To analyze the response to retreatment in patients with chronic/episodic migraine who discontinued therapy with erenumab/fremanezumab after 1 year of treatment.
MethodsObservational, retrospective, single-center, multidisciplinary study in patients with chronic/episodic migraine who received therapy with erenumab/fremanezumab for at least 1 year and discontinued it after achieving an adequate response (optimization). The evaluation of the response after retreatment included the following variables: DMM, MIDAS, and HIT-6 scales at the beginning of retreatment and 3 months later. The response was evaluated in different subgroups (episodic/chronic, erenumab/fremanezumab, and time until retreatment).
Results48 patients were included. 70.8% (n=34) required retreatment with mAb, with a median of 3.9 (2.9–6.4) months until reintroduction. Clinical response after retreatment was achieved in 67.6% (n=23) of patients. No statistically significant differences were found in the analyzed subgroups.
ConclusionInterruption of treatment with erenumab/fremanezumab for chronic/episodic migraine produces a clinical worsening of the disease requiring retreatment in most cases, approximately after 4 months. Two out of three patients respond positively after restarting monoclonal therapy. This response does not appear to be related to the type of migraine, the specific monoclonal antibody prescribed, or the time to retreatment.
Analizar la respuesta al retratamiento en pacientes con migraña crónica/episódica que suspendieron erenumab/fremanezumab tras un año de tratamiento.
MétodosEstudio observacional, retrospectivo, unicéntrico y multidisciplinar en pacientes con migraña crónica/episódica que recibieron tratamiento con erenumab/fremanezumab durante al menos un año y lo suspendieron tras lograr una respuesta adecuada (optimización). La evaluación de la respuesta después del retratamiento incluyó las siguientes variables: DMM, escalas MIDAS y HIT-6 al inicio del retratamiento y 3 meses después. La respuesta se evaluó en diferentes subgrupos (episódica/crónica, erenumab/fremanezumab y tiempo hasta retratamiento).
ResultadosSe incluyeron 48 pacientes. El 70,8% (n = 34) requirió retratamiento con mAb, con una mediana de 3,9 (2,9-6,4) meses hasta la reintroducción. La respuesta clínica tras retratamiento se logró en el 67,6% (n = 23) de los pacientes. No se encontraron diferencias estadísticamente significativas en los subgrupos analizados.
ConclusiónLa interrupción del tratamiento con erenumab/fremanezumab para la migraña crónica/episódica produce un empeoramiento clínico de la enfermedad necesitándose retratamiento en la mayoría de los casos, aproximadamente después de 4 meses. Dos de cada tres pacientes responden positivamente tras el reinicio de la terapia monoclonal. Esta respuesta no parece estar relacionada con el tipo de migraña, el anticuerpo monoclonal específico prescrito o el tiempo hasta retratamiento.
Migraine is a primary headache of moderate–severe intensity characterized by recurrent episodes of pain lasting 4–72 h, often accompanied by gastrointestinal, vestibular and cognitive symptoms, and even stimulus phobia.1 It manifests in the form of crises or attacks and their frequency is variable. Based on this frequency, they are classified into episodic migraine (EM) (<15 headache days/month) and chronic migraine (CM) (≥15 headache days/month for more than 3 months, of which at least 8 days are of the migraine type).2
The therapeutic approach is based on symptomatic treatment of migraine attacks and preventive treatment. The preventive treatment of migraine aims to reduce the frequency, intensity, and duration of the attacks and make them milder, thus easier to manage. It's similar for both EM and CM.
Monoclonal antibodies (mAbs) that act on the calcitonin gene-related peptide (CGRP) pathway, targeting either the receptor (erenumab) or the ligand (fremanezumab, galcanezumab, eptinezumab), have been incorporated into the therapeutic arsenal as a preventive treatment. These mAbs have recently been authorized and financed in Spain and they are the only therapies currently available specifically designed for the prevention of migraine and have shown significant benefits in patients with both EM and CM.3,4
The clinical development of erenumab for migraine prophylaxis included 4 double-blind, placebo-controlled clinical trials, where reductions of 2–3 days for CM and 1–2 days for EM in monthly migraine days (MMD) (primary endpoint) versus placebo were observed. Similarly, the clinical development of fremanezumab for migraine prophylaxis included 2 double-blind, phase III, randomized, placebo-controlled trials, whit the same results. The safety profile of erenumab is characterized by constipation, pruritus, muscle spasms, and injection site reactions. In the case of fremanezumab, injection site reactions have also been reported. In any case, these drugs are well-tolerated.1,5
Treatment with mAbs is chronic in nature, which means that patients are exposed to their adverse events in long-term. Additionally, these drugs have a significant economic impact. In this context, the latest European guidelines suggest considering discontinuing treatment with mAbs if a response is achieved after 12–18 months, also considering the possibility of maintaining treatment if necessary.6 However, it is known that after a period of treatment interruption, the disease worsens in most cases, requiring retreatment.
