Fixed-dose combinations of antiretroviral drugs have meant an important step forward in simplifying treatment and improving compliance and has led to an increased effectiveness of therapy, a viral load decrease and improving the quality of life of patients.
The single-table formulation of dolutegravir with abacavir and lamivudine (DTG/ABC/3TC) is a highly efficacious and well-tolerated once-daily regimen for HIV-infected patients. The objective of the study was to assess the incremental cost-utility ratio of the fixed-dose combination of (DTG/ ABC/3TC) versus the combinations emtricitabine/tenofovir/efavirenz (FTC/TDF/EFV), and darunavir/r (DRV/r) or raltegravir (RAL) with emtrici- tabine/tenofovir (FTC/TDF) or abacavir/lamivudine (ABC/3TC) as initial antiretroviral therapy in patients infected with HIV-1 from the perspective of the Spanish National Health System.
MethodThe ARAMIS model, which uses a microsimulation approach to simulate the individual changes in each patient from the start of treatment to death through a Markov chain of descriptive health states of the disease, was adapted to Spain. The alternatives used for comparison were the fixed-dose combination of emtricitabine/tenofovir/efavirenz (FTC/TDF/ EFV), and the fixed-dose combinations of emtricitabine/tenofovir (FTC/
TDF) or abacavir/lamivudine (ABC/3TC) with darunavir/r (DRV/r) or ral- tegravir (RAL). The probability of achieving virological suppression by the treatments included in the model was obtained from clinical trials SINGLE, SPRING-2 and FLAMINGO and the costs were expressed in € (2015). The model use the perspective of the Spanish National Health System, with a lifetime horizon and a discount rate of 3% was applied to cost and effectiveness.
ResultsTreatment initiation with DTG/ABC/3TC was dominant when it was compared with treatment initiation with all the comparators: vs. FTC/TDF/EFV (-67 210.71€/QALY), vs. DRV/r + FTC/TDF or ABC/3TC (-1 787 341.44€/QALY), and vs. RAL + FTC/TDF or ABC/3TC (-1 005 117.13€/QALY). All the sensitivity analyses performed showed the consistency of these findings.
ConclusionsWith the premises considered, treatment initiation with DTG/ABC/3TC STR appears to be the most cost-effective option in ARTnaive HIV infected patients from the Spanish Health System perspective
Las combinaciones a dosis fijas de medicamentos antirretro- virales han significado un importante paso adelante en la simplificación del tratamiento y la mejora del cumplimiento, así como hacia una mayor eficacia de la terapia, una disminución de la carga viral y una mejora de la calidad de vida de los pacientes.
La formulación de un comprimido único una vez al día con dosis fijas de dolutegravir, abacavir y lamivudina (DTG/ABC/3TC) para pacientes infectados con VIH es un régimen altamente eficaz y bien tolerado. El objetivo del estudio fue evaluar la relación coste-utilidad incremental de la combinación de dosis fija de (DTG/ABC/3TC) versus las combinaciones de emtricitabina/tenofovir/efavirenz (TDF/FTC/EFV) y darunavir/r (DRV/r) o raltegravir (RAL) con emtricitabina/tenofovir (FTC/TDF) o aba- cavir/lamivudina (ABC/3TC) como tratamiento antirretroviral inicial en pacientes infectados con VIH-1 desde la perspectiva del Sistema Nacional de Salud Español.
MétodoSe adaptó en España el modelo ARAMIS. Este utiliza un enfoque de microsimulación para emular los cambios individuales en cada paciente desde el inicio del tratamiento hasta su muerte mediante una cadena de Markov de estados de salud descriptivos de la enfermedad. Las alternativas empleadas para la comparación fueron la combinación de dosis fijas de emtricitabina/tenofovir/efavirenz (TDF/FTC/EFV) y las combinaciones de dosis fijas de emtricitabina/tenofovir (FTC/TDF) o abaca- vir/lamivudina (ABC/3TC) con darunavir/r (DRV/r) o raltegravir (RAL). La
probabilidad de lograr la supresión virológica mediante los tratamientos incluidos en el modelo se ha obtenido de ensayos clínicos individuales, SPRING2 y FLAMINGO, y los costes fueron expresados en € (2015). El uso del modelo de la perspectiva del Sistema Nacional de Salud español, con un horizonte de vida útil y una tasa de descuento del 3% se, aplicó a coste y efectividad.
ResultadosEl inicio de tratamiento con DTG/ABC/3TC fue dominante cuando se comparó con el inicio del tratamiento con el resto de comparadores: frente a TDF/FTC/EFV (-67.210,710 € / AVAC) vs DRV/r FTC/TDF o ABC/3TC (-1,787,341.44 € / AVAC) y vs RAL FTC/TDF o ABC/3TC (-1,005,117.13 € / AVAC). Todos los análisis de sensibilidad realizados demostraron la consistencia de estos hallazgos.
ConclusionesCon las premisas consideradas, el inicio del tratamiento con la combinación a dosis fijas de DTG/ABC/3TC parece ser la opción más rentable para el tratamiento de pacientes infectados con el VIH desde la perspectiva del Sistema Nacional de Salud español.
The natural history of infection by human immunodeficiency virus (HIV) is characterized by a progressive decrease of CD4+ cells and immune function, promoting the occurrence of infections and AIDS-defining malignancies. It is estimated that there are 36.7 million HIV-infected people worldwide and that 1.1 million deaths occur annually from the disease1. In Spain, it affects about 148,785 people2 and its estimated annual mortality is approximately 845 deaths per year3.
Since the introduction of highly active antiretroviral therapy (HAART) as a combination of three antiretroviral drugs, control and maintenance of the disease has been achieved in HIV-infected patients and morbidity and mortality have markedly decreased, converting HIV infection into a chronic disease4.
Although Spanish guidelines have been recommending an initial treatment including two nucleoside reverse transcriptase inhibitors (NRTIs) combined with an integrase inhibitor (INI), a non-nucleoside reverse transcriptase inhibitor (NNRTI) or a protease inhibitor boosted with ritonavir (PI/r), currently only INI-based regimens are preferentially recommended5.
Fixed-dose combinations of antiretroviral drugs have meant an important step forward in simplifying treatment and improving compliance. Better compliance has led to a lower risk of treatment errors and decreased resistance selection, which results in an increased effectiveness of therapy with the consequent decrease in viral load and improvement in quality of life of patients6.
Dolutegravir (DTG) is an integrase inhibitor whose clinical development has shown good tolerability and safety, a high barrier to resistance and a lack of relevant drug interactions. The single-tablet formulation with abacavir and lamivudine (DTG/ABC/3TC) obtained European approval in September 2014 and has been marketed in Spain since May 2015.
To assist in the inclusion of the drug on the formulay, the study objective was to assess the incremental cost-utility ratio (ICUR) in €/QALY of the fixed-dose combination of (DTG/ABC/3TC) versus the combinations Emtrici- tabine/Tenofovir/Efavirenz (FTC/TDF/EFV), and darunavir/r (DRV/r) or Ralte- gravir (RAL) with Emtricitabine/Tenofovir (FTC/TDF) or Abacavir/Lamivudine (ABC/3TC) as initial antiretroviral therapy in patients infected with HIV-1 from the perspective of the Spanish National Health System.
MethodsDescription of the modelThe study consisted of adaptation to Spain of the Anti-Retroviral Analysis by Monte Carlo Individual Simulation (ARAMIS) model7,8, which uses a microsimulation approach to simulate the individual changes in each patient from the start of treatment to death through a chain of descriptive health states of the disease that are mutually exclusive (the patient can only be in one state). Thus, by including the behaviour of each individual generated with baseline characteristics of patients from clinical trials of DTG in naive patients (SINGLE, FLAMINGO and SPRING-2 trials), the model is able to show the biological variability of the responses that may occur, which is an advantage over typical aggregate approaches such as the Markov models in which the aggregate variables only represent the behaviour of the population mean.
