This review was prepared to offer the most complete information about the use of ondansetron in parenteral admixtures with other drugs.
MethodThe search was done from September 2016 to April 2017 by using electronic databases Stabilis® and Micromedex® solutions, Medli- ne/PubMed and Scholar Google searching publications about ondansetron stability in parenteral infusion when is administered by itself or with other medication.
Results49 studies are included with a total of 53 drugs. 15 drugs were found compatible administered with ondansetron in a clinical routine concentration range in intravenous administration. Also, four ternary blends were found compatible and another one was incompatible. Otherwise, 38 drugs were found incompatible.
ConclusionsCompatibility of ondansetron offers a broad number of options to be used to avoid nausea and vomiting symptoms in patients with other concomitant medication.
Esta revisión ha sido preparada para recopilar toda la información referente a la estabilidad del ondansetrón en mezclas parenterales junto a otros fármacos.
MétodoLa búsqueda fue realizada entre septiembre de 2016 y abril de 2017 empleando bases de datos electrónicas como Stabilis® y Micromedex® solutions, Medline/PubMed y Google Académico buscando publicaciones sobre la estabilidad del ondansetrón para infusión vía parenteral cuando es administrado en monoterapia o en una mezcla con otros fármacos.
ResultadosEn este trabajo han sido incluidos 49 artículos con un total de 53 fármacos. 15 fármacos han sido descritos como compatibles con ondan- setrón en concentraciones habituales en la clínica práctica para administración intravenosa. Además, cuatro mezclas ternarias han sido descritas como compatibles y una como incompatible. Por otro lado, 38 fármacos han sido descritos como incompatibles para su administración con ondansetrón.
ConclusionesLa compatibilidad del ondansetrón ofrece un amplio rango de opciones para evitar los síntomas de náuseas y vómitos en pacientes con otra medicación concomitante.
The use of intravenous drugs under combination has advantages against the individual use of each one. But, to mix them is important to know the stability of the admixture. This parameter is usually defined as a remaining 90% of the initial value of each drug. Another important parameter is the compatibility of the admixture and it makes reference to the physicochemical parameters as colour change, presence of turbidity and precipitate or changes in the pH values or osmolality. Changes in the concentration of the drugs and in their compatibility can be due by degradation, chemical reactions or physical incompatibilities. If the value of the concentration decrease under 90% or if a compatibility change is observed, the admixture is not stable and it can not be administered to the patients.
Also, microbiological stability should be taken into account. This parameter depends on the place of preparation, time and the presence of different microorganisms. Microbiological stability usually is assigned by using a microbiological risk matrix1 and it depends of the type of drugs, its preparation procedure or the temperature of storage.
Ondansetron is an antiemetic drug mainly used to avoid nausea and vomiting produced by cancer chemotherapy, radiation therapy, surgery and other. This drug is a highly selective 5HT3 receptor-antagonist. The ondansetron mode of action to control of vomiting and nausea is not described yet. One possibility could be done by blocking the release of 5HT in the small intestine, due by the chemotherapeutic agents and radiotherapy, which initiates a vomiting reflex by activating vagal afferents via 5HT3 receptors. Also, ondansetron can avoid the post-operative nausea and vomiting. Thus, patients who required ondansetron to avoid vomiting and nausea usually have another medication as chemotherapeutics, corticoids or sedative drugs having most of them reported incompatibilities. To administer the correct treatment to the patients is needed to choose a compatible mixture. Ondansetron is mainly administered via parenteral in the clinical routine practice. The availability of venous access is limited and if various drugs are administered to the patients is necessary to study the co-administration of the drugs mixing them in the same solution. Also, mixing drugs a reduction in the liquid volume is acquired. This reduction is highly recommended in renal insufficiency, heart disease or elderly patients and in the subcutaneous administration by infusion bags or infusers. Then, it is interesting to know if the administration of ondansetron with other drugs is possible.
MethodsSearch strategyWe have performed a search for articles published in English language through Medline/PubMed and Scholar Google. The following keywords were used: [“ondansetron”] and [“stability” or “compatibility” or “coadministered” or “admixtures”]. In PubMed the search details were: “ondansetron[Title] AND (stability[All Fields] OR compatibility[All Fields] OR admixtures[All Fields] OR coadministered[All Fields])” founding 61 articles. A similar searching in Scholar Google provides 57 articles. Finally, the data presented in the electronic databases Stabilis® and Micromedex® solutions and in the was taken into account to complete the review adding 19 more articles to the search. The PRISMA2 recommendations have been followed to prepare this review about the ondansetron stability.
