Obesity constitutes a global public health problem, and knowledge about drug dosing in obese patients is limited. Clinical trials in critically ill patients rarely include obese individuals, resulting in a lack of specific dosing information in product data sheets. The aim of this literature review is to provide clinicians with efficient and safe guidelines for this group of patients.
MethodsA multidisciplinary group composed of pharmacists specialised in hospital pharmacy and physicians specialised in intensive care medicine was formed. The therapeutic groups and, in depth, the most commonly used active ingredients in the intensive care unit were identified and reviewed. The bibliographic review was carried out using terms such as: “obese”, “overweight”, “critical illness”, “drug dosification”, and “therapeutic dose monitoring”. All the information was evaluated by the working group, which reached a consensus on dosing recommendations for each drug in obese critically ill patients.
ResultsEighty three drugs belonging to the following therapeutic groups were identified: antivirals, antibacterials, antifungals, immunosuppressants, antiepileptics, vasopressors, anticoagulants, neuromuscular blocking agents, and sedatives. A table with the consensus dosing recommendation for each of these was produced after review.
ConclusionsDrug dosing in obese patients, both in critical and non-critical settings, remains an area with significant uncertainties. This review provides updated and exhaustive information on the dosing of the main therapeutic groups in obese critically ill patients, and is a useful tool for both physicians in critical care units and clinical pharmacists in their practice in this setting.
La obesidad constituye un problema de salud pública global, y el conocimiento sobre la dosificación de fármacos en pacientes obesos es limitado. Los ensayos clínicos en pacientes críticos raramente incluyen individuos obesos, lo que resulta en la falta de información específica sobre la dosificación en las fichas técnicas de los productos. El objetivo de esta revisión bibliográfica es proporcionar a los clínicos pautas eficientes y seguras para este grupo de pacientes.
MétodoSe conformó un grupo multidisciplinar compuesto por farmacéuticos especialistas en farmacia hospitalaria y médicos especialistas en medicina intensiva. Se identificaron y revisaron los grupos terapéuticos y, en profundidad, los principios activos más utilizados en la Unidad de Cuidados Intensivos. La revisión bibliográfica se realizó utilizando términos como: “obese”, “overweight”, “critical illness”, “drug dosification”, y “therapeutic dose monitoring”. Toda la información fue evaluada por el grupo de trabajo, que consensuó recomendaciones de dosificación para cada fármaco en pacientes obesos críticos.
ResultadosSe identificaron 83 fármacos pertenecientes a los siguientes grupos terapéuticos: antivirales, antibacterianos, antifúngicos, inmunosupresores, antiepilépticos, vasopresores, anticoagulantes, bloqueantes neuromusculares y sedantes. Se elaboró una tabla con la recomendación consensuada de dosificación para cada uno de ellos tras su revisión.
ConclusionesLa dosificación de medicamentos en pacientes obesos, tanto en entornos críticos como no críticos, sigue siendo un área con importantes incertidumbres. Esta revisión proporciona información actualizada y exhaustiva sobre la dosificación de los principales grupos terapéuticos en pacientes obesos críticos, siendo una herramienta útil tanto para médicos en unidades de cuidados críticos como para farmacéuticos clínicos en su práctica asistencial en dicho entorno.
Obesity is a global public health problem with increasing prevalence.1 This phenomenon has led to an increase in the proportion of obese patients in intensive care units (ICUs), with an estimated 20%–25% of critically ill patients being obese.2 This scenario poses numerous management challenges, including optimal pharmacological treatment in this specific population. However, knowledge of drug dosing in these patients is very limited due to factors such as the under-representation of obese patients in clinical trials and the still insufficient understanding of changes in pharmacokinetic (PK) and pharmacodynamic (PD) variables.
A critical aspect of the pharmacological management of obese patients is the determination of the body weight to be considered: actual weight, ideal weight, or adjusted body weight. There are discrepancies in the literature on this issue, which may lead to overdosing and consequent toxicity if actual weight is used, or underdosing and possible therapeutic failure if standard drug label recommendations are applied. In light of these considerations, a review of the existing literature on dosing guidelines for commonly used drugs in the ICU was proposed. The objective of this review is to provide clinicians with guidance on the application of the most effective and safe guidelines for this patient population.
MethodsA group of ICU specialist physicians and senior pharmacists identified the therapeutic groups and, more specifically, the most commonly used drugs in our hospital. Physicochemical data (log P, which indicates the hydrophilic or hydrophobic character of a substance), PK data (volume of distribution (Vd), clearance, and plasma protein binding) and the presence or absence of specific information on dosing in the technical data sheet regarding obesity and overweight were collected for each of them. Physicochemical and PK data were obtained from Uptodate, DrugBank, and PubChem.
At the same time, an initial search was conducted in PubMed, Google Scholar, and the Cochrane Library. Systematic reviews, clinical practice guidelines, original scientific studies, and clinical case reports published between 1980 and 2023 were included. The literature review was conducted using the terms: “obese”, “overweight”, “critical illness”, “drug dosification”, and “therapeutic dose monitoring”. Furthermore, the names of the different drugs were cross-referenced with the search terms. The languages accepted for this review were English and Spanish. Articles containing data on children (under 18 years of age) and those written in languages other than English or Spanish were excluded.
