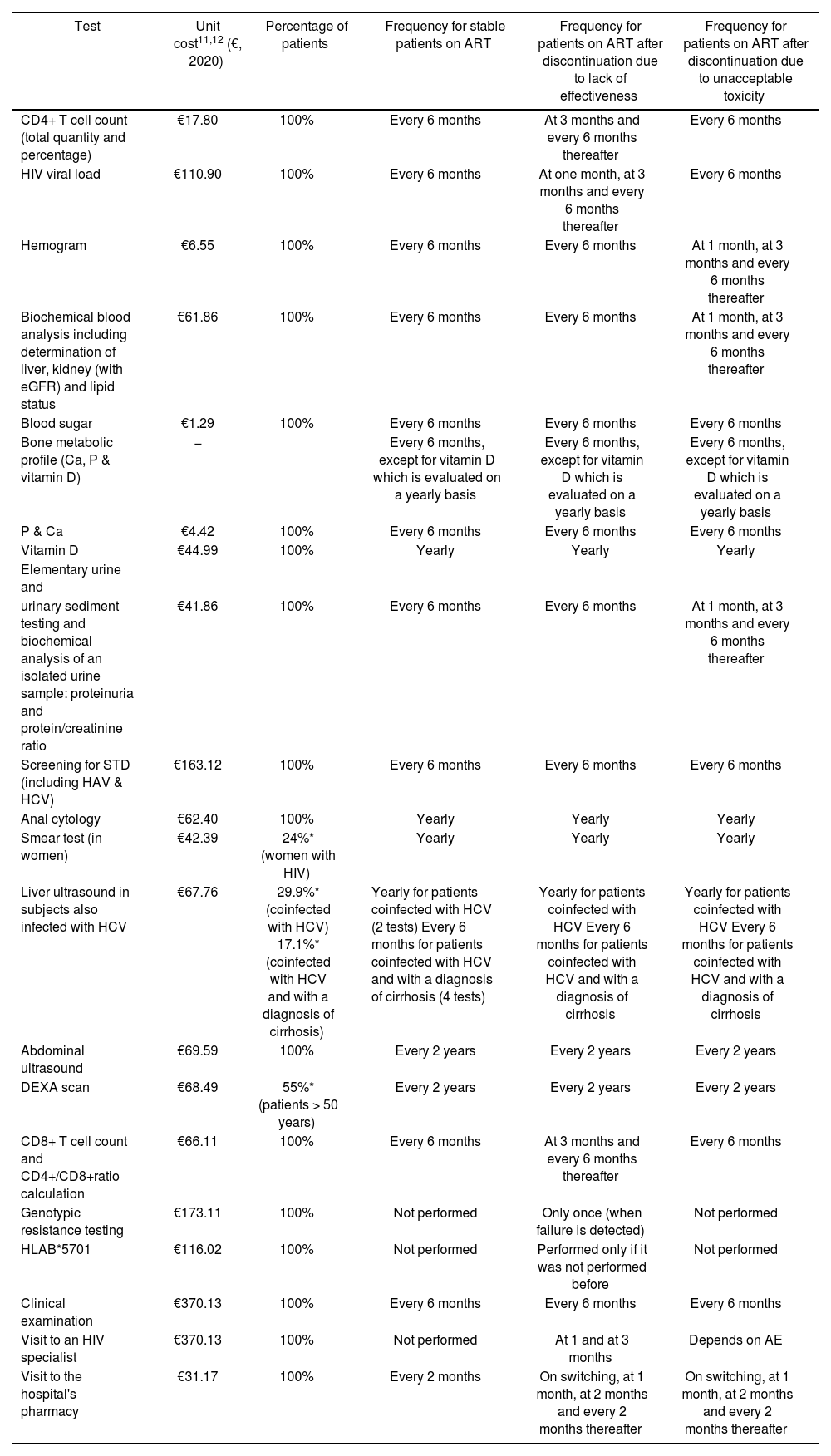
Resource consumption and costsThe analysis only considered direct healthcare costs, including the costs associated with clinical tests, medical visits and hospital pharmacy visits related to the management of the ART and to the follow-up of adverse events (the cost of the adverse events or of the ART themselves was not taken into consideration). The analysis, based on the patient management recommendations made by GeSIDA4,5, identified the main healthcare resources associated with following up the three classes of HIV patients considered (clinical tests and medical visits). The frequency with which these resources were used as well as the amount of resources used, together with the frequency of hospital pharmacy visits over the two-year period, were all estimated by the expert panel (Table 1).