Sampling methodsStudied articles in this review use intravenous admixtures. Plastic syringes and Y-site administration were discarded, except in case of Y-site incompatibilities. They were taken into account because these combinations will be also incompatible for infusion bags administration.
The terms room or ambient temperature are used in this review and they make reference to 20-25 °C. The accepted levels of stability are a remaining percentage of the drugs higher of 90%.
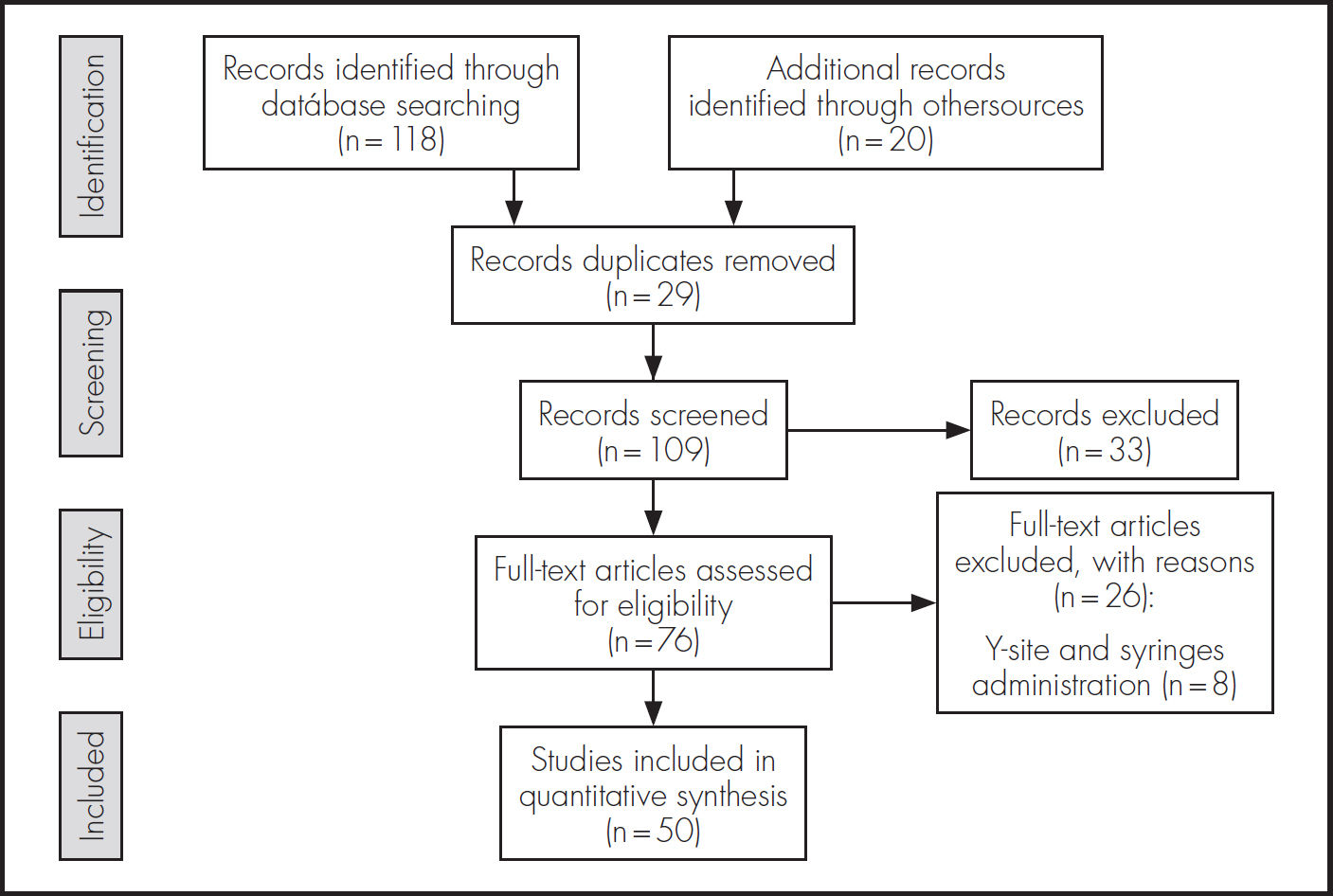
ResultsThe present review describes first the stability of ondansetron hydrochloride at different temperatures. After that, the stability of ondansetron when is co-administered with other drugs in intravenous administration is exposed and finally, the published incompatibilities of ondansetron with other drugs are presented. The summary of the search and work done is presented in a flow diagram in figure 1.