The alternatives used for comparison were the fixed-dose combination of Emtricitabine/Tenofovir/Efavirenz (FTC/TDF/EFV), and the fixed-dose combinations of Emtricitabine/Tenofovir (FTC/TDF) or Abacavir/Lamivudine (ABC/3TC) with Darunavir/r (DRV/r) or Raltegravir (RAL). Thus was obtained the comparing of DTG/ABC/3TC versus standard treatments with NRTIs, INI and a PI/r as a third agent.
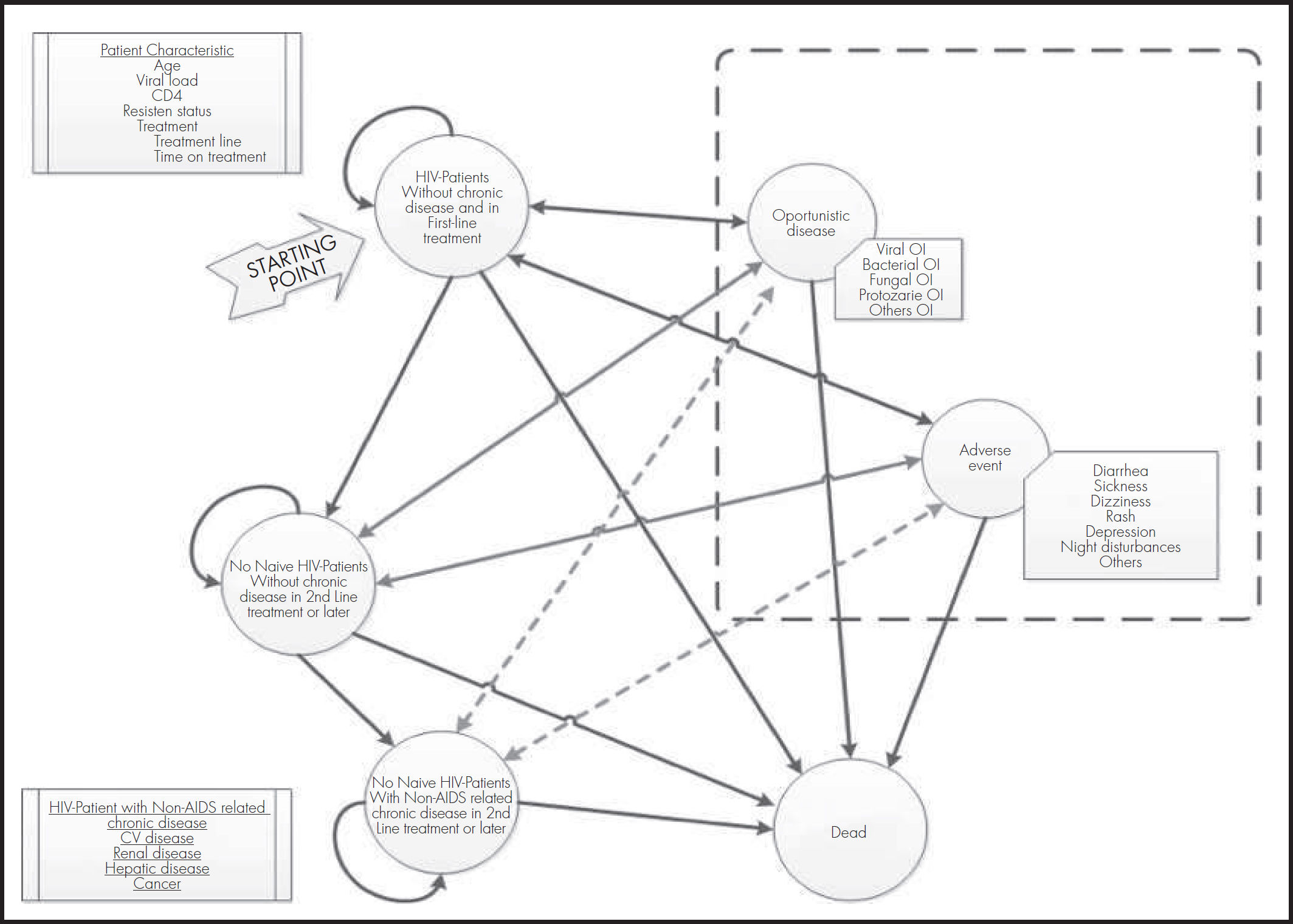
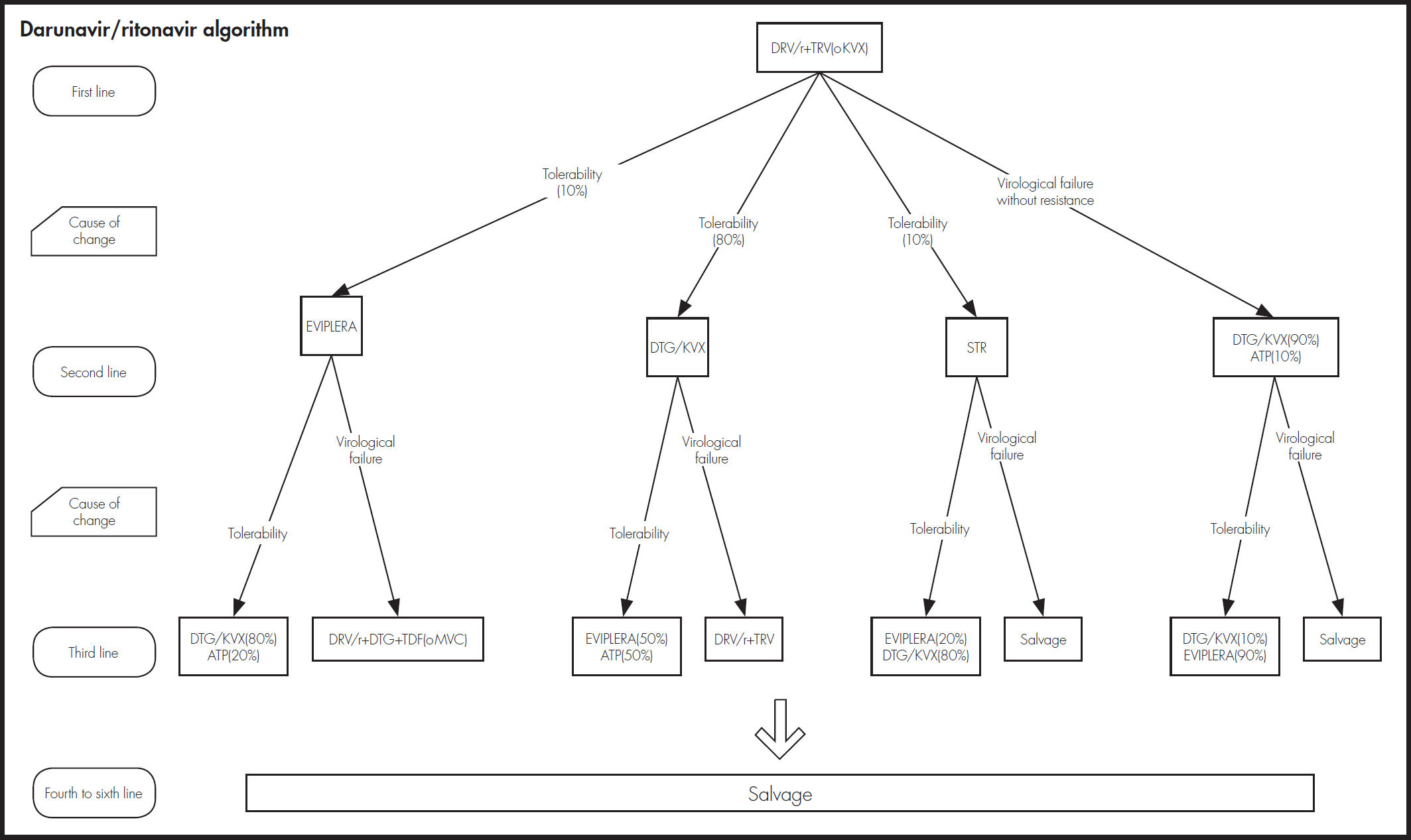
As shown in figure 1, the model defined the following health states: HIV infection without chronic disease as first-line treatment, HIV infection without chronic disease as second-line or subsequent treatment, HIV infection for chronic disease non-related to AIDS (second-line or subsequent treatment), opportunistic disease (viral, bacterial, fungal, protozoan or other), adverse event to the treatment and death (absorbing state).