Use of resources associated with patient follow-up
| Test | Unit cost11,12 (€, 2020) | Percentage of patients | Frequency for stable patients on ART | Frequency for patients on ART after discontinuation due to lack of effectiveness | Frequency for patients on ART after discontinuation due to unacceptable toxicity |
|---|---|---|---|---|---|
| CD4+ T cell count (total quantity and percentage) | €17.80 | 100% | Every 6 months | At 3 months and every 6 months thereafter | Every 6 months |
| HIV viral load | €110.90 | 100% | Every 6 months | At one month, at 3 months and every 6 months thereafter | Every 6 months |
| Hemogram | €6.55 | 100% | Every 6 months | Every 6 months | At 1 month, at 3 months and every 6 months thereafter |
| Biochemical blood analysis including determination of liver, kidney (with eGFR) and lipid status | €61.86 | 100% | Every 6 months | Every 6 months | At 1 month, at 3 months and every 6 months thereafter |
| Blood sugar | €1.29 | 100% | Every 6 months | Every 6 months | Every 6 months |
| Bone metabolic profile (Ca, P & vitamin D) | − | Every 6 months, except for vitamin D which is evaluated on a yearly basis | Every 6 months, except for vitamin D which is evaluated on a yearly basis | Every 6 months, except for vitamin D which is evaluated on a yearly basis | |
| P & Ca | €4.42 | 100% | Every 6 months | Every 6 months | Every 6 months |
| Vitamin D | €44.99 | 100% | Yearly | Yearly | Yearly |
| Elementary urine and | |||||
| urinary sediment testing and biochemical analysis of an isolated urine sample: proteinuria and protein/creatinine ratio | €41.86 | 100% | Every 6 months | Every 6 months | At 1 month, at 3 months and every 6 months thereafter |
| Screening for STD (including HAV & HCV) | €163.12 | 100% | Every 6 months | Every 6 months | Every 6 months |
| Anal cytology | €62.40 | 100% | Yearly | Yearly | Yearly |
| Smear test (in women) | €42.39 | 24%* (women with HIV) | Yearly | Yearly | Yearly |
| Liver ultrasound in subjects also infected with HCV | €67.76 | 29.9%* (coinfected with HCV) 17.1%* (coinfected with HCV and with a diagnosis of cirrhosis) | Yearly for patients coinfected with HCV (2 tests) Every 6 months for patients coinfected with HCV and with a diagnosis of cirrhosis (4 tests) | Yearly for patients coinfected with HCV Every 6 months for patients coinfected with HCV and with a diagnosis of cirrhosis | Yearly for patients coinfected with HCV Every 6 months for patients coinfected with HCV and with a diagnosis of cirrhosis |
| Abdominal ultrasound | €69.59 | 100% | Every 2 years | Every 2 years | Every 2 years |
| DEXA scan | €68.49 | 55%* (patients > 50 years) | Every 2 years | Every 2 years | Every 2 years |
| CD8+ T cell count and CD4+/CD8+ratio calculation | €66.11 | 100% | Every 6 months | At 3 months and every 6 months thereafter | Every 6 months |
| Genotypic resistance testing | €173.11 | 100% | Not performed | Only once (when failure is detected) | Not performed |
| HLAB*5701 | €116.02 | 100% | Not performed | Performed only if it was not performed before | Not performed |
| Clinical examination | €370.13 | 100% | Every 6 months | Every 6 months | Every 6 months |
| Visit to an HIV specialist | €370.13 | 100% | Not performed | At 1 and at 3 months | Depends on AE |
| Visit to the hospital's pharmacy | €31.17 | 100% | Every 2 months | On switching, at 1 month, at 2 months and every 2 months thereafter | On switching, at 1 month, at 2 months and every 2 months thereafter |
ART: antiretroviral treatment; Ca: calcium; gGFR: estimated glomerular filtration rate; HAV: hepatitis A virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; P: phosphorus; STD: sexually transmitted disease.
The percentage of patients has been extracted from a hospital-based survey of patients infected with HIV18.