The literature search included all articles that mentioned the behaviour of the drug in obese patients, critically ill patients, or critically ill patients with obesity. To address the inconsistencies in the literature regarding the dose to be used in critically obese patients, the year of publication, the type of study, and the number of patients included were considered in the selection process. Optimal dose recommendations in critically obese patients were based on PK and clinical studies. In the absence of these, case reports were occasionally used to help determine the dose for this type of patient. Abstracts and, where appropriate, full articles were reviewed to determine whether the information was relevant to the objective. The information collected was peer-reviewed a posteriori by pharmacists. A colour code (traffic light) was used to assess agreement or disagreement with the dosing recommendations for critically obese patients found in the selected literature.
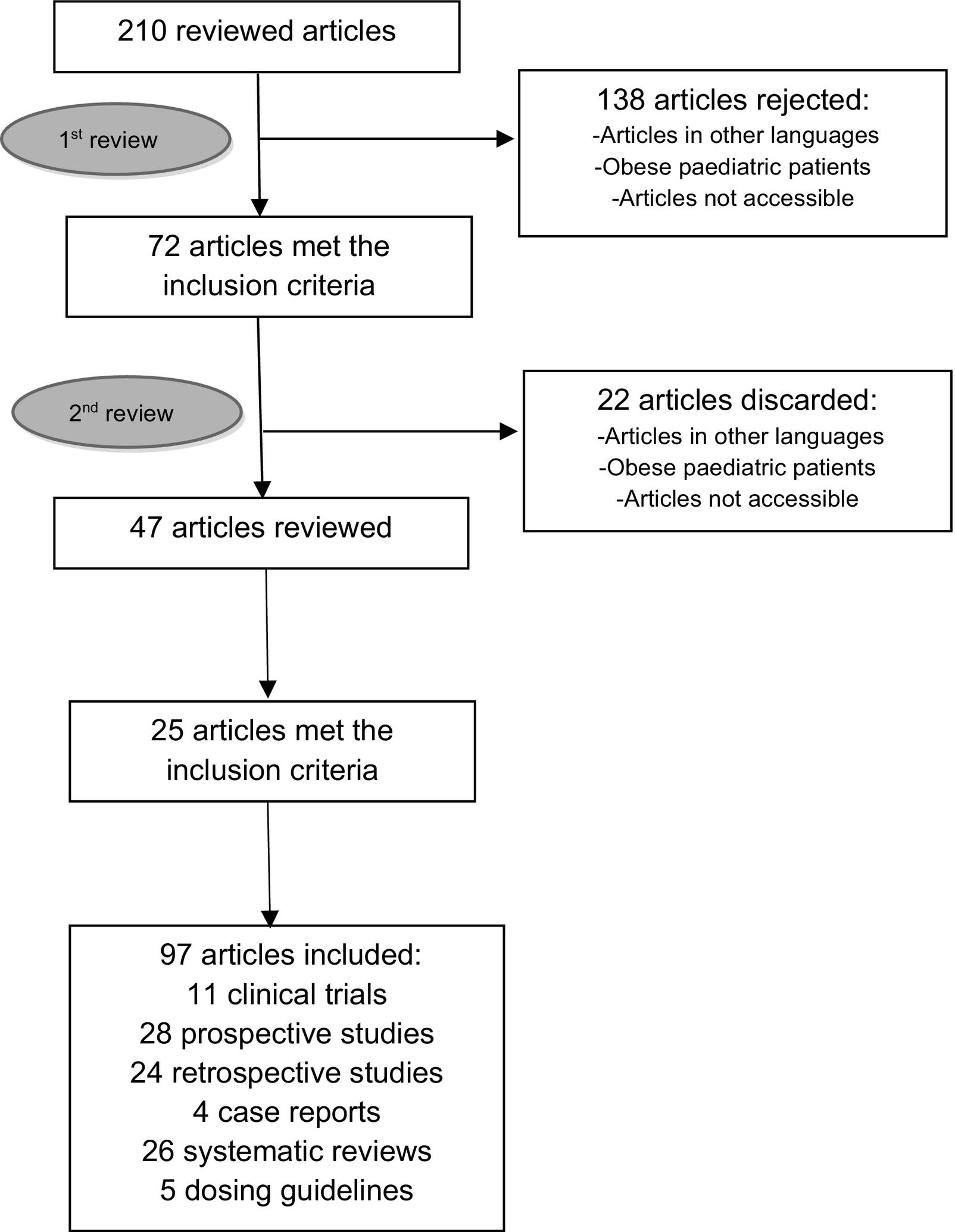
The discrepancies identified were then analysed by the full Working Group. A second peer-reviewed literature search was conducted in PubMed and Google Scholar, specifically for those drugs for which there was no initial consensus. Once this information was gathered, the full Working Group assessed the evidence for each drug and developed consensus recommendations supported by the literature reviewed. Finally, these recommendations were summarised in the form of a literature review. The workflow diagram is shown in Fig. 1.