Each month throughout the simulation and depending on the treatment received, the changes in patient characteristics are determined and if these changes cause the individual to remain in the initial state or change to a new one. These changes are determined by the probability of disease progression, occurrence of opportunistic infections and/or adverse effects, and occurrence of long-term chronic diseases and death. All these probabilities are determined by the CD4+ count the patient has at the start of each cycle.
Thus, depending on the treatment received, the individual will show a given probability of achieving viral suppression (defined as viral load suppression below 50 copies/mL at 48 weeks). This leads to an increase in the CD4+ count that is more marked in the first two months of treatment but which is maintained if viral suppression persists, reaching a maximum values of 1,200 cells/pl. If virological suppression is not achieved with treatment initially, or if it is lost subsequently, the individual moves on the next line of treatment and so on. Once treatment options have been exhausted, the individual experiences a decrease in the CD4+ count as described in previously published models9.
The model includes nine categories of adverse events that individuals may experience during treatment (diarrhoea, nausea, dizziness, vomiting, rash, sleep disturbances, insomnia, depression and other). The probability of experiencing these events in grade 2 or higher and of discontinuing treatment due to the events was obtained from their clinical trials.
The occurrence of opportunistic infections was modelled as a probability dependent on the CD4+ count as described in the literature7.
Cardiovascular disease was modelled as a monthly risk determined by a Framingham equation10 for prediction of coronary heart disease and stroke.
In addition to mortalities due to opportunistic infections and cardiovascular diseases, all-cause mortality and HIV mortality were modelled based on Spanish data from interactive consultation of the National Health System Statistical Portal11.
Thus, at the end of each cycle, a specific effectiveness is obtained and the corresponding allocation of costs generated in the period. These are cumulative throughout the life of each simulated individual and finally allow for obtaining the incremental cost-effectiveness ratio (ICER) of DTG/ABC/3TC versus other treatment options using the formula:
