Mepolizumab is indicated as an additional treatment of severe refractory eosinophilic asthma. The observed differences in population subgroups according to plasma eosinophil count, the existence of patients with high levels of immunoglobulin E who are candidates of omalizumab and mepolizumab, as well as mepolizumab's economic impact, lead to make efficient economic studies for clinical decision making. The aim was to analyze mepolizumab's cost-efficacy and budget impact.
MethodCost comparison and the use of mepolizumab's budgetary impact was performed, from the Spanish National Health System's perspective. Among the assessed alternatives, inhaled systemic corticosteroids, plus long acting beta agonist (β2) and/or oral systemic corticosteroids in patients with non immunoglobulin E-mediated severe allergic asthma, and said treatment along with omalizumab in patients with immunoglobulin E mediated eosinophilic allergic asthma were included. Its efficacy was evaluated through avoided clinically relevant exacerbations. The direct costs associated with exacerbation were assessed.
ResultsMepolizumab's long run average incremental cost regarding omalizumab's is 797 euros per patient a year. Considering omalizumab's alternative discounted price, including mepolizumab for patients with immunoglobulin E mediated eosinophilic allergic asthma would increase public spending from 2.3 to 4.6 million euros. Given omalizumab's notified price, the gradual introduction of mepolizumab in the Spanish National Health System would save 3.6 million euros in three years. For non immunoglobulin E-mediated severe asthma patients, the avoided cost/exacerbation by introducing mepolizumab is 15,085 euros, assuming a gradual market penetration of mepolizumab. In patients with ≥ 500 eosinophils/µL, this cost decreases to 7,767 euros per avoided exacerbation with a budgetary impact of 183.2 million euros in three years with a progressive penetration of mepolizumab.
ConclusionsThe cost comparison between mepolizumab and omalizumab in immunoglobulin E mediated eosinophilic asthma patients suggests a use of the lower cost drug, promoting price competition. Additionally, prioritizing its use among non immunoglobulin E-mediated severe refractory eosinophilic asthma patients and ≥ 500 eosinophils/µL plasma level patients, would improve its efficiency as well as reducing its budgetary impact.
Mepolizumab esta indicado como tratamiento adicional del asma eosinofilica refractaria grave. Las diferencias observadas en subgrupos poblacionales segun recuento eosinofilico plasmatico, existencia de pacientes con altos niveles de inmunoglobulina E candidatos a omalizumab y mepolizumab, e impacto economico de mepolizumab obligan a realizar estudios economicos para tomar decisiones clinicas eficientes. El objetivo fue realizar un analisis de coste/eficacia e impacto presupuestario de mepolizumab.
MétodoSe realizo la comparacion de costes e impacto presupuestario del uso de mepolizumab desde la perspectiva del Sistema Nacional de Salud. Las alternativas valoradas fueron corticosteroides sistemicos inhalados + agonista β2 de larga duracion y/o corticosteroides sistemicos orales en pacientes con asma alergica grave no mediada por inmunoglobulina E, y este tratamiento junto a omalizumab en pacientes con asma eosinofilica alergica mediada por inmunoglobulina E. La eficacia se evaluo mediante exacerbaciones clinicamente relevantes evitadas. Se valoraron los costes directos asociados a exacerbacion.
ResultadosEl coste incremental medio de mepolizumab respecto a omalizumab es de 797 euros por paciente y ano. Considerando precio alternativo con descuento de omalizumab, incluir mepolizumab para pacientes con asma eosinofílica alérgica y mediada por inmunoglobulina E supondría incrementar el gasto público de 2,3 a 4,6 millones de euros. Teniendo en cuenta el precio notificado de omalizumab, la introducción gradual de mepolizumab en el Sistema Nacional de Salud supondría ahorrar 3,6 millones de euros en tres años. Para pacientes con asma grave no mediada por inmunoglobulina E, el coste/exacerbación evitada al añadir mepolizumab es de 15.085 euros, con un impacto presupuestario en tres años de 578,4 millones de euros, asumiendo una penetración progresiva de mepolizumab en el mercado. En los pacientes con ≥ 500 eosinófilos/µl, este coste disminuye a 7.767 euros por exacerbación evitada, con un impacto presupuestario de 183,2 millones de euros en tres años con penetración progresiva de mepolizumab.
