To show the effectiveness and safety of topical sevoflurane after ambulatory and prolonged administration in patients with refractory vascular ulcers .
MethodsRetrospective observational study analysing clinical improvement and vascular ulcers surface area variation after topical application of sevoflurane. Inclusion criteria were patients with painful vascular ulcers refractory to usual therapies and who were treated with topical sevoflurane for at least 36 months. The following variables were collected: age, sex, medical history, associated comorbidity, ulcer aetiology, and medical treatment. The visual analogue scale was used to measure baseline and break through pain intensity before and after treatment.
ResultsNine patients met the inclusion criteria of the total number of patients treated whose median age was 74.8±7.5 years. Cases 2 and 9 died during follow-up. In all cases, the analgesic action of topical sevoflurane was rapid (3.1±2.1 min), intense (visual analogic scale: 7±1.1 to 1.4 ± 1.1 points), and long-lasting (6–24 h). With the exception of case 4, all patients experienced a large reduction in vascular ulcers surface area (15.1±5.0 to 2.7±4.2) and tolerance wasn't observed over time.
ConclusionTopical application of sevoflurane is an analgesic and re-epithelialising strategy for vascular ulcers with a successful safety profile.
Mostrar la efectividad y seguridad del sevoflurano tópico tras la administración ambulatoria y prolongada en pacientes con úlceras vasculares refractarias.
MétodosEstudio observacional retrospectivo de pacientes con úlceras vasculares dolorosas refractarias a terapias habituales y que fueron tratados con sevoflurano tópico durante al menos 36 meses. Se recogieron las variables: edad, sexo, antecedentes médicos, comorbilidad asociada, etiología de úlcera y tratamiento médico. Se analizó la mejoría clínica y la variación de la superficie de las úlceras vasculares. Para cuantificar la intensidad del dolor basal e irruptivo antes y después del tratamiento se utilizó la escala visual analógica .
ResultadosDel total de pacientes tratados, 9 cumplían los criterios de inclusión. La edad media fue de 74,8 ± 7,5 años. Los casos 2 y 9 fallecieron durante el seguimiento. La acción analgésica del sevoflurano tópico fue rápida (3,1 ± 2,1 min), intensa (escala visual analógica: 7 ± 1,1 a 1,4 ± 1,1 puntos) y duradera (de 6 h a 24 h). Salvo el caso 4, todos experimentaron una reducción de la superficie (15,1 ± 5,0 a 2,7 ± 4,2) de las úlceras vasculares y no se observó tolerancia a lo largo del tiempo.
ConclusiónLa aplicación de sevoflurano tópico es una estrategia analgésica y reepitelizante para las úlceras vasculares que presenta un perfil correcto de seguridad.
Peripheral vascular disease is a highly prevalent condition and is mainly caused by atherosclerosis1,2. Restricted blood flow causes progressive symptoms such as ischaemic pain, ulceration, and gangrene. The incidence of vascular ulcers (VU) is 0.1%–0.2% in the adult population. Vascular ulcers are generally chronic, have high recurrence rates, and have a high impact on quality of life. According to previous studies, pain is a common symptom in 17%–64% of patients3. It is therefore considered a relevant public health problem.
Currently, there is no standard analgesic treatment for this condition, but topical treatments are often used due to their efficacy and safety. Examples of such treatments include lidocaine-prilocaine (EMLA), ibuprofen, and morphine creams4.
Sevoflurane is an inhaled anaesthetic used for inducing and maintaining general anaesthesia that has the ability to affect the central and peripheral nervous systems5. There have been recent reports of the efficacy of topical sevoflurane irrigation in the treatment of VU. Currently, such reports remain limited to isolated clinical cases and a few case series. More clinical trials are needed to clarify its role as an analgesic in VU6–9.
The aim of this study was to show the efficacy and safety of this novel topical therapy when administered on an outpatient basis over an extended period in multiple patients with VU. The secondary objective was to measure variations in VU surface area.