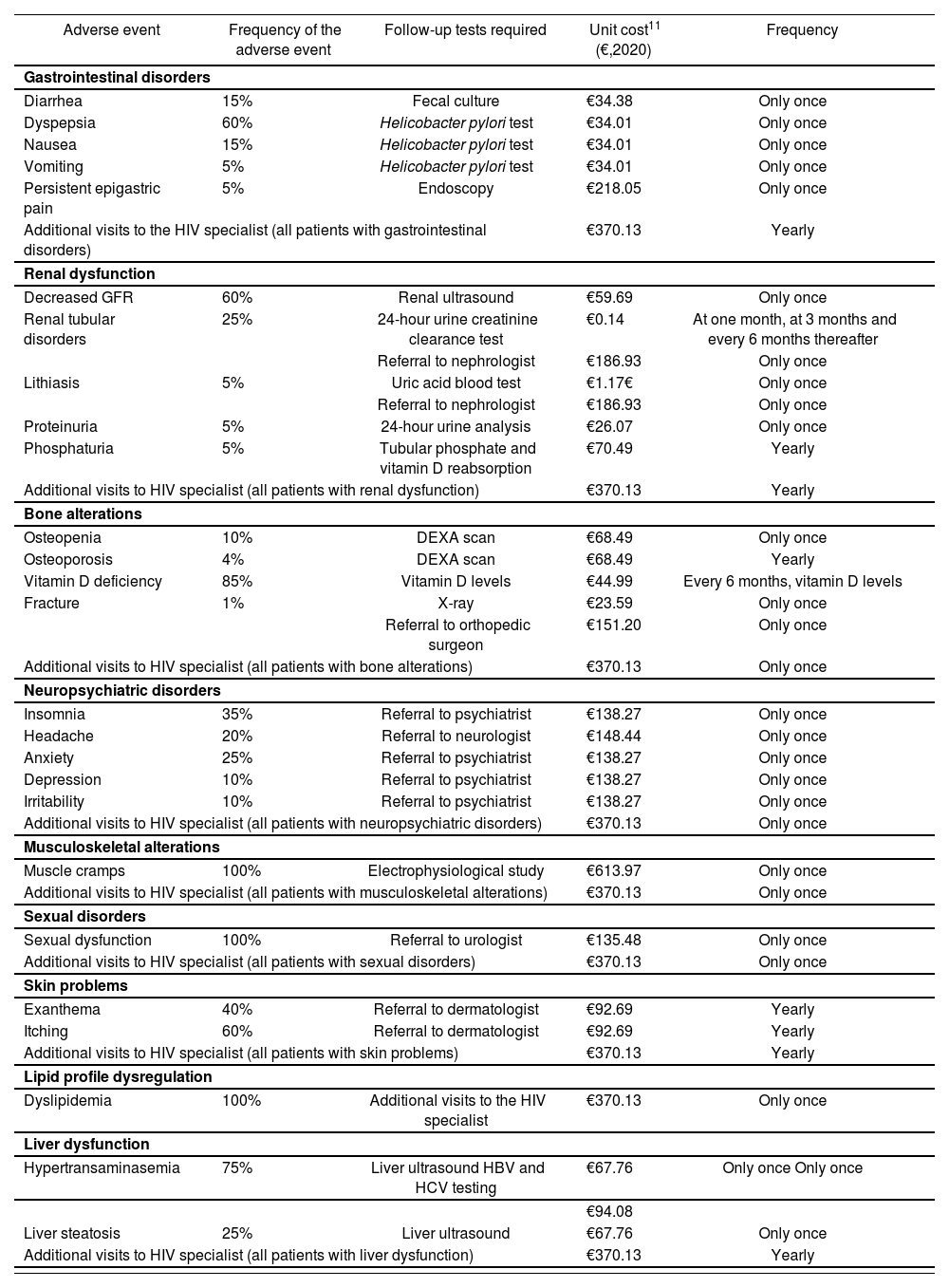
Additionally, for patients who discontinued ART due to unacceptable toxicity, the panel identified the most common adverse events according to their nature: gastrointestinal disorders, renal dysfunction, bone alterations, neuropsychiatric disorders, musculoskeletal alterations, sexual disorders, skin problems, lipid profile dysregulation, and liver dysfunction. They also analyzed the consumption of resources associated with the clinical tests and medical visits required (Table 2). Unit costs were extracted from the eSalud costing database11. The costs of hospital pharmacy visits were obtained from the literature (Table 1)12.