Excluded records were in base of a repetition of the article or because obtained information was based in subcutaneous or Y-site administration, being the required compatibility of the admixture larger in case of parenteral administration.
The stability of ondansetron with other drugs was studied with 53 drugs coming from a total number of publications of 49. In case of ondansetron compatible admixtures fifteen drugs were described. Otherwise, 38 drugs were found incompatible. Also, five ternary blends were studied being four compatible and another incompatible.
Ondansetron chemical stabilityPreviously to describe the ondansetron chemical stability with other compounds, a description of its own stability is necessary. When the drug is stored at -20 °C in normal saline or dextrose in a concentration range from 0.25 to 1 mg/ml the reported chemical stability is at least 90 days3-5. At 4 °C, most of the published articles found a 14 days stability for ondansetron solutions with concentrations from 0.024 to 1 mg/ml in normal saline and dextrose 5%3,4,6,7. Also Ringer injection8 was found compatible with ondansetron hydrochloride (0.016 mg/ml) for seven days at refrigerated temperature. When ondansetron (0.24 mg/ml) is refrigerated in an elastomeric device for pump infusion, its demonstrated chemical stability is 31 days9. Furthermore, in case of ambient temperature (22-25°C), 48 hours of chemical stability are reported by most of the authors in the concentration range between 0.03 and 1 mg/ml in normal saline solution and dextrose3,4 and chemical stability of ondansetron (0.024-0.096 mg/ml) in lactate Ringer injection6 was fourteen days at room or refrigerated temperature.
Finally, when ondansetron is delivered by a portable infusion-pump reservoirs at near-body-body temperature (30 °C) a chemical stability up to 24 hours for 0.24 mg/ml and up to one week was reported for 2 mg/ml samples9.
Ondansetron chemical stability coadministered with other drugsAdmixtures with ondansetron and other medication were evaluated to determine their compatibility.
Therefore, for additional pain treatment, ondansetron can be blended at different conditions/concentrations in binary mixtures with meperidine hy- drochloride10, tramadol11, hydromorphone hydrochloride and with morphine sulfate12. Otherwise, ondansetron hydrochloride and naloxone hydrochloride admixture was also studied13.
Ondansetron plus another immunosuppressant or antiemetic treatment mixed in the same solutions was also demonstrated (dexamethasone sodium phosphate14,15, methylprednisolone16, fosaprepitant17).
Additionally it is compatible with chemotherapeutic agents like cyclophosphamide with dextrose and normal saline solution18, cisplatin19, cytarabine, doxorubicin hydrochloride, etoposide and methotrexate sodium20.
Also for binary mixtures, recently a twelve hours chemical stability has been found for ondansetron/haloperidol admixtures at room temperature in normal saline solution or dextrose21.
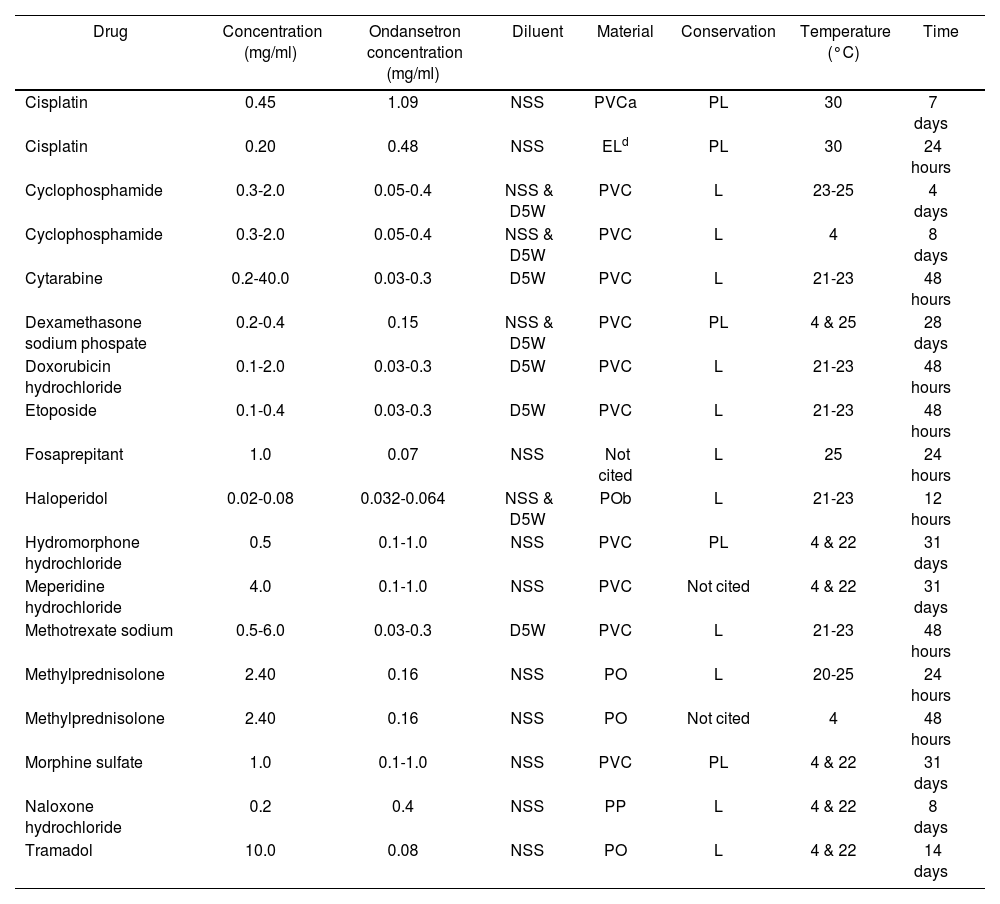