The Body Mass Index (BMI) is calculated in kg/m2 and is a measure of the degree of obesity. In the late 1990s, the World Health Organisation3 and a panel of experts from the US National Institute of Health recommended the classification of BMI into 3 categories. Subsequently, due to the increasing number of patients with severe obesity, the Spanish Society for the Study of Obesity4 in 2007 and the American Heart Association5 in 2010 introduced subcategories 4 and 5, respectively:
- •
Obesity class I: 30–34.9 kg/m2
- •
Obesity class II: 35–39.9 kg/m2
- •
Obesity class III (morbid obesity): 40–49.9 kg/m2
- •
Obesity class IV (super morbid obesity): 50–59.9 kg/m2
- •
Obesity class V: equal to or greater than 60 kg/m2.
Several weight measures were used to recommend drug dosage:
- •
Total weight (TW): Is the actual weight of the patient as measured in the bed, or reported by the patient or carer in the absence of a built-in bed scale.6
- •
Ideal weight (IW): Calculated using mathematical formulae that do not take into account differences in body composition.7
- •
Adjusted weight (AW): Is an intermediate weight between actual and ideal weight, often used to calculate drug dosages.8 It is calculated as IW+ ([TW - IW] × C), where the constant C varies with the drug and represents the percentage of the estimated excess that the drug distributes.9
A total of 83 drugs were identified in the following therapeutic groups: antivirals, antibacterials, antifungals, immunosuppressants, antiepileptics, vasopressors, anticoagulants, neuromuscular blockers, and sedatives. Of these, only 13 out of 83 drugs (15.6%) provided information on dosing in the obese population in their prescribing information. In the first phase of the review, 210 articles were screened and 72 were included. A second review was then carried out to resolve discrepancies, which increased the number of articles to 97. The literature reviewed included 11 clinical trials, 28 prospective studies, 24 retrospective studies, 4 case reports, 26 systematic reviews, and 5 dosing guidelines. Specific information about the drugs included in this review is detailed below.
AntiviralsAcyclovir: Dose adjustment according to AW is recommended. Traditionally, dose adjustment by IW has been preferred.10 However, a comparative PK/PD study in patients with a BMI greater than 40 kg/m2 showed lower systemic exposure with IW-based dosing in morbidly obese patients compared to normal-weight patients. In addition, this study indirectly evaluated AW-based dosing of acyclovir in morbidly obese patients and found similar results to non-obese patients dosed by TW in terms of systemic exposure. Therefore, the use of AW-based dosing is recommended.11 Furthermore, recent research suggests that rates of acute kidney injury are not significantly different between AW and IW dosing.12 Despite these recommendations, it is important to assess each case based on the patient's clinical situation and renal function.
Ganciclovir: Dosage adjustment according to AW is recommended due to its hydrophilic nature and potential myelotoxicity. As of 2023, there were no literature data on dosing in obese patients. A study conducted in 2023 evaluated the efficacy and safety of ganciclovir in obese and overweight patients and found no significant differences between AW- and TW-based dosing regimens.13 A recent review14 suggests that obese patients may benefit from therapeutic drug monitoring (TDM) of ganciclovir plasma levels. However, ganciclovir TDM can currently only be recommended in the context of research studies, as there is no defined therapeutic range and the existing literature is insufficient to justify its clinical use.15
Foscarnet and cidofovir: Dosing of both drugs by AW is recommended due to their hydrophilic nature and potential nephrotoxicity. Both drugs are hydrophilic, with a Vd in non-obese patients of 0.3–0.5 and 0.4–0.5 l/kg, respectively.16 There are no data in the literature on dosing in obese patients.
AntibacterialsAminoglycosidesIt is recommended that aminoglycosides be dosed using the AW with a factor (f) of 0.4. There is a consensus in the literature on the importance of TDM, the patient's clinical status, and renal function of the patient to adjust the dose appropriately.17 For gentamicin, a 2020 study suggests dosing according to the glomerular filtration rate calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in obese patients and suggests a 25% dose reduction in dose in all cases in critically obese patients.18
Beta-lactamsIn this group, dosing is not based on weight but on standard doses. Numerous studies support TDM of beta-lactams in critically ill patients.19–22 In obese patients, administration by extended or continuous infusion is the preferred approach, with the frequency adjusted to maximum doses within established intervals, taking into account factors such as glomerular filtration rate and the clinical condition of the patient. Recommendations are based on the minimum inhibitory concentration (MIC) of the target microorganism. Recommended doses are detailed in Supplementary table 1 and, in some cases, TDM is suggested to ensure adequate therapeutic levels. In addition, certain specific drugs require special attention.
- •
Ceftriaxone: The recommended dose is 2 g every 12 h, taking into account the patient's clinical and renal function. In a study of 101 patients (39 obese and 62 non-obese), the majority of participants (94.1%) received a dose of 1 g every 24 h. Although no statistically significant differences in dosing regimens were found between the two groups, obese patients were more likely to receive 2 g of ceftriaxone (46.2% vs. 30.6%; P = .115). Clinical failure occurred in 61.5% of obese patients compared with 40.3% of non-obese patients (P = .038).23 In addition, the high plasma protein binding of ceftriaxone may affect its PK in critically obese patients, particularly in the presence of hypoalbuminemia, which is common in these patients. Another study included 137 patients, 34 of whom had serum albumin levels below 2.5 g/dl. Hypoalbuminemia was associated with a lower clinical success rate in obese patients treated with 2 g of ceftriaxone every 12 hours. 30-day-mortality (13.7% vs. 0%, P < .001) and 30-day hospital readmission (31.6% vs. 12.0%, P = .008) were more common in the hypoalbuminemic group.24 Because this study was conducted in a non-critical population, further clinical trials evaluating clinical failure in critically ill patients treated with ceftriaxone 2 g/12 h are needed to consider a higher dose, such as 2 g/8 h.