Use of resources associated with adverse event follow-up
| Adverse event | Frequency of the adverse event | Follow-up tests required | Unit cost11 (€,2020) | Frequency |
|---|---|---|---|---|
| Gastrointestinal disorders | ||||
| Diarrhea | 15% | Fecal culture | €34.38 | Only once |
| Dyspepsia | 60% | Helicobacter pylori test | €34.01 | Only once |
| Nausea | 15% | Helicobacter pylori test | €34.01 | Only once |
| Vomiting | 5% | Helicobacter pylori test | €34.01 | Only once |
| Persistent epigastric pain | 5% | Endoscopy | €218.05 | Only once |
| Additional visits to the HIV specialist (all patients with gastrointestinal disorders) | €370.13 | Yearly | ||
| Renal dysfunction | ||||
| Decreased GFR | 60% | Renal ultrasound | €59.69 | Only once |
| Renal tubular disorders | 25% | 24-hour urine creatinine clearance test | €0.14 | At one month, at 3 months and every 6 months thereafter |
| Referral to nephrologist | €186.93 | Only once | ||
| Lithiasis | 5% | Uric acid blood test | €1.17€ | Only once |
| Referral to nephrologist | €186.93 | Only once | ||
| Proteinuria | 5% | 24-hour urine analysis | €26.07 | Only once |
| Phosphaturia | 5% | Tubular phosphate and vitamin D reabsorption | €70.49 | Yearly |
| Additional visits to HIV specialist (all patients with renal dysfunction) | €370.13 | Yearly | ||
| Bone alterations | ||||
| Osteopenia | 10% | DEXA scan | €68.49 | Only once |
| Osteoporosis | 4% | DEXA scan | €68.49 | Yearly |
| Vitamin D deficiency | 85% | Vitamin D levels | €44.99 | Every 6 months, vitamin D levels |
| Fracture | 1% | X-ray | €23.59 | Only once |
| Referral to orthopedic surgeon | €151.20 | Only once | ||
| Additional visits to HIV specialist (all patients with bone alterations) | €370.13 | Only once | ||
| Neuropsychiatric disorders | ||||
| Insomnia | 35% | Referral to psychiatrist | €138.27 | Only once |
| Headache | 20% | Referral to neurologist | €148.44 | Only once |
| Anxiety | 25% | Referral to psychiatrist | €138.27 | Only once |
| Depression | 10% | Referral to psychiatrist | €138.27 | Only once |
| Irritability | 10% | Referral to psychiatrist | €138.27 | Only once |
| Additional visits to HIV specialist (all patients with neuropsychiatric disorders) | €370.13 | Only once | ||
| Musculoskeletal alterations | ||||
| Muscle cramps | 100% | Electrophysiological study | €613.97 | Only once |
| Additional visits to HIV specialist (all patients with musculoskeletal alterations) | €370.13 | Only once | ||
| Sexual disorders | ||||
| Sexual dysfunction | 100% | Referral to urologist | €135.48 | Only once |
| Additional visits to HIV specialist (all patients with sexual disorders) | €370.13 | Only once | ||
| Skin problems | ||||
| Exanthema | 40% | Referral to dermatologist | €92.69 | Yearly |
| Itching | 60% | Referral to dermatologist | €92.69 | Yearly |
| Additional visits to HIV specialist (all patients with skin problems) | €370.13 | Yearly | ||
| Lipid profile dysregulation | ||||
| Dyslipidemia | 100% | Additional visits to the HIV specialist | €370.13 | Only once |
| Liver dysfunction | ||||
| Hypertransaminasemia | 75% | Liver ultrasound HBV and HCV testing | €67.76 | Only once Only once |
| €94.08 | ||||
| Liver steatosis | 25% | Liver ultrasound | €67.76 | Only once |
| Additional visits to HIV specialist (all patients with liver dysfunction) | €370.13 | Yearly | ||
GFR: glomerular filtration rate; HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus.