A summary with the stability results of the admixtures that can be used in clinical practice is presented in table 1.
Described stabilities of ondansetron with other drugs
| Drug | Concentration (mg/ml) | Ondansetron concentration (mg/ml) | Diluent | Material | Conservation | Temperature (°C) | Time |
|---|---|---|---|---|---|---|---|
| Cisplatin | 0.45 | 1.09 | NSS | PVCa | PL | 30 | 7 days |
| Cisplatin | 0.20 | 0.48 | NSS | ELd | PL | 30 | 24 hours |
| Cyclophosphamide | 0.3-2.0 | 0.05-0.4 | NSS & D5W | PVC | L | 23-25 | 4 days |
| Cyclophosphamide | 0.3-2.0 | 0.05-0.4 | NSS & D5W | PVC | L | 4 | 8 days |
| Cytarabine | 0.2-40.0 | 0.03-0.3 | D5W | PVC | L | 21-23 | 48 hours |
| Dexamethasone sodium phospate | 0.2-0.4 | 0.15 | NSS & D5W | PVC | PL | 4 & 25 | 28 days |
| Doxorubicin hydrochloride | 0.1-2.0 | 0.03-0.3 | D5W | PVC | L | 21-23 | 48 hours |
| Etoposide | 0.1-0.4 | 0.03-0.3 | D5W | PVC | L | 21-23 | 48 hours |
| Fosaprepitant | 1.0 | 0.07 | NSS | Not cited | L | 25 | 24 hours |
| Haloperidol | 0.02-0.08 | 0.032-0.064 | NSS & D5W | POb | L | 21-23 | 12 hours |
| Hydromorphone hydrochloride | 0.5 | 0.1-1.0 | NSS | PVC | PL | 4 & 22 | 31 days |
| Meperidine hydrochloride | 4.0 | 0.1-1.0 | NSS | PVC | Not cited | 4 & 22 | 31 days |
| Methotrexate sodium | 0.5-6.0 | 0.03-0.3 | D5W | PVC | L | 21-23 | 48 hours |
| Methylprednisolone | 2.40 | 0.16 | NSS | PO | L | 20-25 | 24 hours |
| Methylprednisolone | 2.40 | 0.16 | NSS | PO | Not cited | 4 | 48 hours |
| Morphine sulfate | 1.0 | 0.1-1.0 | NSS | PVC | PL | 4 & 22 | 31 days |
| Naloxone hydrochloride | 0.2 | 0.4 | NSS | PP | L | 4 & 22 | 8 days |
| Tramadol | 10.0 | 0.08 | NSS | PO | L | 4 & 22 | 14 days |
NSS: Normal saline solution; D5W: 5% dextrose; a=PVC: polyvinyl chloride;b= PO: Polyolefin;c=PP:Polypropylene;d= EL: Elastomer, L=light exposure; PL=protection from light
Finally, ternary blends have been studied. Ondansetron (0.44 mg/ml), dexamethasone sodium phosphate (0.28 mg/ml) and lorazepam (0.028 mg/ml) in dextrose 5% solution at ambient light and temperature have demonstrate a 24 hours of chemical stability22. Admixtures with ondansetron, doxorubicin and other chemotherapeutic agent were also tested at 30 °C23, but it is not cited the light storage conditions; ondansetron (0.48 mg/ml), doxorubicin (0.4 mg/ml) and vincristine sulphate (0.014 mg/ml) in normal saline and elastomer have shown 24 hours of chemical stability. Change the material to PVC and increasing the concentrations of each drug to the double, the shown chemical stability increases until five days. With dacar- bazine (8 mg/ml), ondansetron (0.64 mg/ml) and doxorubicin (0.8 mg/ ml) at 30 °C in elastomer or PVC have shown 24 hours stability. Recently a physicochemical stability of 15 days for mixtures containing fosaprepi- tant (0.6-3.0 mg/ml), ondansetron (0.03-0.16 mg/ml) and dexamethasone (0.03-0.16 mg/ml)24 in viaflo infusion bags have been established under different storage conditions of light (4 and 25 °C) and temperature (under light exposure or protected from light).