The model was developed in Microsoft Excel 2007 and Microsoft Visual Basic for Applications as embedded code. The results shown correspond to 1 million simulated individuals, from the perspective of the Spanish national health system. The base case was performed for a time horizon of the patient's whole lifetime and applying a discount rate of 3%.
Model parameters
Study populationInitially patients were described with demographic variables (age, sex), disease defining parameters (plasma viral load, CD4+ cell count), and covariates used for measuring cardiovascular risk in the Framingham equation (systolic pressure, total cholesterol, high-density lipoprotein [HDL] cholesterol, history of smoking, diabetes and left ventricular hypertrophy).
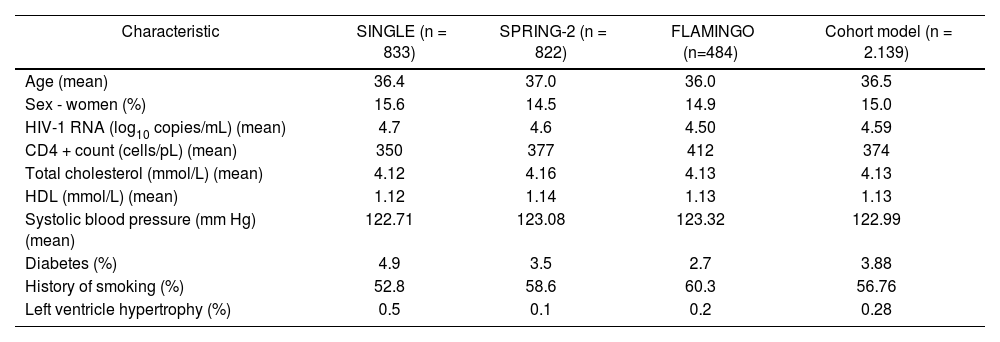
Table 1 shows a summary of the baseline characteristics used in the model. These were obtained from patients participating in the SINGLE, SPRING-2 and FLAMINGO clinical trials of DTG in treatment-naive patients.
Baseline characteristics of the patients included in the model
| Characteristic | SINGLE (n = 833) | SPRING-2 (n = 822) | FLAMINGO (n=484) | Cohort model (n = 2.139) |
|---|---|---|---|---|
| Age (mean) | 36.4 | 37.0 | 36.0 | 36.5 |
| Sex - women (%) | 15.6 | 14.5 | 14.9 | 15.0 |
| HIV-1 RNA (log10 copies/mL) (mean) | 4.7 | 4.6 | 4.50 | 4.59 |
| CD4 + count (cells/pL) (mean) | 350 | 377 | 412 | 374 |
| Total cholesterol (mmol/L) (mean) | 4.12 | 4.16 | 4.13 | 4.13 |
| HDL (mmol/L) (mean) | 1.12 | 1.14 | 1.13 | 1.13 |
| Systolic blood pressure (mm Hg) (mean) | 122.71 | 123.08 | 123.32 | 122.99 |
| Diabetes (%) | 4.9 | 3.5 | 2.7 | 3.88 |
| History of smoking (%) | 52.8 | 58.6 | 60.3 | 56.76 |
| Left ventricle hypertrophy (%) | 0.5 | 0.1 | 0.2 | 0.28 |
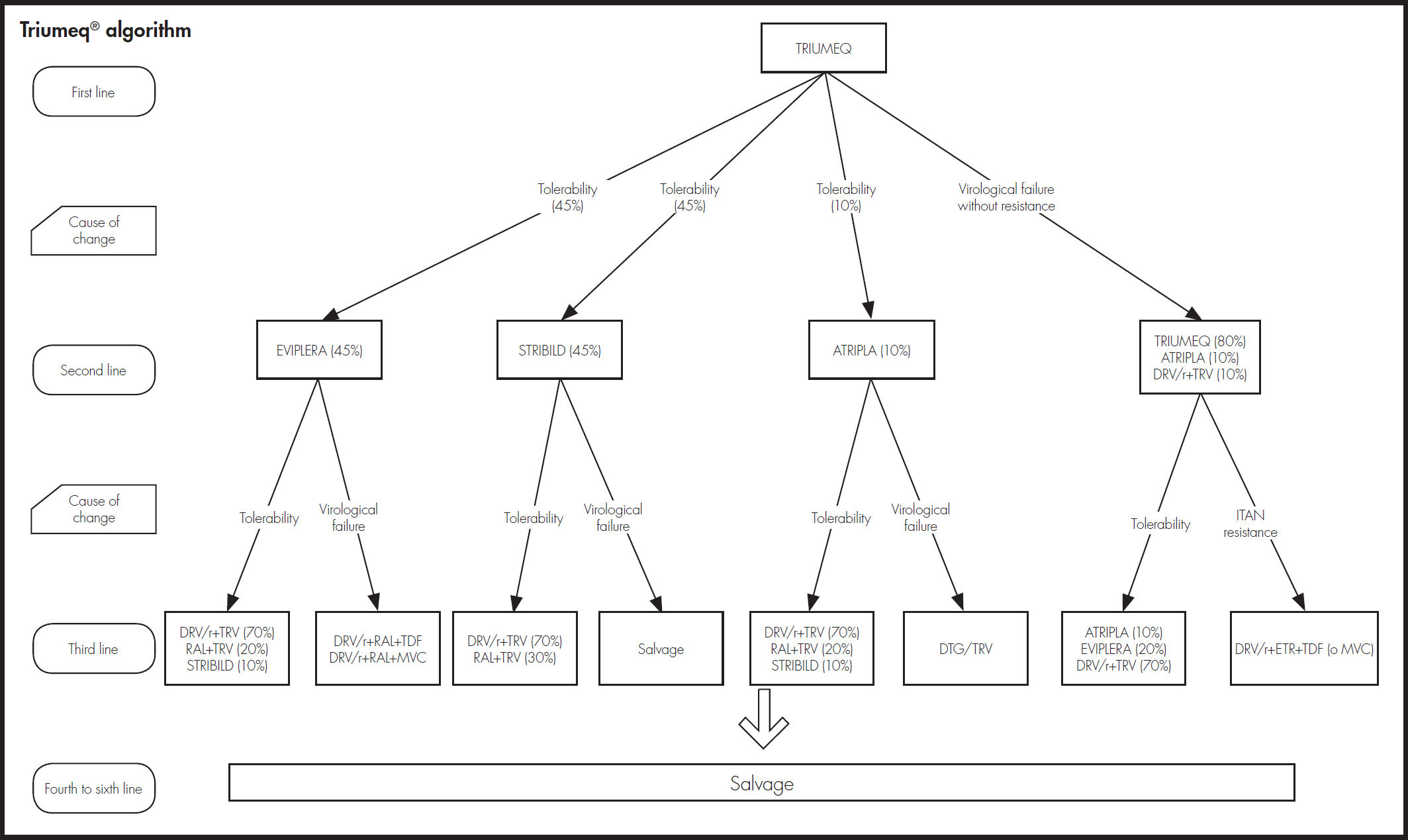
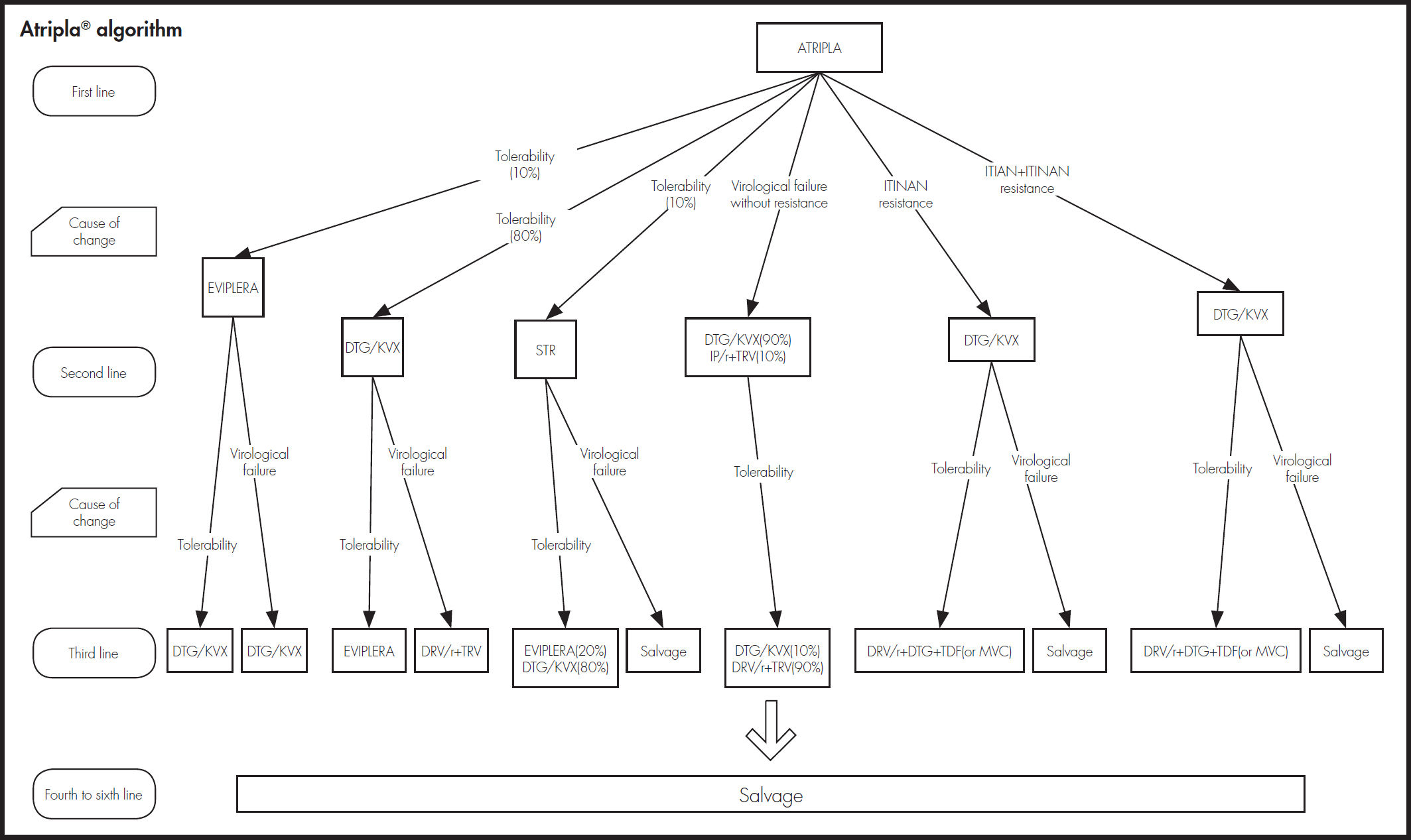
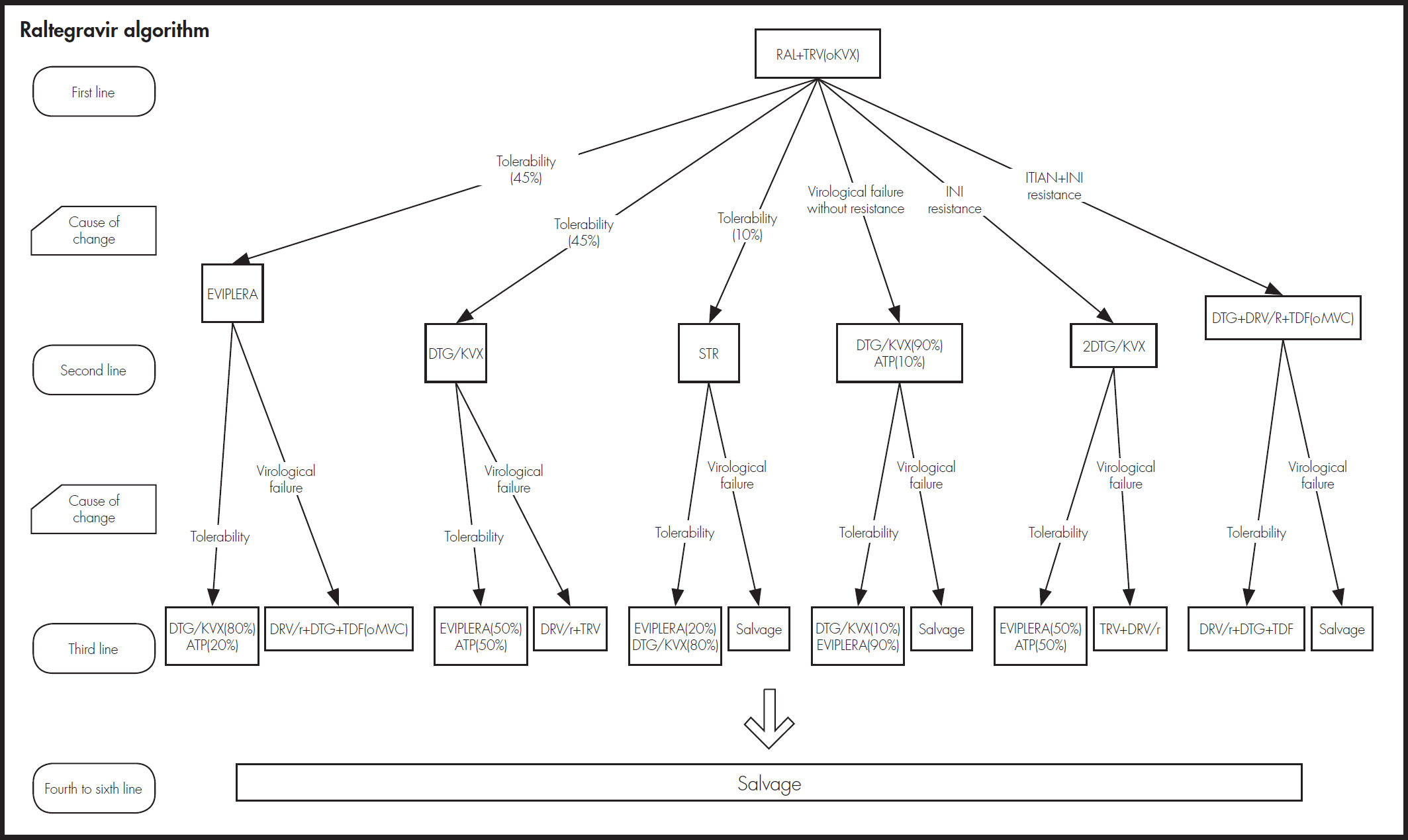
As shown in figure 2, an expert panel determined the four treatment algorithms, one for each initial treatment. The choice of treatment in the second and subsequent lines was made attempting to prevent problems of tolerability and resistance that could compromise efficacy. The model took into account resistance to NRTIs, NNRTIs, and INI.