For each adverse event considered, a comparison was made between the cost associated with stable patients on ART, the cost of following up patients who discontinued an existing ART and were switched to a new one due to lack of effectiveness and the cost of following up a patient further to discontinuation of an existing ART and initiation of a new one due to unacceptable toxicity. A distinction was made between the cost of clinical tests and that corresponding to medical visits.
Sensitivity analysisWith a view to determining the potential effect of modifying the level of resource consumption (which could be different across different hospitals depending on individual patient management policies) and the costs included in the assessment, a sensitivity analysis was performed where the total costs of following up stable and discontinuing patients (due to lack of effectiveness or unacceptable toxicity) were made to vary by 25%.
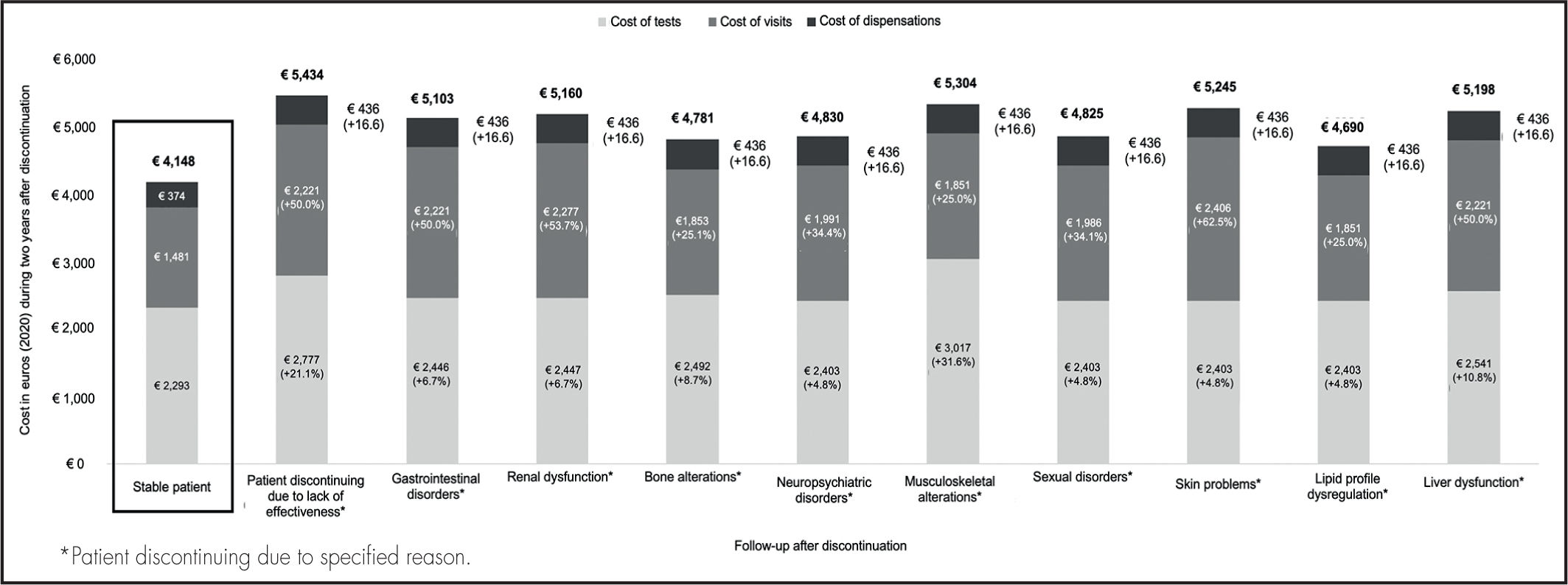
ResultsStable patientsThe total cost inherent in following up a stable patient on ART for up to two years was estimated at €4,148, of which €2,293 corresponded to clinical tests, €1,481 to medical visits, and €374 to visits to the hospital’s pharmacy (Figure 2).
Patients discontinuing ART due to lack of effectivenessThe total cost of following up a patient on ART for up to two years from discontinuation of one regimen and initiation of a new one due to lack of effectiveness was found to be 31.0% higher than the usual cost of following up a stable patient on ART. The cost was calculated to be €5,434, of which €2,777 corresponded to clinical tests (21.1% more than for stable patients), €2,221 to medical visits (50.0% more than for stable patients), and €436 to visits to the hospital’s pharmacy (16.6% more than for stable patients) (Figure 2).