The main objetive of the present study was to conduct a real-world investigation to assess the response to retreatment in patients with chronic/episodic migraine who suspended erenumab/fremanezumab after 1 year of treatment.
MethodsThis study was an observational, retrospective, and multidisciplinary real-life study. It included all patients with chronic/episodic migraine who received treatment with erenumab/fremanezumab (options available in hospital therapeutic guide at the time of study) for at least 1 year (April 2023) and discontinued it after achieving an adequate response. Patients who were lost to follow-up were excluded.
An adequate response was defined as meeting one of the following criteria:
- 1
A 50% reduction in MMD.
- 2
Clinical improvement in any of the validated migraine scales:
- •
Migraine disability assessment (MIDAS): Reduction of >5 points when the baseline score is 11–20, and reduction of >30% when the baseline score is >20.
- •
Headache impact test (HIT-6): Reduction of >5 points.
- •
Sociodemographic variables (age, sex), clinical variables (type of migraine: episodic/chronic), and pharmacotherapeutic variables (type of antibody, number of prior antibody therapies, and whether a combination of preventive drugs in the case of EM or botulinum toxin for CM had been received) were analyzed. Data were obtained from the patient clinical records.
When patients suspended treatment, the following variables were assessed: retreatment with mAb, time until retreatment and response 3 months after retreatment. The response was evaluated based on MMD, MIDAS, and HIT-6 scales at the moment of the retreatment and 3 months later.
Response after retreatment was analyzed in different subgroups (episodic/chronic migraine, treatment with erenumab/fremanezumab, and time until retreatment).
For data presentation, qualitative variables were shown as frequency and percentage, while quantitative variables were presented as medians and IQR (interquartile range) as appropriate.
For subgroup analysis, relationships between qualitative variables were examined using chi-square test. Student's t-test or non-parametric Mann–Whitney U test were applied for non-normal distribution quantitative variables. The analysis of time until retreatment was conducted using survival curves through the Kaplan–Meier method. The statistical software SPSS v. 25 was used for the analysis.
The research was conducted in accordance with the principles of the Declaration Of Helsinki and received approval from the ethics committee “Comité Ético de Investigación del Sur de Sevilla” (Seville, Spain) (reference 0922-N-22; July 2022).
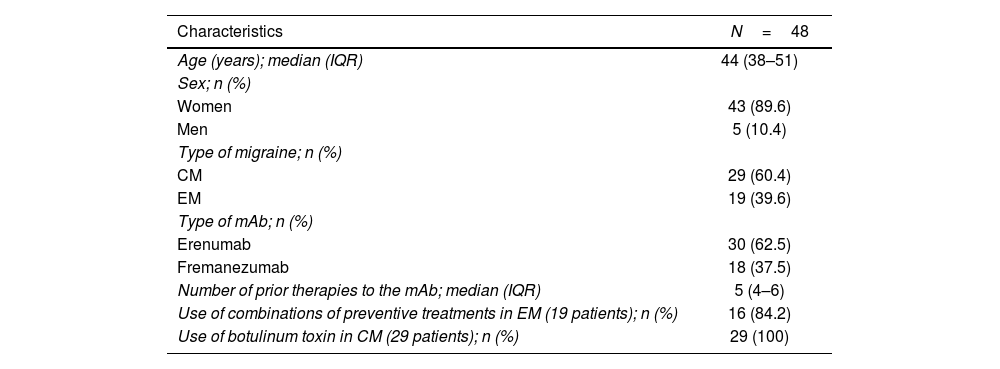
ResultsForty-eight patients treated with erenumab/fremanezumab for at least 1 year were included. All of them discontinued erenumab/fremanezumab due to a satisfactory response. The baseline demographic, clinical, and pharmacotherapeutic characteristics of patients are shown in Table 1.