- •
Cefiderocol: Dosing according to standard guidelines is recommended. There are no specific PK studies on the effect of obesity in critically ill patients treated with cefiderocol. However, a recent real-world study25 in which 28.6% of 112 patients were obese showed no worse clinical outcomes compared with the pivotal CREDIBLE-CR study.26
Ertapenem: In critically obese patients infected with microorganisms with MIC >0.25–0.50 μg/ml, a dose of 2 g ertapenem every 24 h is recommended. TDM can always be used to assess adequate drug exposure, especially in situations of hypoalbuminemia.27 Due to its high lipophilicity, ertapenem has a high binding to plasma proteins (85%–95%). In ICU patients, the incidence of hypoalbuminemia (less than 2.5 g/dl) ranges from 40% to 50%, which leads to an increase in the Vd of the drug and, consequently, to a reduction in its systemic exposure, compromising its therapeutic efficacy.28 For this reason, some authors suggest a loading dose regimen of 2 g of ertapenem followed by a maintenance dose of 1 g every 12 h in critically ill patients with hypoalbuminemia. Studies suggest an increased risk of 30-day mortality in patients with hypoalbuminemia treated with standard doses of ertapenem compared with other carbapenems such as meropenem or imipenem.29 In this context, meropenem is recommended for use in obese critically ill patients due to its better PK profile. It is important to consider that the dose of ertapenem may vary according to the site of infection, the MIC of the pathogen, hypoalbuminemia in critically ill patients, or increased renal clearance in obese critically ill patients. In early PK/PD studies in obese patients, a dose of 1 g every 24 h did not provide sufficient blood exposure to achieve a bacteriostatic effect on microorganisms with MICs greater than 0.25–0.50 μg/ml.30 Therefore, some guidelines recommend 2 g every 24 h if the MIC is greater than 0.25–0.50 μg/ml in obese patients.31,32 In one case report, a TDM-guided dose of 1.5 g/day was used in a patient with extreme obesity, which provided adequate exposure to the MIC of the pathogen.33 However, more recent reviews and studies suggest that there are no clinically significant differences between the obese and standard populations in surgical prophylaxis,34 intra-abdominal infection,35 or pneumonia.36 For osteoarticular infections, a dose of 1 g every 24 h may not be sufficient to treat infections caused by organisms with high MICs.37
Quinolones- •
Ciprofloxacin: It is generally recommended that higher than standard doses be used in obese critical patients by administering 400 mg intravenously every 8 h and 750 mg orally every 12 h. Evidence on the use of ciprofloxacin in obese patients is limited. However, a recent study in morbidly obese patients does not recommend routinely increasing the dose of ciprofloxacin, as no significant relationship was observed between obesity and the PK parameters of the drug. However, higher doses may be needed to treat infections in tissues where ciprofloxacin penetration is expected to be poor, such as skin and soft tissue infections.38 A dose of 400 mg every 8 hours has also been used in patients on renal replacement therapy39 for bacteria with MICs above 0.5 mg/l, such as Pseudomonas aeruginosa or Acinetobacter baumannii.40
- •
Levofloxacin: It is recommended to maintain a dose of 750 mg every 24 hours and, in cases where the calculated clearance exceeds 110 ml/min (calculated based on IW), to increase the dose to 1000 mg every 24 hours for infections caused by Gram-negative bacteria.41,42
- •
Vancomycin: Recommended dosage is based on TW. A loading dose of 20–25 mg/kg is suggested, with a maximum of 2.5 g, and a maintenance dose of 15–20 mg/kg every 8–12 h, with a maximum limit of 4 g per day is suggested. In patients with a BMI greater than 40 kg/m,2 a dose of 10–12.5 mg/kg every 12 h is recommended. It is recommended that maintenance doses be adjusted according to TDM.
- •
Teicoplanin: The recommended dose is based on TW. There is limited literature on teicoplanin dosing in obese patients, but it is recommended to be based on the TW. A loading dose of 12 mg/kg every 12 hours is suggested for the first 3 doses, followed by a daily maintenance dose of 6–12 mg/kg. There are studies suggesting a dose of 15 mg/kg in those cases where an increase in the minimum concentration (Cmin) is required.43 It is recommended that maintenance doses be adjusted on the basis of TDM.44
Linezolid and tedizolid: In obese patients with coagulase-negative staphylococcal infections and a CrCL (calculated by CKD-EPI) of less than 30 ml/min/1.73 m,2 one study suggests reducing the dose to 450 mg every 12 h. For those patients with a CrCL (CKD-EPI) greater than 60 ml/min/1.73 m,2 the dose is recommended to be increased to 450 mg every 8 h. However, if TDM is available and the MIC of the pathogen is greater than 2 mg/l, the dose of 600 mg/8 h is recommended, although the high risk of thrombocytopenia should be considered.45 Several studies suggest that the standard dose of linezolid (600 mg/12 h) is insufficient to achieve adequate plasma concentrations in critically obese patients.46 In view of the increased Vd in these patients, one study recommends a loading dose of 600 mg or even 900 mg, followed by a standard dose of 600 mg every 12 h, with the next dose starting after 8 h instead of 12 h and preferably as a prolonged infusion.47 In general, TDM is recommended in critically obese patients.48,49 There are no data on the dosing of tedizolid in the critically obese patient.