Ondansetron incompatibilitiesSeveral incompatibilities of ondansetron with other drugs have been reported (physicochemical incompatibility, precipitation due to pH of the so- lution)25-51. For example, dantrolene sodium precipitates from solutions with a pH less than 8.8, including dextrose and sodium chloride solutions. Then the combination with ondansetron is not possible in these solutions43.
Another like azathioprine sodium or cefepime hydrochloride and ondansetron (1 mg/ml) in normal saline or dextrose were found incompatible by Y-site administration simulation at room temperature37,38.
Also, biologic compounds as trastuzumab49, rituximab50 and gemtuzu- mab ozogamicin51 were found incompatible with ondansetron in normal saline solution.
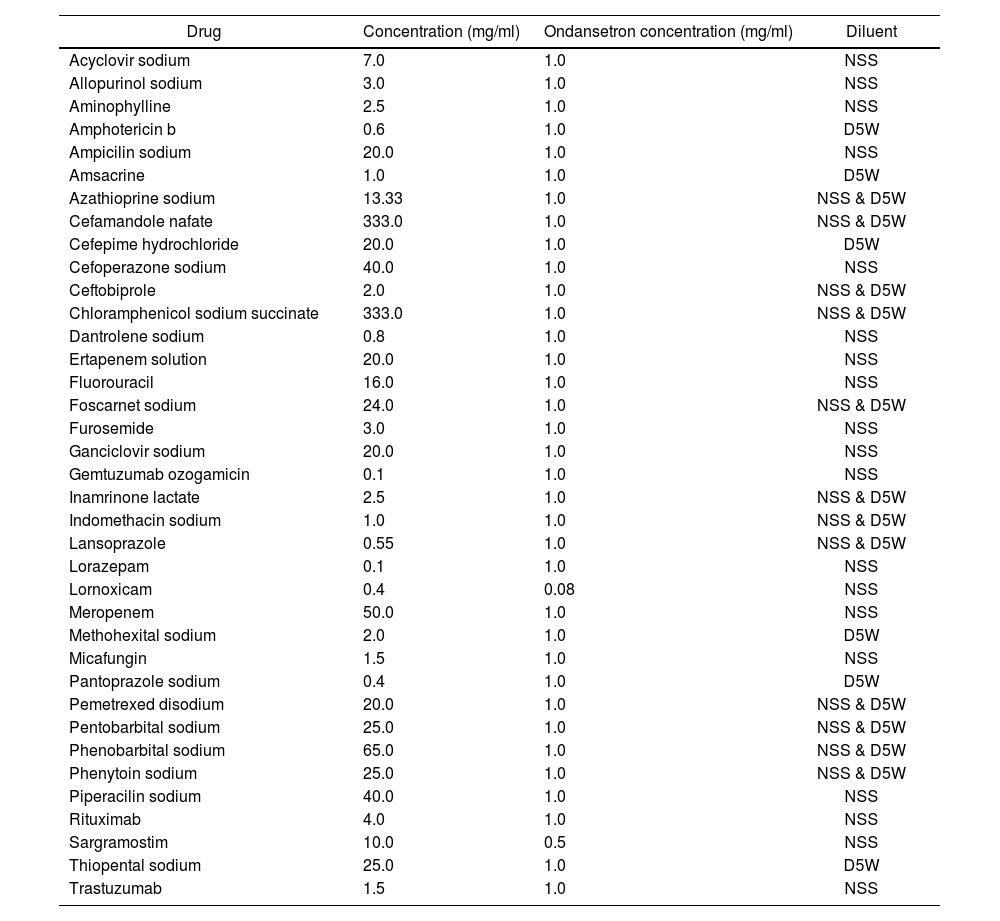
As a general view, table 2 summarizes the incompatibilities.