Baseline demographic and disease characteristics.
| Characteristics | N=48 |
|---|---|
| Age (years); median (IQR) | 44 (38–51) |
| Sex; n (%) | |
| Women | 43 (89.6) |
| Men | 5 (10.4) |
| Type of migraine; n (%) | |
| CM | 29 (60.4) |
| EM | 19 (39.6) |
| Type of mAb; n (%) | |
| Erenumab | 30 (62.5) |
| Fremanezumab | 18 (37.5) |
| Number of prior therapies to the mAb; median (IQR) | 5 (4–6) |
| Use of combinations of preventive treatments in EM (19 patients); n (%) | 16 (84.2) |
| Use of botulinum toxin in CM (29 patients); n (%) | 29 (100) |
CM: chronic migraine; EM: episodic migraine; IQR: interquartile range; mAb: monoclonal antibody.
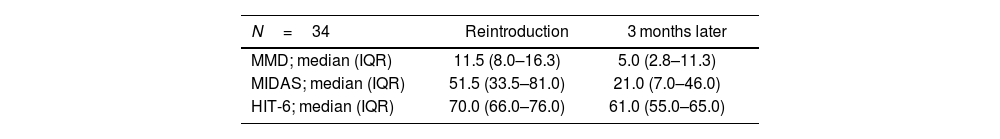
Among the patients, 70.8% (n=34) required retreatment with mAb, with a median time of 3.9 months (IQR: 2.9–6.4) until retreatment. The median values of MMD, MIDAS, and HIT-6 scales at the time of retreatment and 3 months later are detailed in Table 2.
Response outcomes.
| N=34 | Reintroduction | 3 months later |
|---|---|---|
| MMD; median (IQR) | 11.5 (8.0–16.3) | 5.0 (2.8–11.3) |
| MIDAS; median (IQR) | 51.5 (33.5–81.0) | 21.0 (7.0–46.0) |
| HIT-6; median (IQR) | 70.0 (66.0–76.0) | 61.0 (55.0–65.0) |
HIT-6: Headache impact test; IQR: interquartile range; MIDAS: migraine disability assessment; MMD: Monthly migraine days.
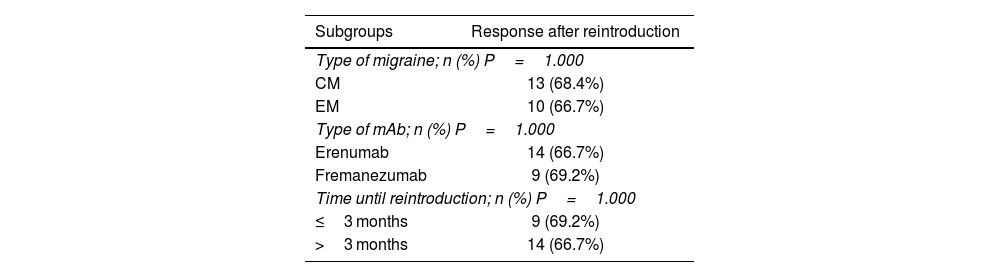
The clinical response after retreatment with mAb was achieved in 67.6% (n=23) of the patients. There were no statistically significant differences (P>.05) in the subgroups based on the type of migraine, type of mAb, or time until retreatment (≤3 months or >3 months) (Table 3).
Response analysis by subgroups.
| Subgroups | Response after reintroduction |
|---|---|
| Type of migraine; n (%) P=1.000 | |
| CM | 13 (68.4%) |
| EM | 10 (66.7%) |
| Type of mAb; n (%) P=1.000 | |
| Erenumab | 14 (66.7%) |
| Fremanezumab | 9 (69.2%) |
| Time until reintroduction; n (%) P=1.000 | |
| ≤3 months | 9 (69.2%) |
| >3 months | 14 (66.7%) |
CM: chronic migraine; EM: episodic migraine; mAb: monoclonal antibody.
Technique used: Chi-Square test (Fischer exact test); significance level: P<.05.