Clindamycin: In general, a dose of 900 mg every 8 hours is recommended in obese patients.50 In life-threatening emergencies, doses of up to 4.8 g per day have been administered intravenously according to the product data sheet,51 although the maximum recommended dose is 2.7 g per day. Data on the use of clindamycin in critically obese patients are not available.
Daptomycin: Dosing according to AW is recommended as recent studies have shown that this practice reduces adverse effects compared to TW dosing without compromising clinical efficacy. This study showed a reduction in adverse effects when AW was used instead of TW.52 Expert consensus also supports AW dosing as it maintains efficacy parameters and improves safety by administering lower doses in obese patients. Although a recent review suggests dosing according to lean body weight, the recommendation to dose according to AW is maintained due to the difficulty in calculating lean body weight and its similarity to adjusted weight.53 Traditionally, daptomycin has been dosed according to total body weight, but it has been observed that drug exposure after a dose of 4 mg/kg increases by approximately 25%–60% in obese patients compared to non-obese patients.54,55 In a retrospective observational study of 326 patients, a signal of muscle toxicity was observed independent of statin use (ROR, 6.82; 95% CI [4.56–10.22]; P < .001). A multiple logistic analysis in 250 patients showed a significant association between toxicity and BMI greater than 25 kg/m2 (OR, 3.57; 95% CI [1.58–8.09]; P = .002).56 In addition, other studies suggest higher rates of CPK elevation and discontinuation due to adverse events when daptomycin is dosed based on total body weight in obese patients.57,58
AntifungalsAzoles- •
Fluconazole: Dosing should be adjusted according to TW, up to a maximum daily dose of 1600 mg. Some authors suggest giving a loading dose of 12 mg/kg followed by 6 mg/kg every 24 h or 12 mg/kg every 24 h, depending on the PK/PD target.59 A study60 conducted in 17 morbidly obese patients undergoing laparoscopic gastric surgery demonstrated the involvement of sex in the drug's Vd in the obese population. Therefore, they conclude that to achieve a 24- hour AUC/ MIC greater than 100 for pathogens with an MIC less than 2 mg/l, loading doses can be as usual (800 mg on the first day of treatment), except in male patients weighing more than 140 kg, in whom 600 mg every 12 h should be administered as a loading dose on the first day of treatment. In all patients, target concentrations were achieved with a maintenance dose of 400 mg every 24 h. Importantly, this study was conducted in obese, non-critically ill patients.
- •
Voriconazole: Dosing is recommended according to the AW, following the standard 4 mg/kg every 12 h, with the possibility of a first-day loading dose of 6 mg/kg every 12 h. Some authors suggest that oral dosing could be adjusted to a standard dose of 200–300 mg every 12 h in patients with a BMI greater than 35 kg/m2.61 In all circumstances, close monitoring with TDM is recommended.
- •
Posaconazole: Standard dosing is recommended, except in patients weighing more than 140 kg who require 400 mg intravenously every 24 hours for the treatment of fungal infections.62 Although there have been studies conducted with the tablet formulation, where lower drug exposure was observed in patients weighing 120 kg and above, no dose adjustment is recommended, although TDM is advised.63
- •
Isavuconazole: No adjustment is recommended. However, a PK study64 in 41 critically ill patients showed that 51.4% of patients with a BMI greater than 25 kg/m2 had a Cmin less than 1 μg/ml. Although the majority of the literature does not recommend routine TDM with isavuconazole, it may be considered in obese critically ill patients. Further studies are needed to justify TDM with isavuconazole.