ConclusionesLa comparación de costes entre mepolizumab y omalizumab en pacientes con asma eosinofílica mediada por inmunoglobulina E señala como razonable utilizar el fármaco de menor coste, promoviendo competencia de precios. Asimismo, priorizar su uso en pacientes con asma eosinofílica refractaria grave no mediada por inmunoglobulina E y niveles plasmáticos ≥ 500 eosinófilos/µl permitiría mejorar la eficiencia y disminuir el impacto presupuestario.
It is estimated that asthma affects approximately 4.9% of adults1. In Spain, the prevalence of patients with uncontrolled or refractory severe asthma to corticosteroids and β2 long acting beta agonist (LABA) treatment is approximately 3.9% of asthmatics2. Within this group, about 25% have eosinophilic asthma, characterized by a late onset, presence of eosinophils in bronchial biopsies and is usually associated with nasal polyps, rhinosinusitis and respiratory infections3,4.
Omalizumab is a monoclonal antibody indicated in uncontrolled severe allergic asthma authorized in Spain in 20065. The dosage of omalizumab is variable, ranging from a minimum of 75 mg every 4 weeks up to 600 mg every 2 weeks5. In 2015, mepolizumab is marketed. This monoclonal antibody is indicated as an additional treatment for adult patients with severe refractory eosinophilic asthma6. Mepolizumab acts by binding to interleukin 5 and preventing its interaction with the surface of eosinophils. This causes a reduction in their production and survival. The recommended dose is 100 mg every 4 weeks. Studies evaluating the dose of mepolizumab and eosinophilic response show a similar pharmacodynamics between 100 mg and 75 mg7.
In the pivotal clinical trials for authorizing mepolizumab, the effect as a main variable on the frequency of exacerbations that are clinically relevant was measured8,9. A clinically relevant exacerbation is an acute asthma attack requiring the use of systemic corticosteroids for at least three days and/or hospitalization and/or emergency room visits, or doubling the dose of systemic corticosteroids for at least three days in patients treated with oral corticosteroids as maintenance therapy8,9. Mepolizumab has proven to be effective in reducing exacerbations and daily doses of oral systemic corticosteroids (OCS) in patients with severe eosinophilic asthma not adequately controlled with high doses of inhaled systemic corticosteroids (ICS) + LABA and/or OCS (usual treatment).
However, a higher frequency of asthma attacks is associated with a high eosinophils count (> 300-400 cells/μL)10,11. In the subgroup analysis of the pivotal clinical trials, it is also observed that the relative benefit is greater in patients with higher blood levels of eosinophils8,9. Subgroup analysis is pre-specified and shows statistical interaction. The difference is consistent in studies7,12 and there is biological plausibility, as an inhibitor for eosinophils could exert a greater action, the bigger the contribution of eosinophilia is to the asthmatic process.
It should be noted that approximately 30% of diagnosed eosinophilic asthmatic patients show signs and symptoms that are consistent with the IgE-mediated persistent allergic asthma phenotype13, meeting omalizumab's treatment criteria. However, no evidence exists to opt for either therapy in this subpopulation12.
Given the differences in subgroups according to eosinophils count in severe refractory eosinophilic asthma, the existence of candidates for omalizumab patients or mepolizumab, and the economic impact resulting from the use of mepolizumab, it seems crucial to conduct a study of economic evaluation and budgetary impact that helps making efficient clinical decisions. At the time of this work, other similar action mechanism drugs to mepolizumab –reslizumab and benralizumab– were pending funding and price in Spain14,15. These drugs were not compared to mepolizumab, and it is difficult to differentiate between them. The economic comparison of these therapies in the same group is not the subject of this study.
The aim of this work is to perform a cost-efficacy and budgetary impact analysis (BIA) of mepolizumab's as treatment for severe refractory eosinophilic asthma, mediated and non mediated by elevated IgE levels in adult patients who are not adequately controlled with high dose of ICS + LABA and/or OCS in Spain.
MethodsThe cost-efficacy analysis and the BIA were developed from the perspective of the Spanish National Health System (NHS). Only direct costs were quantified in euros in 2018. The BIA was carried out for a period of three years (2018-2020). Analyses were performed taking into account the latest economic assessment and BIA guidelines16,17.