It's important to know how migraine evolves after discontinuation the drug in order to be able to specify the mAb long-term effectiveness and what extent is retreatment necessary. There are many publications that approach these issues, nevertheless we have limited information on how patients respond to a second cycle of mAbs, and whether it differs according to their characteristics. This study aims to provide more information about these questions.
The findings of this study align with previous publications, showing a high percentage of retreatment with mAb due to the worsening of the disease after suspension. Specifically, in the study by Gantenbein et al, which has a sample size similar to ours, a higher percentage of retreatment was obtained (88.9%) than in our cohort (70.8%).7
Studies agree that the disease worsens about 3–4 months after the last treatment, concluding that the therapeutic effect of mAbs seems to be maintained up to 12 weeks after ending therapy.8 This fact is reflected in the current study being 3.9 (IQR: 2.9–6.4) the median number of months until retreatment. This period of time can be explained by a decrease in plasma antibody concentrations after 3 months of discontinuation as described in the prospective analysis by Raffaelli et al.9
After retreatment with mAbs, 2 out of 3 patients achieved a response in this study (67.6%). We don't know why a drug that had been effective months ago is not effective later when reintroduced in 100% of cases. Resistance may be created, or it's necessary to extend the evaluation of treatment efficacy beyond 3 months in a second mAb cycle.
Few are the studies we currently have that describe how patients respond to a second cycle of mAbs by measuring specific response variables. A similar response rate (72.8%) was found in a study conducted in Berlin with a cohort almost the same as ours,10 obtaining an approximate reduction in MMD while the improvement in the HIT-6 value was more striking in our case. We also found a Spanish analysis in which there was less retreatment with mAb, and those who required it obtained a response. Although, they did not specify what this response was like by providing values from validated scales, neither they could establish a clear response predictor.11
Recently, other studies with greater follow-up have been published that collected specific variables related to the response to retreatment where the evaluations were positive, confirming the long-term efficacy of mAbs.12,13
Regarding the response analysis in different subgroups (type of migraine, type of mAb, and time until retreatment), no significant differences were observed in this study. In the search for factors that may condition this response, we found in the literature some studies that evaluate possible predictors. Iannone et al found that patients who reported better quality of life indices before starting mAbs showed sustained benefit during discontinuation and did not require retreatment.14 In parallel, the real-life study by Guerzoni et al points out that a high body mass index and the presence of aura were positively correlated with relapse of migraine and medication overuse headache.15
The main limitations of this study are those to the inherent biases of retrospective studies, and further assessment of quality-of-life questionnaires or to extend the follow-up time would be beneficial. However, it contributes valuable information to the current literature by providing data on the response after a new mAb cycle, including migraine frequency (MMD) and validated pain intensity scales, and examining potential patient-specific influences.
In conclusion, interruption of treatment with erenumab/fremanezumab for chronic/episodic migraine produces a clinical worsening of the disease requiring retreatment in most cases, approximately after 4 months. Two out of three patients respond positively after restarting monoclonal therapy. This response does not appear to be related to the type of migraine, the specific mAb prescribed, or the time to retreatment.
Contribution to the scientific literatureThe present work adds valuable information to the current literature by providing concrete data on response after a new cycle of monoclonal antibodies, including migraine frequency (MMD) and validated pain intensity scales.
This study allows for the examination of possible patient-specific influences on this response, performing an analysis by subgroups (type of migraine, type of monoclonal antibody or time until retreatment).
Declaration of authorshipAll authors have contributed to the concept, design, data collection, and review of the manuscript, and have approved the final version for publication.
Authors 1–3 have contributed to the analysis and interpretation of the data.
Patricia García-Lloret and Mercedes Galván-Banqueri contributed to the preparation and editing of the manuscript.
Ethical responsibilitiesEthical responsibilities have been taken into account.
FundingThis study did not require funding of any kind from public sector, commercial or not-for-profit agencies.
CRediT authorship contribution statementPatricia García-Lloret: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Data curation, Conceptualization. Mercedes Galván-Banqueri: Writing – review & editing, Validation, Supervision, Data curation, Conceptualization. María de las Aguas Robustillo-Cortés: Validation, Methodology, Formal analysis, Data curation, Conceptualization. María Fernández-Recio: Validation, Data curation, Conceptualization.