Recent studies have highlighted the risk of low exposure to echinocandins in critically obese patients. However, whether this affects clinical outcomes has not established. Due to the discrepancy in recommended doses in existing studies, TDM is suggested to determine the appropriate dose in these patients.65 The International Association of Therapeutic Drug Monitoring and Clinical Toxicology considers the use of TDM for echinocandins in critically obese patients to be valuable.66
- •
Micafungin: For Candida albicans infection, a daily dose of 150 mg every 24 hours is recommended for patients weighing less than 115 kg and 200 mg every 24 hours for those weighing more than 115 kg. For Candida glabrata infection, regardless of weight, a maximum dose of 200 mg every 24 hours is recommended.67
- •
Anidulafungin: It is recommended that the dose be increased by 25% in patients weighing more than 140 kg and by 50% in those weighing more than 200 kg, i.e. to 125 mg every 24 hours and 150 mg every 24 hours, respectively.68 Studies have shown that anidulafungin clearance increases with body weight, necessitating an increase in the loading and maintenance doses.69
Liposomal amphotericin B: Dosing by TW is recommended up to a maximum of 100 kg, i.e., up to a maximum dose of 500 mg, depending on the indication. There is limited data in the literature on the dosing of liposomal amphotericin B in obese patients. A recent study evaluated clinical outcomes depending on whether the dose was adjusted according to AW or TW. Patients on AW-adjusted dosing showed a significantly lower rate of nephrotoxicity and a (non-significant) trend towards lower mortality. However, these results were not obtained in critically ill patients, and dosing according to TW is recommended in these cases.70 According to a study conducted in 16 obese patients, body size does not influence the clearance of liposomal amphotericin B.71
ImmunosuppressantsIn general, there is a consensus in the literature on the need for weight-based dosing and performing TDM in all types of patients (obese and non-obese) for this group of drugs.
CorticosteroidsDosing by IW is recommended, except for short courses where AW may be used to avoid possible underdosing.
- •
Methylprednisolone: The use of IW or AW is recommended, particularly in patients with more severe forms of obesity (BMI of 40 kg/m2 or more).72
- •
Hydrocortisone: Dosing is not based on weight, so the intravenous dose should be the same for non-obese patients.
- •
Tacrolimus: Dosing by IW or AW is recommended. The usual standard practice for initial tacrolimus dosing after transplantation is based on the patient's total weight, as recommended in the data sheet. However, recent studies suggest that this approach may not be appropriate and suggest that the dose should be reduced in overweight and obese individuals.73–75 Higher BMI has been identified as a risk factor for being a slow metaboliser, requiring a lower tacrolimus dose per kilogram of TW.76 In contrast, other studies have shown that there are no differences in tacrolimus Cmin between obese and non-obese patients after weight-based dosing in the immediate post-transplant period.77
- •
Mycophenolate mofetil: A study in kidney transplant patients suggests that higher doses should be used in patients weighing more than 100 kg to avoid underdosing.78 This study suggests that doses should be personalised according to patient characteristics or guided by TDM.
For drugs that are administered by weight, it is generally recommended that a loading dose be administered using the TW and that a maintenance dose be continued adjusted to the ideal weight IW, while monitoring plasma levels. The dose should always be adjusted according to the patient's clinical status, renal and/or hepatic function, depending on the PK characteristics of the drug. For drugs administered at standard doses, there are no studies to support higher doses, so it is recommended to dose according to the indication and, whenever possible, to use TDM to adjust drug doses.
Phenytoin: Phenytoin is not distributed proportionally with body weight. A recent study79 suggests that a loading dose of 20 mg/kg administeredby AW, followed by maintenance dosing with conventional daily doses or IW, may be more likely to achieve the desired therapeutic concentrations. Subsequent titration should be based on drug monitoring by TDM and clinical efficacy.
Levetiracetam: In general, maximum doses of 1500–2000 mg every 12 hours are recommended. Levetiracetam has linear PKs with low intra- and inter-individual variability, suggesting that TDM is not required, unlike other antiepileptic drugs. Nevertheless, there is controversy80 in this regard, due to the exclusion of critically ill and obese patients, and other factors, in clinical trials. Recent research has shown that increased renal clearance in certain groups of critically ill patients, such as septic, neurocritical, and polytrauma patients, has a significant impact on levetiracetam plasma levels, resulting in lower than expected concentrations.81,82 Therefore, TDM is recommended whenever possible.
VasopressorsWhen administering vasopressors to obese patients, it is of essential importance to carefully consider the dosing approach, whether weight-based or based on clinical parameters. It is recommended that consistency in the use of IW or AW is maintained if a weight-based dosing strategy is chosen when prescribing different vasoactive drugs.
Norepinephrine: In general, a dosing strategy based on clinical goals is preferred. A retrospective study evaluated the effect of TW and BMI on norepinephrine and other vasopressor requirements. It was found that obese patients required lower total vasopressor doses per kilogram to achieve clinical goals such as mean arterial pressure.83 Another study, using a weight-based dosing strategy, showed that norepinephrine accumulation in morbidly obese patients was associated with lower in-hospital mortality. However, 1-year mortality was higher in morbidly obese patients.84
Vasopressin: It is recommended that the dose of vasopressin should not be modified because of obesity.
In 2016, a study of 40 patients with septic shock concluded that increasing the dose of vasopressin according to weight did not correlate with changes in mean arterial pressure when used in conjunction with catecholamine vasopressors in septic shock. However, fixed-dose vasopressin administration may not be sufficient in obese patients with septic shock.85 Subsequently, another study compared standard and high-dose vasopressin regimens in obese patients with septic shock. This study showed that high-dose vasopressin administration was not associated with differences in catecholamine requirements or improved clinical outcomes.86
AnticoagulantsUnfractionated heparin: Traditionally, it has been recommended to start therapy with AW until activated partial thromboplastin time control is achieved. This is because heparin dosing in obese patients should take into account the increased vasculature and blood volume, ignoring the weight of adipose tissue, which is less vascularised. A study in a cohort of critically obese patients recommends dosing according to AW in obese patients weighing more than 165 kg and according to TW for patients weighing less than 165 kg.87
Low molecular weight heparin (LMWH): LMWHs are hydrophilic molecules, which means that standard weight-based dosing in obese patients may increase the risk of bleeding complications due to overdosing. Moreover, it is also crucial to highlight that obesity is an independent risk factor for thromboembolism. It is estimated that the thromboembolic risk in obese patients is double that of non-obese patients and can be up to six times higher in those with a BMI greater than 35 kg/m2.