Incompatibilities of ondansetron with other drugs
| Drug | Concentration (mg/ml) | Ondansetron concentration (mg/ml) | Diluent |
|---|---|---|---|
| Acyclovir sodium | 7.0 | 1.0 | NSS |
| Allopurinol sodium | 3.0 | 1.0 | NSS |
| Aminophylline | 2.5 | 1.0 | NSS |
| Amphotericin b | 0.6 | 1.0 | D5W |
| Ampicilin sodium | 20.0 | 1.0 | NSS |
| Amsacrine | 1.0 | 1.0 | D5W |
| Azathioprine sodium | 13.33 | 1.0 | NSS & D5W |
| Cefamandole nafate | 333.0 | 1.0 | NSS & D5W |
| Cefepime hydrochloride | 20.0 | 1.0 | D5W |
| Cefoperazone sodium | 40.0 | 1.0 | NSS |
| Ceftobiprole | 2.0 | 1.0 | NSS & D5W |
| Chloramphenicol sodium succinate | 333.0 | 1.0 | NSS & D5W |
| Dantrolene sodium | 0.8 | 1.0 | NSS |
| Ertapenem solution | 20.0 | 1.0 | NSS |
| Fluorouracil | 16.0 | 1.0 | NSS |
| Foscarnet sodium | 24.0 | 1.0 | NSS & D5W |
| Furosemide | 3.0 | 1.0 | NSS |
| Ganciclovir sodium | 20.0 | 1.0 | NSS |
| Gemtuzumab ozogamicin | 0.1 | 1.0 | NSS |
| Inamrinone lactate | 2.5 | 1.0 | NSS & D5W |
| Indomethacin sodium | 1.0 | 1.0 | NSS & D5W |
| Lansoprazole | 0.55 | 1.0 | NSS & D5W |
| Lorazepam | 0.1 | 1.0 | NSS |
| Lornoxicam | 0.4 | 0.08 | NSS |
| Meropenem | 50.0 | 1.0 | NSS |
| Methohexital sodium | 2.0 | 1.0 | D5W |
| Micafungin | 1.5 | 1.0 | NSS |
| Pantoprazole sodium | 0.4 | 1.0 | D5W |
| Pemetrexed disodium | 20.0 | 1.0 | NSS & D5W |
| Pentobarbital sodium | 25.0 | 1.0 | NSS & D5W |
| Phenobarbital sodium | 65.0 | 1.0 | NSS & D5W |
| Phenytoin sodium | 25.0 | 1.0 | NSS & D5W |
| Piperacilin sodium | 40.0 | 1.0 | NSS |
| Rituximab | 4.0 | 1.0 | NSS |
| Sargramostim | 10.0 | 0.5 | NSS |
| Thiopental sodium | 25.0 | 1.0 | D5W |
| Trastuzumab | 1.5 | 1.0 | NSS |
NSS=Normal saline solution; D5W: 5% dextrose.
Finally, the ternary blend with ondansetron (0.008 mg/ml), mesna (1.25 mg/ml) and furosemide (0.02 mg/ml) was found chemically unstable36.
DiscussionThe described combinations of ondansetron with other drugs offers a wide range of therapeutic options and also, avoids the fallacious use of many combinations. But, there is a lack in the published chemical stability works of ondansetron with other concomitant medication in admixtures and there are unpublished possible clinical routine combinations in infusion bags for palliative medicine.
Then, new research studies can be developed to cover the lacks. The study of the chemical stability of ondansetron with other drugs in infusion bags for parenteral administration used in cancer treatment, palliative medicine or others can improve the treatment of many patients.
AcknowledgmentsFunding: This work was partially supported by European Regional Development Fund (ERDF) and the European Social Fund (ESF) [grant number PEJ-2014-A-06341].
Conflict of Interest: All the authors of this manuscript declare that they have no conflict of interest.