Estimation of effectivenessThe probability of achieving virological suppression by the treatments included in the model was obtained from clinical trials comparing DTG/ ABC/3TC versus the other alternatives, SINGLE trial for FTC/TDF/EFV12,13, SPRING-2 trial14,15 for RAL + (FTC/TDF) or + (ABC/3TC) and FLAMINGO trial16,17 for DRV/r + (FTC/TDF) or + (ABC/3TC). Based on their clinical practice, the expert panel considered that the proportions for applying FTC/ TDF and ABC/3TC in these two alternatives are 80 and 20, respectively. Since there are no direct trials of DTG/ABC/3TC versus Rilpivirine/Emtrici- tabine/Tenofovir (RPV/FTC/TDF) and Emtricitabine/Tenofovir/ Elvitegravir/ Cobicistat (FTC/TDF/EVG/cob), the results used for comparison of these regimens were obtained by a network meta-analysis18. The evidence used for showing the long-term efficacy of all treatments was the STARTMRK trial19,20 and assumptions validated by the expert panel.
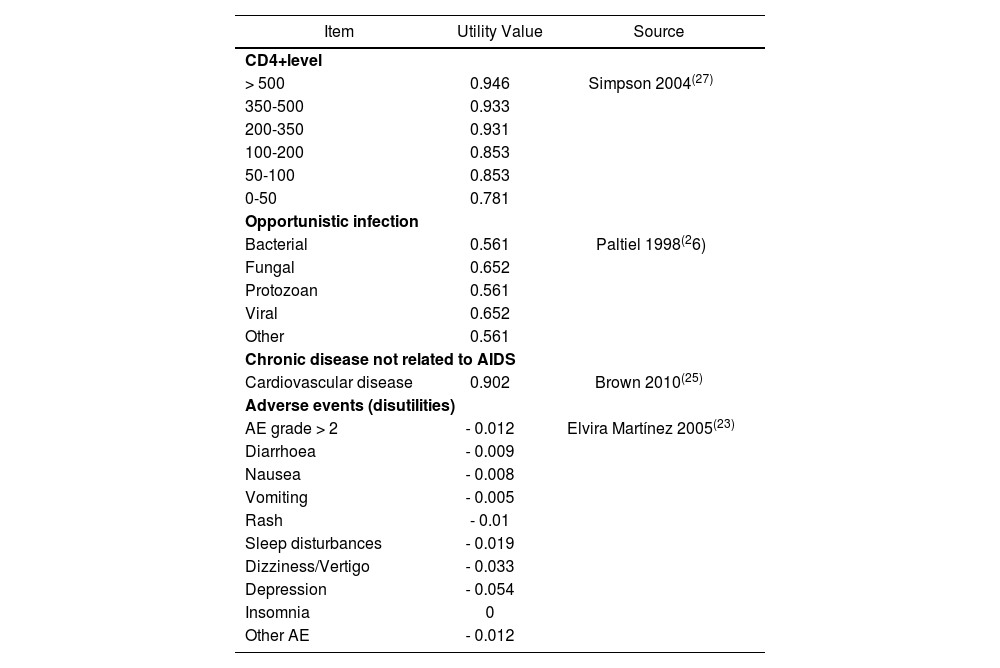
The efficacy of treatment alternatives was measured as life years gained (LYG) and quality-adjusted life years (QALYs) by applying the corresponding utilities associated with CD4+ level, having an opportunistic infection and its sequelae, having an adverse event and those associated with chronic diseases not related to AIDS. These utilities shown in table 2 were obtained from the literature7,21-24.
Utilities used in the model
| Item | Utility Value | Source |
|---|---|---|
| CD4+level | ||
| > 500 | 0.946 | Simpson 2004(27) |
| 350-500 | 0.933 | |
| 200-350 | 0.931 | |
| 100-200 | 0.853 | |
| 50-100 | 0.853 | |
| 0-50 | 0.781 | |
| Opportunistic infection | ||
| Bacterial | 0.561 | Paltiel 1998(26) |
| Fungal | 0.652 | |
| Protozoan | 0.561 | |
| Viral | 0.652 | |
| Other | 0.561 | |
| Chronic disease not related to AIDS | ||
| Cardiovascular disease | 0.902 | Brown 2010(25) |
| Adverse events (disutilities) | ||
| AE grade > 2 | - 0.012 | Elvira Martínez 2005(23) |
| Diarrhoea | - 0.009 | |
| Nausea | - 0.008 | |
| Vomiting | - 0.005 | |
| Rash | - 0.01 | |
| Sleep disturbances | - 0.019 | |
| Dizziness/Vertigo | - 0.033 | |
| Depression | - 0.054 | |
| Insomnia | 0 | |
| Other AE | - 0.012 | |
While quality of life stratified by CD4 was calculated in each cycle in which the patient was in the health state, in the case of opportunistic infections, it was calculated in three cycles, assuming that this opportunistic infection had a duration of three months. The effect of adverse events was applied in the cycle in which they occurred, except for cases of depression, whose disutility was imputed over a period of three cycles (3 months).