- •
Thromboembolic prophylaxis: For patients with a BMI greater than or equal to 40 kg/m2, several studies recommend administration of 40 mg enoxaparin subcutaneously twice daily, 5000 IU of dalteparin every 12 hours, and 75 IU/kg/24 hours of tinzaparin. For patients with a BMI greater than or equal to 50 kg/m2, 60 mg of enoxaparin every 12 hours is recommended.88 Other studies suggest a dose of 0.5 mg/kg BW of enoxaparin per day, with no evidence of excessive anti-Xa activity.89
- •
Anticoagulant therapy: In patients with a BMI greater than or equal to 40 kg/m2, it is recommended to use TW weight and administer enoxaparin at a dose of 0.7–0.8 mg/kg subcutaneously every 12 h, with a maximum dose of 150 mg per dose, avoiding the use of a single daily dose.90–92 These data are confirmed in a recent systematic review,93 which also supports the adjustment of the LMWH regimen by monitoring anti-Xa levels, especially in the treatment setting.
Although there is some evidence suggesting that the dosing of atracurium and succinylcholine should be based on the TW, in critically obese patients, it is recommended to use small, serial loading doses, titrated according to clinical effect. Another option is to administer a single loading dose based on the IW, bearing in mind that additional doses may be required, followed by initial maintenance infusions also based on the IW, with subsequent titration according to clinical effect and peripheral nerve stimulation.94
Cisatracurium: AW-based dosing is recommended. There are no data available on the use of cisatracurium in critically obese patients; however, there is one study in obese patients undergoing bariatric surgery. Compared with TW dosing, cisatracurium infusion based on AW dosing results in a more favourable postoperative situation, lower cisatracurium consumption, and shorter recovery time. In contrast to the AW dosing group, the IW dosing group required more rescue doses during maintenance of anaesthesia than the TW group.95
Rocuronium: Dosing based on IW is recommended. A study comparing IW and TW-based dosing of rocuronium for intubation has recently been published. The results suggest similar efficacy under optimal intubation conditions between IW (73.8%) and TW (68.5%, P = .12 [0.8–2.5]), and a shorter duration of paralysis when dosing based on IW (43 min vs. 71 min, P < .001).96
Succinylcholine: Dosing after TW is recommended because morbid obesity increases the amount of pseudocholinesterase and the volume of extracellular fluid. A comparison of 1 mg/kg after TW with 1 mg/kg after AW showed that the former provided better intubation conditions without significant postoperative myalgias.97
SedativesSedation dosing should be guided by specific clinical goals to optimise patient management and minimise risk. Due to the excess fat and wide distribution of sedative drugs in obese patients, it is suggested to start with initial loading doses based on the TW. In addition, the strategy of using small serial loading doses until the desired clinical effect is achieved, especially in morbidly obese patients, may be an alternative to avoid excessive sedation. Several studies suggest that maintenance doses of sedatives should be based on the IW or AW.98
Midazolam: It is recommended that the loading dose be based on the AW and the maintenance dose on the IW. Midazolam is a lipophilic benzodiazepine with a Vd of 2 l/kg, which is increased in obese compared to non-obese patients (2.66 ± 0.16 vs. 1.74 ± 0.11 l/kg; P < .001), although its elimination is not affected by obesity. This results in a prolonged half-life in obese patients, so it is recommended that IW or AW be used for initial dosing and smaller additional doses administered as needed, due to the risk of accumulation and supratherapeutic concentrations with TW-based dosing. A retrospective study evaluated the amount of sedation and analgesia administered in 2 groups of critically obese patients (AW and TW dosing). The groups differed only in the amount of midazolam administered; no differences were found in other drugs, nor in days of mechanical ventilation, ICU stay, or self-extubation.99
Propofol: Although various guidelines for propofol have been proposed over time, more recently its prescription by AW has been recommended due to its adverse effects on haemodynamics.100–103
Dexmedetomidine: It is recommended to dose dexmedetomidine based on AW. In the last year, 2 studies have been published comparing the sedation target with dexmedetomidine between patient groups dosed based on TW or AW. After drug administration, no differences were found on the RASS scale.104,105
A supplementary table is included that liststhe different drugs analysed, their log P value, the dosing information in relation to the obese population present in the technical data sheet (AEMPS), our recommendation regarding the dosing weight, the dosing recommendation, the need for TDM follow-up, and a space for observations are given.
DiscussionIn Spain, the prevalence of obesity in the adult population is estimated to be 18.7%.106 In addition, one study showed that 24.5% of ICU patients were obese.107 In other countries, such as France and the United Kingdom, the prevalence of obese ICU patients was 25.8%108 and 30.5%,109 respectively, according to published studies.