MethodsA retrospective follow-up study was performed of an off-label protocol of topical sevoflurane as an analgesic in patients with vascular diseases of various aetiologies. The study was approved by the hospital's Clinical Research Ethics Committee and the Spanish Medicines Agency. The inclusion criteria were as follows: patients classified by the Vascular Surgery Service of the Hospital Universitario Torrecárdenas de Almería (Spain) as having chronic critical limb ischaemia without the option of revascularisation10; poor pain management with standard therapies; and over 36 months of treatment with topical sevoflurane. In all cases, lifestyle adjustments were made at the outset to control the various risk factors.
The protocol consisted of cleaning the ulcer with normal saline 0.9%, followed by its irrigation with sevoflurane at a dose of 1.5 mL/cm2 surface area. The ulcer beds were irrigated directly while avoiding the edges of the healthy skin. If needed, dressings and compressive bandages were applied after completion of the procedure. In cases of chronic disease (>12 months), a biopsy of the VU was performed.
We collected data on age, sex, medical history, comorbidity, ulcer aetiology, and treatment. Before starting treatment and every 2 months thereafter, patients underwent complete blood count, renal and liver function tests, VU depth and surface measurements, and serial cultures of the lesions. We evaluated outcome variables related to the clinical reduction in pain using a visual analogue scale, ulcer healing rate, complications of the intervention, analgesic use, quality of life, and mortality. The Patient Global Impression of Improvement (PGI-I) and the Clinical Global Impression of Improvement (CGI-I) were used to assess the patients' and healthcare staff's perception of the treatment11.
ResultsIn total, 144 patients with VU have been treated since the start of the off-label protocol with topical sevoflurane in 2013. The mean treatment duration was 5.21±3.17 months. Biopsy results were negative in all but 3 cases (2 cases of Marjolin's ulcer and 1 tuberculous ulcer).
Nine patients with high comorbidity associated with refractory painful VU received outpatient pain control with continuous or discontinuous topical sevoflurane for more than 3 years. The time course of the condition prior to inclusion in the protocol was 42.9±40.2 months (range 8–129 months). The mean age of the patients in the case series was 74.8±7.5 years. During the study period, 2 patients died due to pre-existing ischaemic heart disease and cerebral ischaemic heart disease (patients 2 and 9).
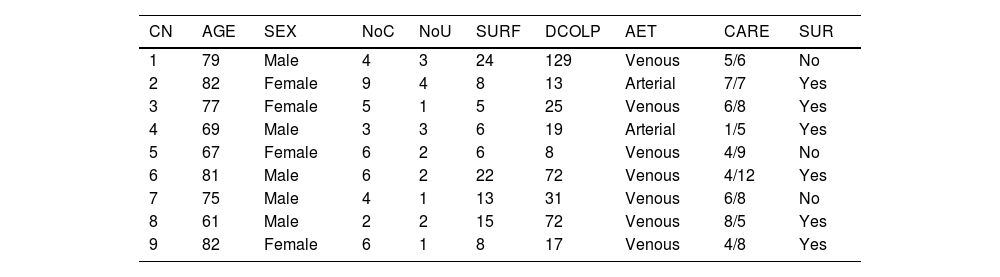
Table 1 shows the baseline characteristics of the cohort. In total, 77.77% of VUs were of venous origin. All the arterial ulcers were due to atheromatous arteriosclerosis, whereas venous ulcers were of varicose and post-phlebitic origin.