Resources and costsAs the model uses the perspective of the Spanish National Health System, only direct health care costs expressed in € (2015) were considered.
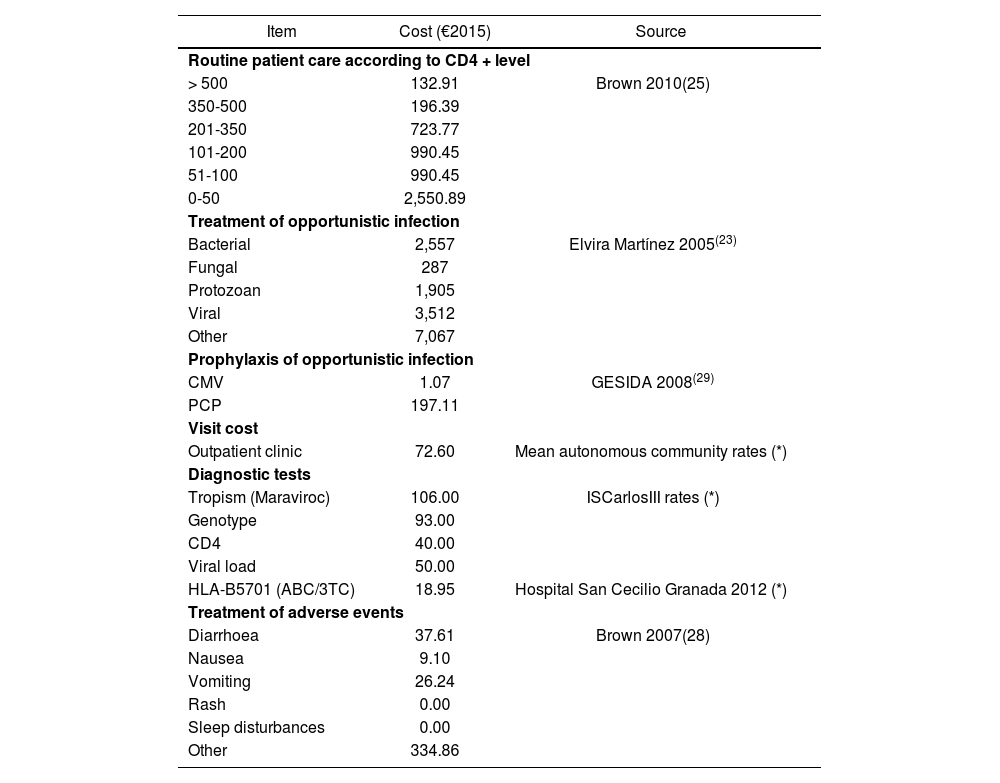
Thus, standard patient care costs were considered such as those derived from medical visits, diagnostic tests, management of adverse events, care of chronic diseases and drug costs of antiretroviral therapy and prophylaxis and treatment of opportunistic infections.
Table 3 shows the costs attributable to each event and health state from the official rates and were calculated from the clinical trial protocols and stratified by CD4+ levels22. The costs of diagnostic tests come from the rates of the Instituto de Salud Carlos III. The costs derived from treatment of adverse events for Spain were obtained from the literature25 and the costs derived from the prophylaxis and treatment of opportunistic infections from the BotPlus-Portsalfarma database 2.0 of the General Council of Official Pharmacists Associations (https:/botplusweb.portalfarma.com)21,26. The drug acquisition costs of the different antiretroviral therapies by the National Health System were obtained from the SESCAM27.
Monthly costs
| Item | Cost (€2015) | Source |
|---|---|---|
| Routine patient care according to CD4 + level | ||
| > 500 | 132.91 | Brown 2010(25) |
| 350-500 | 196.39 | |
| 201-350 | 723.77 | |
| 101-200 | 990.45 | |
| 51-100 | 990.45 | |
| 0-50 | 2,550.89 | |
| Treatment of opportunistic infection | ||
| Bacterial | 2,557 | Elvira Martínez 2005(23) |
| Fungal | 287 | |
| Protozoan | 1,905 | |
| Viral | 3,512 | |
| Other | 7,067 | |
| Prophylaxis of opportunistic infection | ||
| CMV | 1.07 | GESIDA 2008(29) |
| PCP | 197.11 | |
| Visit cost | ||
| Outpatient clinic | 72.60 | Mean autonomous community rates (*) |
| Diagnostic tests | ||
| Tropism (Maraviroc) | 106.00 | ISCarlosIII rates (*) |
| Genotype | 93.00 | |
| CD4 | 40.00 | |
| Viral load | 50.00 | |
| HLA-B5701 (ABC/3TC) | 18.95 | Hospital San Cecilio Granada 2012 (*) |
| Treatment of adverse events | ||
| Diarrhoea | 37.61 | Brown 2007(28) |
| Nausea | 9.10 | |
| Vomiting | 26.24 | |
| Rash | 0.00 | |
| Sleep disturbances | 0.00 | |
| Other | 334.86 | |
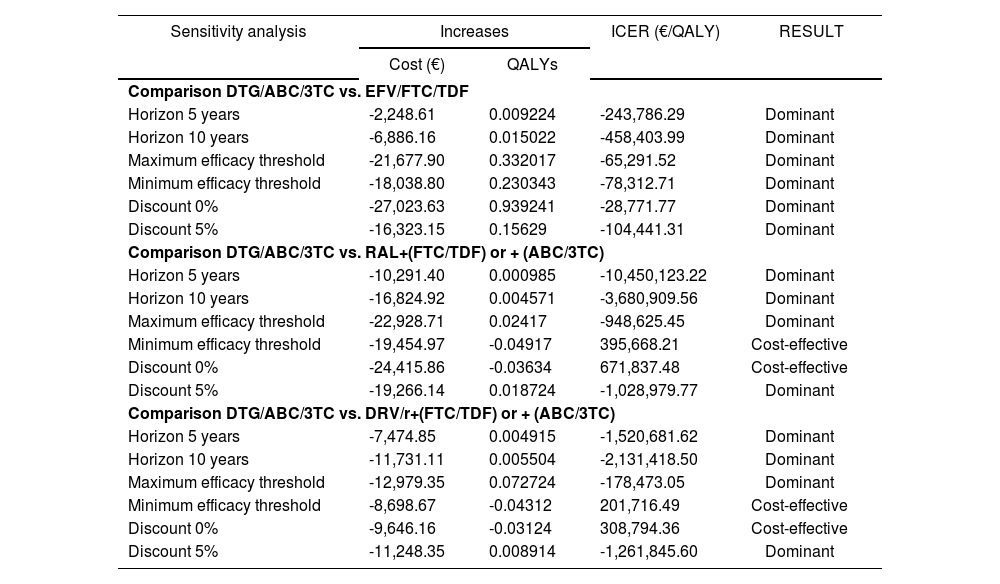
In order to assess the consistency of the model and the effect on its results of the relative efficacy of DTG/ABC/3TC versus its alternatives, the time horizon and discount rate applied, as recommended by the proposed Spanish guidelines for economic evaluation28, the following sensitivity analyses were performed:
Model using a time horizon of 5 and 10 years.
Application of higher relative and lower efficacy using the maximum and minimum values of the confidence intervals of clinical trials12-17.
Model without application of a discount rate and using a rate of 5%.