Factors inherent to obesity, such as increased adipose tissue, altered blood flow, and changes in organ size, may contribute to PK variations in drug distribution, plasma protein binding and drug elimination.110 In addition, critically ill patients have additional conditions that influence PKs, such as mechanical ventilation, use of extracorporeal circuits, severe inflammatory states, renal, cardiac and/or hepatic failure, concomitant medications, and high administration volumes received.111 These changes have implications for drug dosing in critical care, where accurate dosing is essential for patient safety and therapeutic efficacy.
It is important to understand drug properties and their relationship to PK changes in critically obese patients. Hydrophilic drugs with low, negative, or close to 0 log P are mainly distributed in aqueous compartments such as interstitial fluid, plasma, and muscle. In obese patients, the Vd of hydrophilic drugs is slightly increased due to increased plasma volume and lean muscle mass. Therefore, when calculating the dose per weight, the IW or AW weight should be considered, as excess fat mass does not affect the distribution of the drug. On the other hand, lipophilic drugs are mainly distributed in the intracellular milieu and in adipose tissue, and in obese patients, where there is a greater presence of adipose tissue, a significant increase in the Vd of these drugs is observed. In these cases, it is preferable to dose according to the TW.
In this context, the lack of specific data and studies hinders the establishment of clear recommendations for drug dosing in critically obese patients. Furthermore, despite the increasing incidence of obesity, this information is rarely included in the documentation approved by regulatory authorities. An American study112 revealed that only 30% of the molecules reviewed included information on dosing for obesity in the ICU setting, an increase of only 3% compared to data from 10 years earlier.113 In our study, only 16.5% of the drugs analysed included specific information on dosing in the obese population in their data sheet.
In this review, we have compiled the physicochemical characteristics of the most commonly used drugs in the ICU and attempted to resolve discrepancies according to the sources of information that provide dosing advice in situations of obesity and/or critical patients. For this group of patients, dosing recommendations were established and summarised in a table. Other reviews have offered dosing recommendations focused monographically on different groups of drugs, including antimicrobials,6,10,22,32 thromboembolic prophylaxis and supportive medication,72 neuromuscular blockers,94 analgesics, and sedatives.98 However, this guide provides an overview of the most common therapy in a tertiary hospital ICU.
Our article has several limitations due to the high heterogeneity of the studies reviewed. Some studies were conducted in a non-critically obese population, which precludes extrapolation of results to ICU patients. In addition, the studies are not consistent in terms of patient inclusion, with some using BMI greater than 30 kg/m2 or BMI greater than 40 kg/m2, and others classifying patients by weight ranges without mentioning BMI. Theres is no classification of studies according to their design and level of evidence has been performed. In some cases, the evidence is so sparse and ambiguous that it has limited our ability to choose a dosage weight by offering more than one option. Another limitation is the selection of drugs, which was limited to those used in our ICU.
Challenges include establishing weight ranges for dosing in the obese population, using dosing parameters that take into account phenotype and body composition (such as the proportion of lean tissue), and reviewing clinical trials that report on drug dosing in obesity. Further knowledge is also needed to adjust oral the dosing of oral medications in obesity and reduce the risk of underdosing.
The global increase in the prevalence of obesity correlates with an increase in the number of obese patients in the ICU, posing a significant challenge to their management, including more effective and safer drug treatment. Drug dosing in obese patients, both in critical care and non-critical care settings, remains an area of considerable uncertainty due to several factors, such as the under-representation of obese patients in clinical trials and the lack of knowledge of how PK and PD variables are affected in this group. In this review, we provide an updated and detailed overview of the dosing of drugs belonging to the main therapeutic groups used in the pharmacological treatment of critically obese patients. This information will be useful to both physicians working in ICUs and clinical pharmacists in their practice in this setting.
FundingNo funding has been obtained for the study.
Presentation at conferencesThe 66th Congress of the SEFH, organised by the Spanish Society of Hospital Pharmacy and held in A Coruña on 18–21 October 2021 (online).
Liability and Assignment of RightsAll authors accept the responsibility defined by the International Committee of Medical Journal Editors (available at http://www.icmje.org/).
In the event of publication, the authors grant exclusive rights of reproduction, distribution, translation and public communication (by any means or audio, audiovisual or electronic support) of their work to Farmacia Hospitalaria and, by extension, to the SEFH. To this end, a letter of assignment of rights must be signed at the time of sending the work through the online manuscript management system.
CRediT authorship contribution statementHector Carlos García-Díaz: Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Data curation, Conceptualization. Pablo Sánchez-Sancho: Writing – original draft, Formal analysis, Data curation, Conceptualization. Pilar Lalueza-Broto: Writing – review & editing, Validation, Supervision, Conceptualization. Xavier Nuvials: Validation, Supervision, Investigation, Data curation. María Queralt Gorgas Torner: Writing – review & editing, Validation, Supervision, Data curation. Laura Domenech Moral: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Investigation, Formal analysis, Data curation, Conceptualization.
We would like to thank Jose Manuel del Río Gutierrez, Carlota Varón Galcera and Marcos Pérez for their collaboration in the review of drugs for the preparation of this guide.