Study populationThe study population included patients over 12 years with severe refractory asthma. Adult asthmatic population estimates and the prevalence of severe refractory asthma in Spain were employed for the BIA1,2. Subsequently, the percentage of patients to treatment with severe refractory asthma, diagnosed with eosinophilic asthma was calculated. Asthmatic population mediated with elevated IgE levels who is candidate for therapy with omalizumab were also calculated by using data from the Spanish National Statistics Institute18. In addition, a population subgroups BIA was performed according to plasma eosinophil count (Table 1).
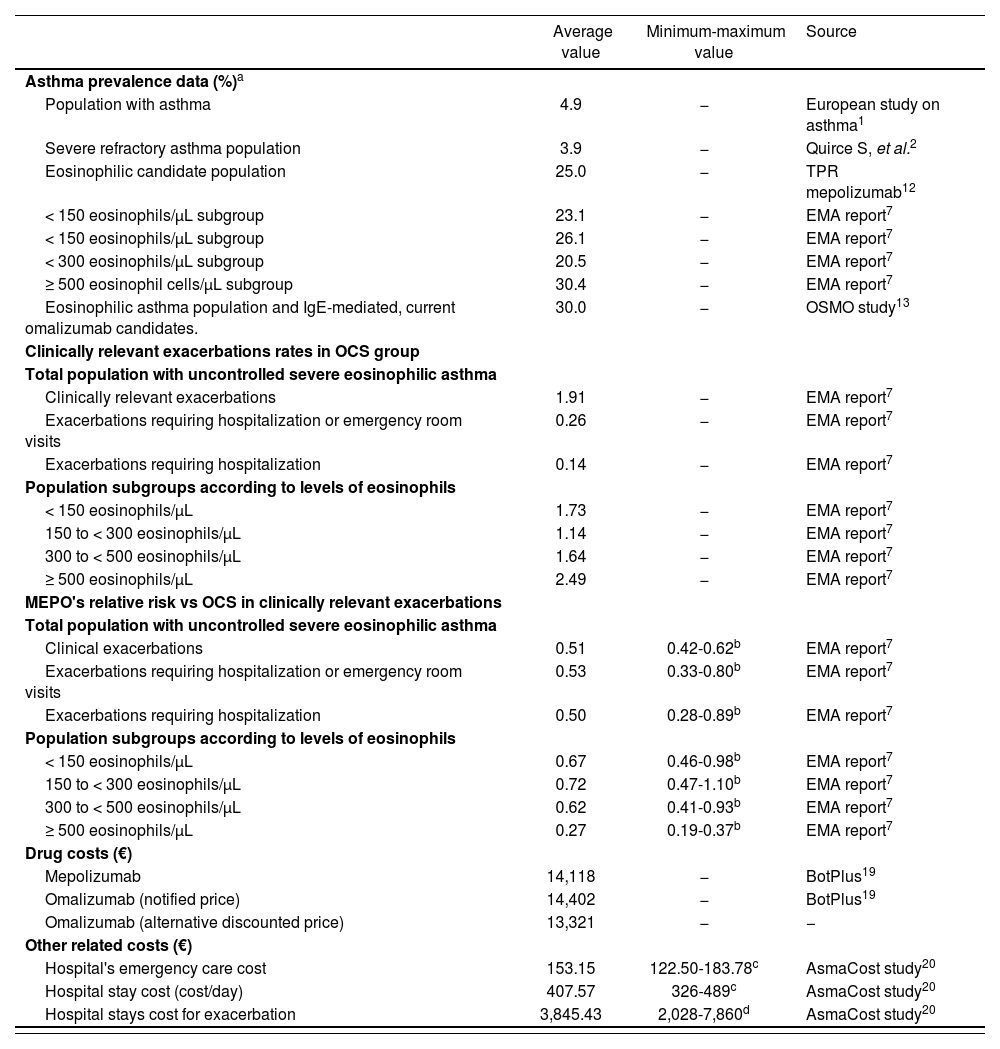
Prevalence values, efficiency and cost used in budget impact analysis
| Average value | Minimum-maximum value | Source | |
|---|---|---|---|
| Asthma prevalence data (%)a | |||
| Population with asthma | 4.9 | − | European study on asthma1 |
| Severe refractory asthma population | 3.9 | − | Quirce S, et al.2 |
| Eosinophilic candidate population | 25.0 | − | TPR mepolizumab12 |
| < 150 eosinophils/μL subgroup | 23.1 | − | EMA report7 |
| < 150 eosinophils/μL subgroup | 26.1 | − | EMA report7 |
| < 300 eosinophils/μL subgroup | 20.5 | − | EMA report7 |
| ≥ 500 eosinophil cells/μL subgroup | 30.4 | − | EMA report7 |
| Eosinophilic asthma population and IgE-mediated, current omalizumab candidates. | 30.0 | − | OSMO study13 |
| Clinically relevant exacerbations rates in OCS group | |||
| Total population with uncontrolled severe eosinophilic asthma | |||
| Clinically relevant exacerbations | 1.91 | − | EMA report7 |
| Exacerbations requiring hospitalization or emergency room visits | 0.26 | − | EMA report7 |
| Exacerbations requiring hospitalization | 0.14 | − | EMA report7 |
| Population subgroups according to levels of eosinophils | |||
| < 150 eosinophils/μL | 1.73 | − | EMA report7 |
| 150 to < 300 eosinophils/μL | 1.14 | − | EMA report7 |
| 300 to < 500 eosinophils/μL | 1.64 | − | EMA report7 |
| ≥ 500 eosinophils/μL | 2.49 | − | EMA report7 |
| MEPO's relative risk vs OCS in clinically relevant exacerbations | |||
| Total population with uncontrolled severe eosinophilic asthma | |||
| Clinical exacerbations | 0.51 | 0.42-0.62b | EMA report7 |