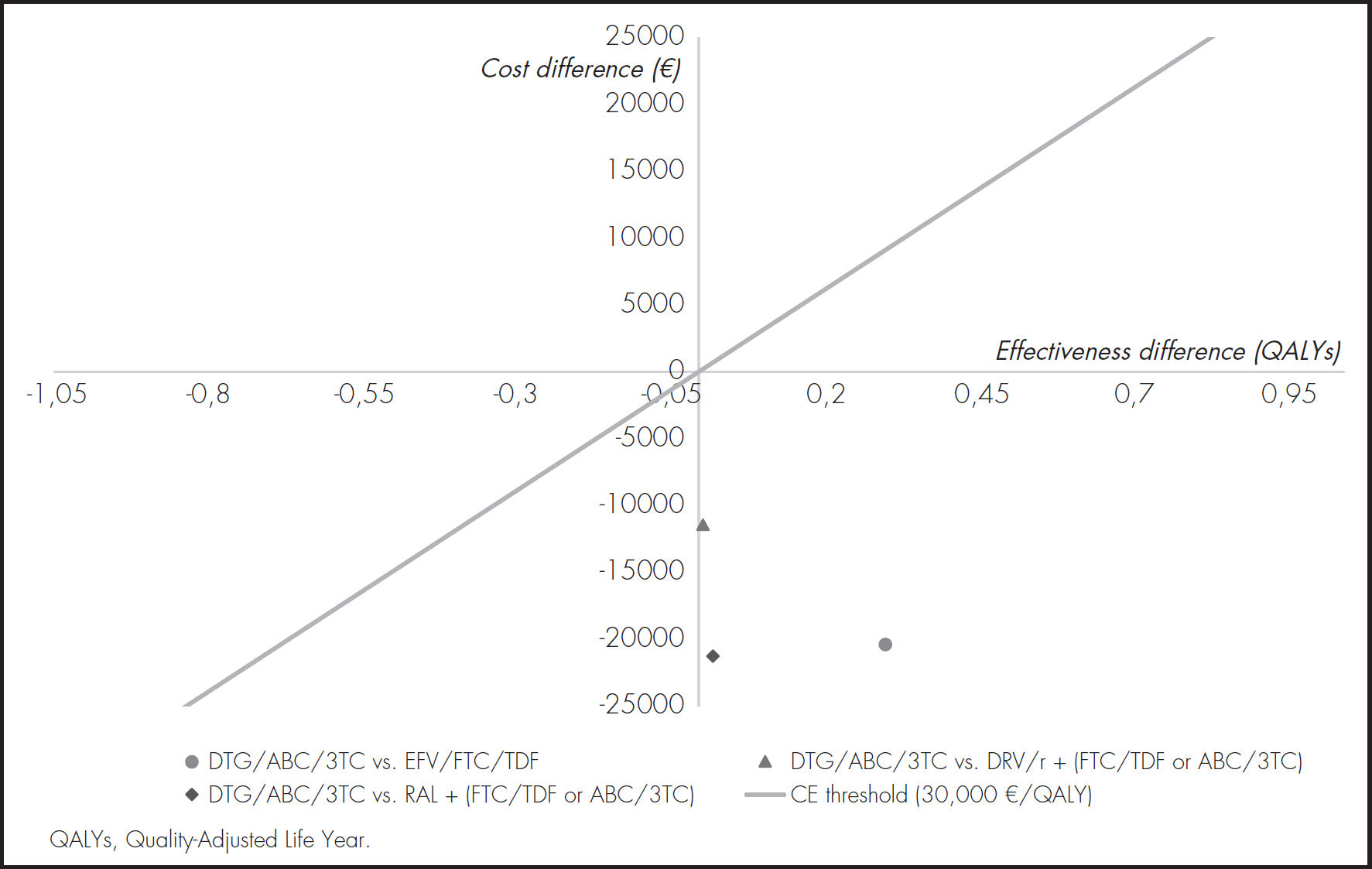
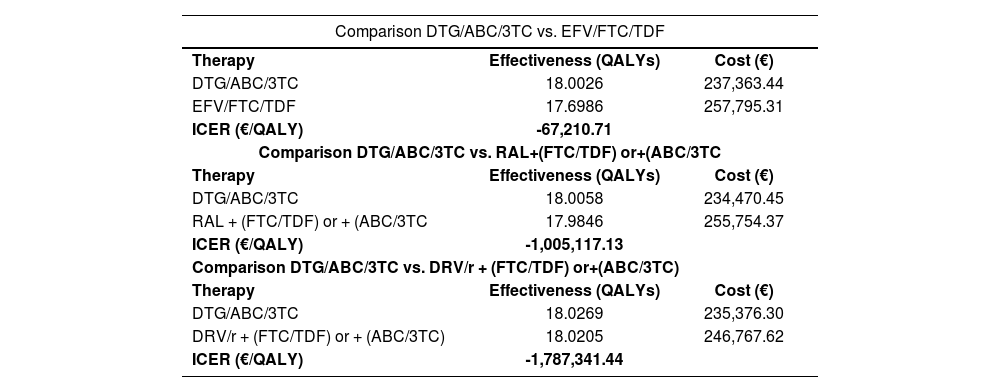
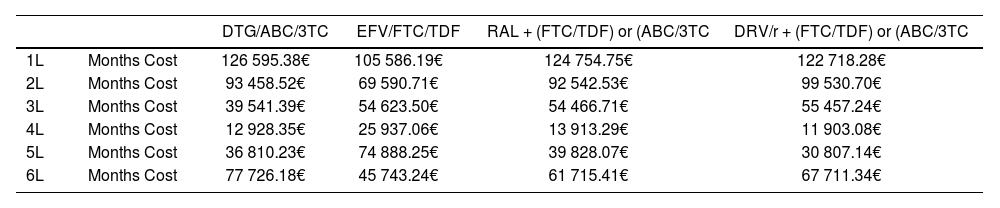
ResultsBase caseOf all the initial treatment regimens studied, DTG/ABC/3TC was the dominant combination by achieving greater effectiveness at a lower cost (Table 4). The incremental cost-effectiveness plane is shown in figure 3. Table 5 shows the duration and average monthly cost of each strategy evaluated.
Cost-effectiveness results of comparisons analyzed
| Comparison DTG/ABC/3TC vs. EFV/FTC/TDF | ||
|---|---|---|
| Therapy | Effectiveness (QALYs) | Cost (€) |
| DTG/ABC/3TC | 18.0026 | 237,363.44 |
| EFV/FTC/TDF | 17.6986 | 257,795.31 |
| ICER (€/QALY) | -67,210.71 | |
| Comparison DTG/ABC/3TC vs. RAL+(FTC/TDF) or+(ABC/3TC | ||
| Therapy | Effectiveness (QALYs) | Cost (€) |
| DTG/ABC/3TC | 18.0058 | 234,470.45 |
| RAL + (FTC/TDF) or + (ABC/3TC | 17.9846 | 255,754.37 |
| ICER (€/QALY) | -1,005,117.13 | |
| Comparison DTG/ABC/3TC vs. DRV/r + (FTC/TDF) or+(ABC/3TC) | ||
| Therapy | Effectiveness (QALYs) | Cost (€) |
| DTG/ABC/3TC | 18.0269 | 235,376.30 |
| DRV/r + (FTC/TDF) or + (ABC/3TC) | 18.0205 | 246,767.62 |
| ICER (€/QALY) | -1,787,341.44 | |
QALYs, Quality-Adjusted Life Year; ICER, Incremental Cost-Effectiveness Ratio.