Baseline characteristics of the study cohort prior to inclusion in the off-label protocol.
| CN | AGE | SEX | NoC | NoU | SURF | DCOLP | AET | CARE | SUR |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 79 | Male | 4 | 3 | 24 | 129 | Venous | 5/6 | No |
| 2 | 82 | Female | 9 | 4 | 8 | 13 | Arterial | 7/7 | Yes |
| 3 | 77 | Female | 5 | 1 | 5 | 25 | Venous | 6/8 | Yes |
| 4 | 69 | Male | 3 | 3 | 6 | 19 | Arterial | 1/5 | Yes |
| 5 | 67 | Female | 6 | 2 | 6 | 8 | Venous | 4/9 | No |
| 6 | 81 | Male | 6 | 2 | 22 | 72 | Venous | 4/12 | Yes |
| 7 | 75 | Male | 4 | 1 | 13 | 31 | Venous | 6/8 | No |
| 8 | 61 | Male | 2 | 2 | 15 | 72 | Venous | 8/5 | Yes |
| 9 | 82 | Female | 6 | 1 | 8 | 17 | Venous | 4/8 | Yes |
CARE, Emergency and scheduled health care in the 12 months prior to inclusion in the off-label protocol; SUR, Previous vascular surgery; AET, Aetiology of the vascular ulcer; CN, Case number; NoC, Number of comorbidities; NoU, Number of ulcers; SURF, Surface area of the ulcer prior to treatment (cm2); DCOLP, Duration of condition prior to inclusion in the off-label protocol (months).
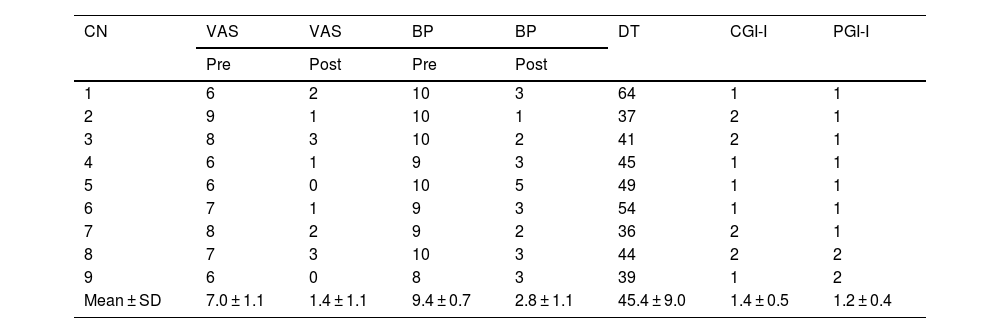
Table 2 shows pre-treatment values, analgesic response to baseline and breakthrough pain related to pre- and post-treatment cleaning, and patient and clinician satisfaction with the therapy.
Analgesic response to topical sevoflurane treatment.
| CN | VAS | VAS | BP | BP | DT | CGI-I | PGI-I |
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||
| 1 | 6 | 2 | 10 | 3 | 64 | 1 | 1 |
| 2 | 9 | 1 | 10 | 1 | 37 | 2 | 1 |
| 3 | 8 | 3 | 10 | 2 | 41 | 2 | 1 |
| 4 | 6 | 1 | 9 | 3 | 45 | 1 | 1 |
| 5 | 6 | 0 | 10 | 5 | 49 | 1 | 1 |
| 6 | 7 | 1 | 9 | 3 | 54 | 1 | 1 |
| 7 | 8 | 2 | 9 | 2 | 36 | 2 | 1 |
| 8 | 7 | 3 | 10 | 3 | 44 | 2 | 2 |
| 9 | 6 | 0 | 8 | 3 | 39 | 1 | 2 |
| Mean ± SD | 7.0 ± 1.1 | 1.4 ± 1.1 | 9.4 ± 0.7 | 2.8 ± 1.1 | 45.4 ± 9.0 | 1.4 ± 0.5 | 1.2 ± 0.4 |
CGI-I, Clinical Global Impression Scale Improvement since start of treatment (1 = Very much improved, 2 = Much improved, 3 = Minimally improved, 4 = No change, 5 = Minimally worse, 6 = Much worse, 7 = Very much worse); BP Post, Breakthrough pain related to cleaning after start of treatment with topical sevoflurane; BP Pre, Breakthrough pain related to cleaning prior to the start of treatment with topical sevoflurane; VAS Pre, Visual Analogue Scale prior to treatment with sevoflurane; VAS Post, Visual Analogue Scale after start of treatment with sevoflurane; PGI-I, Patient Global Impression Scale Improvement since the start of treatment (same scale as CGI-I); DT, Duration of treatment with topical sevoflurane.