Patients discontinuing ART due to unacceptable toxicityThe total cost of following up a patient for up to two years after toxicityrelated discontinuation and initiation of a new ART varied depending on the nature of the adverse event experienced (Figure 2).
The total increase in the cost of following up a patient discontinuing an existing ART regimen and initiating a new one due to unacceptable toxicity as compared with the usual follow-up costs for stable patients ranged between 13.1%, when the adverse event was associated with a lipid profile dysregulation, to 27.9%, when the adverse event was a musculoskeletal alteration.
The total cost of following up a patient on ART following discontinuation of an existing ART and initiation of a new one due to unacceptable toxicity was estimated to stand between €4,690, when the adverse event was a dysregulation of the patient’s lipid profile, and €5,304, when the problem was a musculoskeletal alteration. Of the €4,690 spent on lipid profile dysregulations, €2,403 corresponded to clinical tests (4.8% more than for stable patients), €1,851 to medical visits (25.0% more than for stable patients), and €436 to visits to the hospital’s pharmacy (16.6% more than for stable patients). Of the €5,304 spent on musculoskeletal alterations, €3,017 corresponded to clinical tests (31.6% more than for stable patients), €1,851 to medical visits (25.0% more than for stable patients), and €436 to visits to the hospital’s pharmacy (16,6% more than for stable patients) (Figure 2). Supplementary Table 1 provides a detailed account of the additional costs associated with each of the adverse events analyzed, distinguishing between the costs corresponding to the discontinuation and those related to the adverse event itself.
Sensitivity analysisVarying total follow-up costs by 25% in order to evaluate potential differences in the management of patients by different hospitals showed that the total cost of following a stable patient treated with ART for up to two years ranged between €3,111 and €5,185. In the case of patients switched to a different treatment due to lack of effectiveness, the cost ranged between €4,076 and €6,793. As regards patients who discontinued their treatment due to unacceptable toxicity, the cost ranged from €3,518, when lipid profile dysregulations were present, to €6,630 for musculoskeletal alterations.
DiscussionThe results of this analysis demonstrate that the consumption of resources and the costs associated to following up HIV patients after discontinuation of an existing ART and initiation of a new one are higher than those corresponding to stable HIV patients, mainly due to the higher number of medical visits required. In the case of patients discontinuing ART due to lack of effectiveness, the amount spent on medical visits was 50% higher than that for stable patients, whereas in patients discontinuing ART due to unacceptable toxicity, the increase ranged from 25% when patients experienced musculoskeletal alterations and lipid profile dysregulations, and 63% when they experienced skin problems. As far as hospital pharmacy visits are concerned, an increase of nearly 17% was observed across all patient groups. Two additional visits to the hospital’s pharmacy were considered to result from the change in treatment.
The sensitivity analysis performed showed that the total costs associated with managing an HIV patient whose ART is switched due to lack of effectiveness or unacceptable toxicity could be twice as high as those corresponding to stable patients (considering that stable patients are usually treated conservatively whereas patients whose treatment regimen is modified tend to be managed more comprehensively).
Although the advent of new ART regimens has resulted in improved efficacy and tolerability, switching ART regimens is not unusual particularly during the first year (around 30% of patients)13,14. Changes in treatment are often motivated by poor tolerability, drug-drug interactions, appearance of new comorbidities, dietary requirements, the patient’s wishes, unspecified doctor’s decisions, the cost of treatment and virologic failure15. Given its negative impact on adherence and virologic efficacy, the initial ART regimen should be administered unchanged for a considerable period of time15. Moreover, when changes are made to an ART regimen a more comprehensive patient follow-up should be made4.
The current therapeutic modifications recommended for virologically suppressed patients, take into account the antiretroviral drugs included in the patient’s regimen, and are grouped into switch to triple therapies (that include change of the NRTI, switch to regimens based on non-nucleoside reverse transcriptase inhibitors (NNRTIs) or switch to an INI-based regimen), or a change to regimens with less than three antiretrovirals (which may be dual therapies made up of a bPI + 3TC, DGT + rilpivirine (RPV), or DGT+ (3TC)4). In the case of virologically suppressed patients, an ART regimen with three, or at least two, active antiretrovirals is recommended including, if possible, a next-generation agent4.