Duration and average monthly cost per treatment line
| DTG/ABC/3TC | EFV/FTC/TDF | RAL + (FTC/TDF) or (ABC/3TC | DRV/r + (FTC/TDF) or (ABC/3TC | ||
|---|---|---|---|---|---|
| 1L | Months Cost | 126 595.38€ | 105 586.19€ | 124 754.75€ | 122 718.28€ |
| 2L | Months Cost | 93 458.52€ | 69 590.71€ | 92 542.53€ | 99 530.70€ |
| 3L | Months Cost | 39 541.39€ | 54 623.50€ | 54 466.71€ | 55 457.24€ |
| 4L | Months Cost | 12 928.35€ | 25 937.06€ | 13 913.29€ | 11 903.08€ |
| 5L | Months Cost | 36 810.23€ | 74 888.25€ | 39 828.07€ | 30 807.14€ |
| 6L | Months Cost | 77 726.18€ | 45 743.24€ | 61 715.41€ | 67 711.34€ |
L, treatment line.
Table 6 presents the results of the comparisons used in sensitivity analyses. In these comparisons, DTG/ABC/3TC was the dominant combination (representing a lower cost and greater effectiveness) in all cases versus FTC/TDF/EFV.
Results of sensitivity analyses
| Sensitivity analysis | Increases | ICER (€/QALY) | RESULT | |
|---|---|---|---|---|
| Cost (€) | QALYs | |||
| Comparison DTG/ABC/3TC vs. EFV/FTC/TDF | ||||
| Horizon 5 years | -2,248.61 | 0.009224 | -243,786.29 | Dominant |
| Horizon 10 years | -6,886.16 | 0.015022 | -458,403.99 | Dominant |
| Maximum efficacy threshold | -21,677.90 | 0.332017 | -65,291.52 | Dominant |
| Minimum efficacy threshold | -18,038.80 | 0.230343 | -78,312.71 | Dominant |
| Discount 0% | -27,023.63 | 0.939241 | -28,771.77 | Dominant |
| Discount 5% | -16,323.15 | 0.15629 | -104,441.31 | Dominant |
| Comparison DTG/ABC/3TC vs. RAL+(FTC/TDF) or + (ABC/3TC) | ||||
| Horizon 5 years | -10,291.40 | 0.000985 | -10,450,123.22 | Dominant |
| Horizon 10 years | -16,824.92 | 0.004571 | -3,680,909.56 | Dominant |
| Maximum efficacy threshold | -22,928.71 | 0.02417 | -948,625.45 | Dominant |
| Minimum efficacy threshold | -19,454.97 | -0.04917 | 395,668.21 | Cost-effective |
| Discount 0% | -24,415.86 | -0.03634 | 671,837.48 | Cost-effective |
| Discount 5% | -19,266.14 | 0.018724 | -1,028,979.77 | Dominant |
| Comparison DTG/ABC/3TC vs. DRV/r+(FTC/TDF) or + (ABC/3TC) | ||||
| Horizon 5 years | -7,474.85 | 0.004915 | -1,520,681.62 | Dominant |
| Horizon 10 years | -11,731.11 | 0.005504 | -2,131,418.50 | Dominant |
| Maximum efficacy threshold | -12,979.35 | 0.072724 | -178,473.05 | Dominant |
| Minimum efficacy threshold | -8,698.67 | -0.04312 | 201,716.49 | Cost-effective |
| Discount 0% | -9,646.16 | -0.03124 | 308,794.36 | Cost-effective |
| Discount 5% | -11,248.35 | 0.008914 | -1,261,845.60 | Dominant |
Identical behaviour was shown in the comparison with RAL + (FTC/ TDF) or + (ABC/3TC), except in cases of without discounting in which DTG/ ABC/3TC represented a saving of 24,415.86 € for a lower effectiveness of 0.0396 QALYs, and when the lower limit of efficacy was considered for DTG/ABC/3TC, a saving of 19,454.97 € for a lower effectiveness of 0.0511 QALYs. In both cases, the strategy was found to be cost-effective.
Similar results occurred with DRV/r + (FTC/TDF) or + (ABC/3TC), which were shown to be cost-effective in the non-discounted analyses (saving of 9,646.16 € and lower efficacy of 0.036 QALYs) and in the lower limit of efficacy of DTG/ABC/3TC (saving of 8,698.67 € and lower efficacy of 0.049 QALYs). In the rest of the cases, DTG/ABC/3TC was shown to be the dominant alternative.
DiscussionSince the introduction of HAART, control and maintenance of HIV infection has been achieved, decreasing its morbidity and reducing costs associated with its medical care. However, the increased survival produced causes costs of treatment and patient care to occur over a much longer time period, normally causing overall costs to become larger than other treatment alternatives and making it necessary to evaluate its cost-effectiveness.
The ARAMIS model used for this adaptation has been verified and previously used in several studies7-8. This is the first research that uses microsimulation to assess the cost-utility of a VIH fixed dose combination in Spain. The stochastic approach used in this study enabled us to show the biological variability and to determine that the fixed-dose combination DTG/ABC/3TC is the most efficient alternative among the initial treatment regimens evaluated. It was to be the dominant strategy in the comparisons considered as the base case and in 14 of 18 sensitivity analyses, and was cost-effective in the remaining four.
This greater efficiency is determined by the savings derived from starting treatment with DTG/ABC/3TC and because this alternative was the most efficient in all cases, except in the sensitivity analyses without discounting and using the lower efficacy level of the trials with dolutegravir (DTG) when compared to the alternatives RAL and DRV. In these cases, the lower efficacy of the alternative to starting with DTG/ABC/3TC was shown to be marginal with values between -0.04 and 0.03 QALYs, which over the lifetime of the patient are equivalent to a lifetime benefit of between 10 days and two weeks. This marginality of the unprovided benefit causes the savings produced to make the alternative appear cost-effective with respect to the compared alternatives.
This effectiveness is consistent with the proposed treatment algorithms since all alternatives are favoured by having treatments including DTG in subsequent lines. Thus, the effectiveness shown in the first lines of treatments is consistent with the efficacy results of the comparative clinical trials of the treatments evaluated12-17. And this dominance is consistent with the results of previous research compararing DTG with ABC/3TC or FTC/ TDF in Canada29.
As shown in Table 5, this efficiency of the first lines of the alternative to starting with DTG/ABC/3TC is boosted by a lower mean monthly cost of treatment than the other compared strategies. Furthermore, it can be seen how other alternatives are benefited by the use of DTG/ABC/3TC in the second lines of treatment.
Although the model has limitations due to the lack of data on the efficacy of subsequent treatment lines and the costs derived from visits in actual clinical practice, these have been treated conservatively. Thus, the high efficacy applied to treatments after the first failure will mean that patients continue with high CD4+ levels. Hence, the benefit of postponing the change of initial treatment shown in the alternative to starting with DTG/ABC/3TC is masked by the effect of subsequent lines. With regard to the case to imputing only protocol visits, this is also a consequence of a conservative approach because it does not consider unexpected visits occurring in actual clinical practice as a result of adverse effects and interactions, regardless of whether or not it involves discontinuation. It is considered that this assumption could penalize the strategy of starting with DTG/ABC/3TC since it safety profile, tolerability and interactions suggests that this treatment generates a lower number of these visits, which leads to underestimating the costs attributable to the other compared strategies. Finally, another limitation has been that the study has not taken into account the subsequent introduction of the generic ABC/3TC
Cost-utility analyses of these alternatives based on real World data of their clinical effectiveness and associated resource consumption will be recommended in the future.
Since the results obtained with this model are favourable to the strategy of starting with DTG/ABC/3TC, it may be concluded that, this is the most efficient option of the alternatives evaluated for the Spanish National Health System. This result could assist informed decision-making about inclusion of the fixed-dose combination DTG/ABC/3TC into the hospital formularies.