In all cases, resting pain underwent rapid reductions (3.1±2.1 min), intense reductions (7.0±1.1 to 1.4±1.1 points), and long-lasting reductions (6–24 h). The intensity of the analgesic effect allowed debridement to be completed with an 80% decrease on the numerical pain scale (Table 2). All debridements were completed, except for one which required the use of transmucosal fentanyl. The interval between the application of sevoflurane and the onset of a sufficiently strong analgesic effect to start debridement was 4 min (range 2–8 min). The patients' subjective sensation of post-debridement analgesia lasted 8.5 h. In addition, the reduction in pain achieved by sevoflurane facilitated a decrease in the use of analgesics.
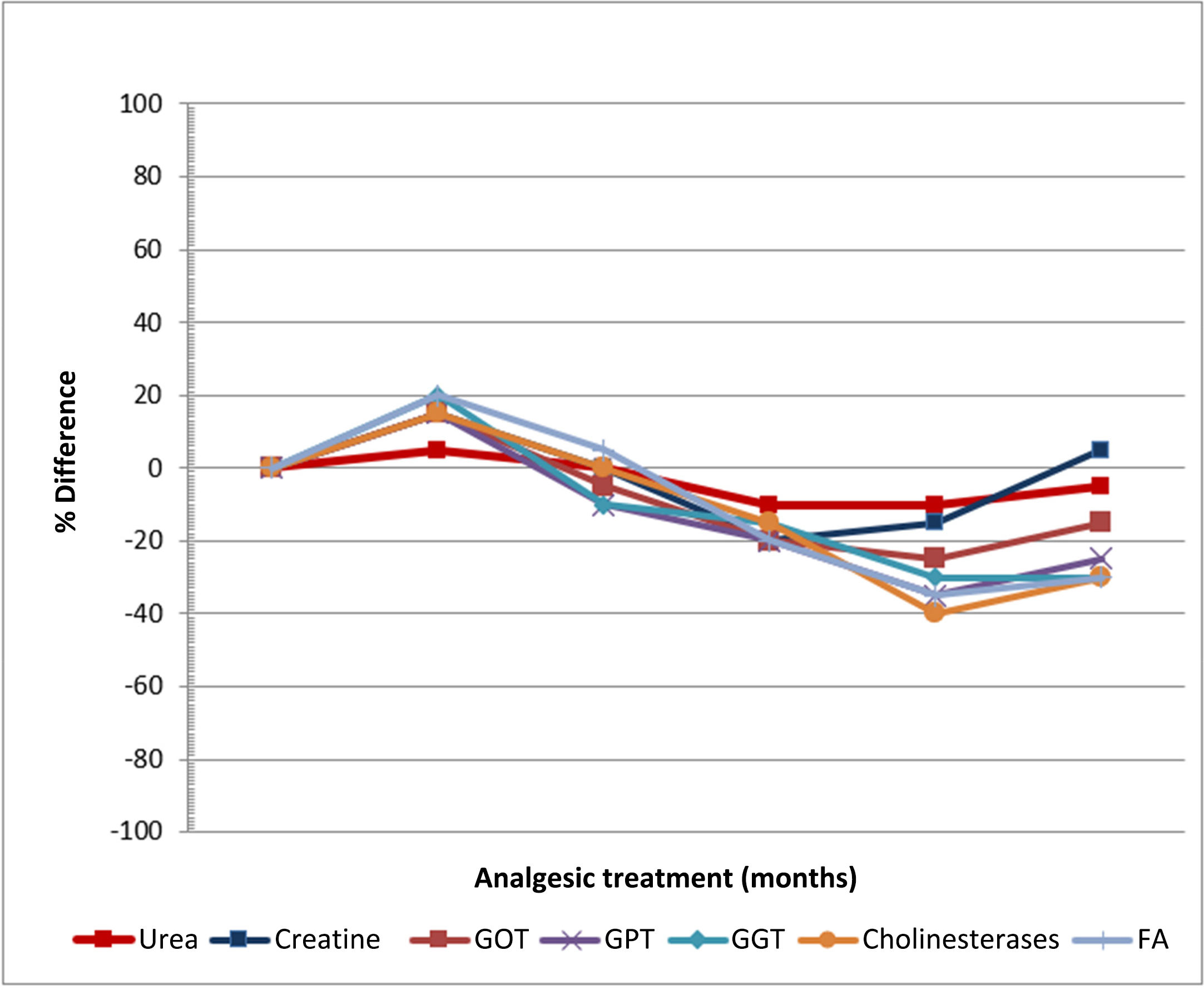
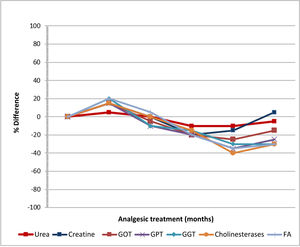