Two real-world analyses based on a Spanish cohort found that, when the above recommendations are followed, there is a higher risk of discontinuation due to virologic failure when patients are treated with dual vs. triple therapy (analysis 1: HR = 2.06 [1.54; 2.77] for the entire population, HR = 2.78 [1.71; 4.51] for patients on dual or triple therapy based on DTG, and HR = 1.86 [1.15; 3.02] for virologically suppressed patients at initiation [HIV RNA <50 copies/mL]8; analysis 2: patients switched to dual DTG-based therapy or triple INI-based therapy: HR = 2.3 [1.3; 4.1])9. These results are in line with those of other international studies including the observational OPERA trial, based on the OPERA database, which contains data from 79,803 HIV patients in the United States gathered between 2010 and 2016). The OPERA trial also found a significantly higher discontinuation rate in patients on double therapy as compared with those on triple therapy (HR = 1.51 [1.41; 1.61])16.
Although the pharmacological cost is the most significant outlay in the treatment of HIV patients3,17, it should be investigated whether other expenses associated with patient follow-up may be avoided. Taking into consideration that the virologic failure risk of patients switched to dual therapy is twice higher than that of patients switched to triple therapy (HR between 1.86 and 2.78), this study may contribute to more judicious choice of the most appropriate ART regimen as the cost of follow-up after discontinuation of an existing ART and initiation of a new ART due to either lack of effectiveness or unacceptable toxicity is higher than that of following up stable patients. This increased use of resources should be taken into consideration.
This article is not without limitations. These are mainly related to the fact that costs were estimated based on a review of the literature and consultations with experts. Moreover, the analysis is somewhat oversimplified as it did not consider HIV patients on ART who require specific management and follow-up adapted to their needs. Nonetheless, the analysis was based on the resources consumed in the course of treating a standard HIV patient with ART, following the recommendations in the guidelines4,5. Furthermore, no consideration was given to the pharmacological cost of ART as it was hypothesized that the management of stable or discontinuing patient does not vary as a function of the type of treatment administered and, therefore, the analysis could be applied to any of the combinations available. Also, as our goal was to focus our analysis on the cost of tests and visits, the costs associated with the concomitant medication prescribed to address the toxicities observed was not part of the analysis. Finally, the indirect costs related to loss of productivity were ignored as the focus was on taking the perspective of the Spanish National Health System.
In spite of the aforementioned limitations, our analysis provides an approximate idea of the cost associated to discontinuation of ART, which may help make better decisions in routine clinical practice.
In conclusion, our study brings to the fore the importance of considering the rates of discontinuation of ART when electing the most appropriate ART regimen for HIV patients as, apart from the clinical consequences associated with such choices, ART regimen selection is a key factor for the consumption of resources, significantly influencing in many cases the overall cost of ART.
FundingThis study was funded by Gilead Sciences.
Conflict of interestÁngeles Castro declared no conflict of interest. Pilar Díaz declared to have received financial support from Gilead Sciences for her participation in the present study as well consultancy and speaker’s fees, and stipends for participating in conferences, bureaus, educational events and the preparation of manuscripts for Gilead Sciences and MSD. She also declared to have received fees for participating as an expert and financial support for attending meetings and/or travelling to events organized by Gilead Sciences prior to the preparation of this study. Pere Domingo declared to have received grants from Gilead Sciences, Janssen & Cilag, and ViiV Healthcare as well as consultancy and speaker’s fees, and stipends for participating in conferences, bureaus, educational events and the preparation of manuscripts for Gilead Sciences, Janssen & Cilag, and ViiV Healthcare, MSD, Theratechnologies, and Roche prior to the preparation of this study. Juan E. Losa-García declared no have no conflict of interest. Antonio Castro is a Gilead Sciences staff member. Neus Vidal-Vilar and work for Outcomes’10, an independent research organization, and have received fees for their contribution to the development of this project and to the preparation of the manuscript.
Presentation at congresses- •
Organization: International Health Economics Association Congress.
- •
Venue: Online.
- •
Date: 12–15 July 2021.
Discontinuation of an existing antiretroviral regimen and initiation of a different one increases resource consumption and costs for the National Health System. This should be taken into consideration every time a given antiretroviral therapy is selected.
Early Access date (09/30/2022).