The only adverse effects were itching at the wound edges and irritation of the surrounding skin in 2 patients, which occurred with repeated applications. Fig. 1 shows the evolution of the analytical parameters over the study period. No abnormalities in haematological, renal, or hepatic function were observed.
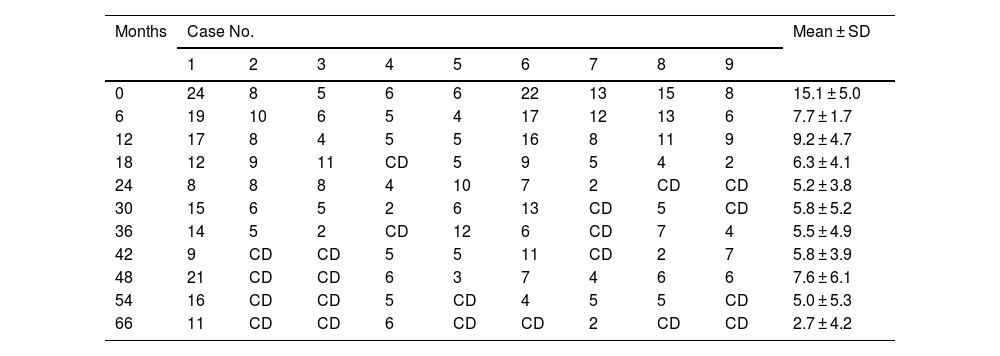
Table 3 shows variations in VU surface area, as well as clinical discharge and recurrence. No increase was observed in drug tolerance or sensitisation over the study period. After 66 months of treatment, there was a reduction in VU surface area (15.1±5.0 to 2.7±4.2 cm2), in all patients except in the case of patient 4. Of note, the Pain Unit discharged 57.14% of the patients as cured (excluding cases 2 and 9 due to death).
Variation in vascular ulcer surface area (cm2) after starting topical treatment with sevoflurane.
| Months | Case No. | Mean ± SD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| 0 | 24 | 8 | 5 | 6 | 6 | 22 | 13 | 15 | 8 | 15.1 ± 5.0 |
| 6 | 19 | 10 | 6 | 5 | 4 | 17 | 12 | 13 | 6 | 7.7 ± 1.7 |
| 12 | 17 | 8 | 4 | 5 | 5 | 16 | 8 | 11 | 9 | 9.2 ± 4.7 |
| 18 | 12 | 9 | 11 | CD | 5 | 9 | 5 | 4 | 2 | 6.3 ± 4.1 |
| 24 | 8 | 8 | 8 | 4 | 10 | 7 | 2 | CD | CD | 5.2 ± 3.8 |
| 30 | 15 | 6 | 5 | 2 | 6 | 13 | CD | 5 | CD | 5.8 ± 5.2 |
| 36 | 14 | 5 | 2 | CD | 12 | 6 | CD | 7 | 4 | 5.5 ± 4.9 |
| 42 | 9 | CD | CD | 5 | 5 | 11 | CD | 2 | 7 | 5.8 ± 3.9 |
| 48 | 21 | CD | CD | 6 | 3 | 7 | 4 | 6 | 6 | 7.6 ± 6.1 |
| 54 | 16 | CD | CD | 5 | CD | 4 | 5 | 5 | CD | 5.0 ± 5.3 |
| 66 | 11 | CD | CD | 6 | CD | CD | 2 | CD | CD | 2.7 ± 4.2 |
CD, Clinical discharge from the Pain Unit due to successful treatment; M ± SD, Mean ± Standard Deviation. Cases 2 and 9 were excluded from statistical calculations following their death.
The main finding of this study is that the use of liquid sevoflurane to irrigate chronic and treatment-resistant VU produces a rapid, intense, and long-lasting analgesic effect. It also has a good safety profile and is well received by patients, making it suitable in outpatient settings over a prolonged period. Our results confirm those of Geronimo et al.12 and Martinez et al.13 regarding the strong analgesic effect of its topical application on ulcerative lesions. The duration of the analgesic effect is variable, but usually lasts for hours. Debridements reduce VU infections and improve the results of skin grafts14.
Earlier publications have documented a cumulative total of 1,850 days of treatment, whereas our series of just 9 patients has a cumulative total of approximately 12, 270 days of treatment.
Other studies have shown that the application of sevoflurane significantly reduces the area of ulcers and the time needed for their re-epithelialisation. Our own study supports these findings: however, we noted that prolonged use was needed due to the high rate of chronicity and recurrence of ulcerative venous disease. This aspect could also be attributed to the characteristics of the off-label protocol approved by our hospital, which only includes patients with chronic critical ischaemia without the option of revascularisation and without adequate pain control with conventional therapies.
The most commonly used therapeutic options are EMLA anaesthetic cream and the topical application of ibuprofen. Sevoflurane only takes a few minutes to take effect, whereas EMLA cream requires 30–45 min. EMLA has also been associated with delayed healing and a non-negligible rate of local adverse effects such as burning and itching4. Topical application of ibuprofen has a milder analgesic effect and has a Number Needed to Treat (NNT) of 6 (95% confidence interval: 4–12)4.
In our case series, the initiation of sevoflurane treatment was associated with improved quality of life and a marked reduction in analgesic usage. These results are in line with those of Cortiñas-Saenz et al.9, who demonstrated improved pain management and reduced opioid consumption following the initiation of topical sevoflurane therapy. The decreased need for high doses of non-steroidal anti-inflammatory drugs and opioids may help to avoid secondary effects and preserve gastric, renal, and hepatic function.
In summary, the topical application of sevoflurane could be a novel therapeutic option for pain management in VU patients due to its rapid and intense action as an analgesic and re-epithelialising agent. Furthermore, it has a good safety profile and improves the patients' quality of life.
Contribution to the scientific literatureThis work is novel in that no other study in the scientific literature on this topic covers such a long follow-up period. This study addresses a novel analgesic treatment of interest for patients with VU that is relevant to daily clinical practice. It is aimed at healthcare professionals involved in their treatment, in which hospital pharmacists play a key role. We present the results obtained by our chronic pain treatment unit on the use of topical sevoflurane in patients with VU, who experience challenging pain control issues.
Presentation at congressesA report containing the results of 5 patients was sent to the European Association of Hospital Pharmacists (EAHP) in Lisbon in March 2023.
FundingNone declared.
Ethical responsibilitiesThe study was authorised by the hospital's Clinical Research Ethics Committee.
ContributionsAll authors contributed equally to the study.