| Exacerbations requiring hospitalization or emergency room visits | 0.53 | 0.33-0.80b | EMA report7 |
| Exacerbations requiring hospitalization | 0.50 | 0.28-0.89b | EMA report7 |
| Population subgroups according to levels of eosinophils | |||
| < 150 eosinophils/μL | 0.67 | 0.46-0.98b | EMA report7 |
| 150 to < 300 eosinophils/μL | 0.72 | 0.47-1.10b | EMA report7 |
| 300 to < 500 eosinophils/μL | 0.62 | 0.41-0.93b | EMA report7 |
| ≥ 500 eosinophils/μL | 0.27 | 0.19-0.37b | EMA report7 |
| Drug costs (€) | |||
| Mepolizumab | 14,118 | − | BotPlus19 |
| Omalizumab (notified price) | 14,402 | − | BotPlus19 |
| Omalizumab (alternative discounted price) | 13,321 | − | − |
| Other related costs (€) | |||
| Hospital's emergency care cost | 153.15 | 122.50-183.78c | AsmaCost study20 |
| Hospital stay cost (cost/day) | 407.57 | 326-489c | AsmaCost study20 |
| Hospital stays cost for exacerbation | 3,845.43 | 2,028-7,860d | AsmaCost study20 |
EMA: European Medicines Agency; MEPO: mepolizumab; OCS: oral systemic corticosteroids; TPR: Therapeutic Positioning Report.
The cost-efficacy analysis, as well as BIA on the use of mepolizumab was performed using two different analysis according to the studied population.
In analysis 1, the analyzed population was diagnosed with eosinophilic allergic asthma and IgE mediated. In these patients, the high dose association of ICS + LABA and/or OCS along with mepolizumab was compared to the same medication associated with omalizumab.
In analysis 2, the study population was suffering from non IgE-mediated severe refractory asthma, and other alternatives to mepolizumab were not considered. Thus, the use of ICS + LABA and/or OCS with mepolizumab in high doses was assessed against high doses of ICS + LABA and/or OCS. This second analysis excludes 30% of patients with eosinophilic asthma (who were treated with omalizumab).
The evaluated mepolizumab dosage is 100 mg every 4 weeks7. Omalizumab is dosed based on body weight and basal IgE levels. The dose ranges from 75 mg every 4 weeks to 600 mg every 2 weeks5. Regarding cost analysis, an average of these values was employed (Table 1).
Measure health outcomesThe efficacy of the therapies was obtained from the Therapeutic Positioning Report on mepolizumab12 and from the European Medicines Agency's assessment report on mepolizumab7. Clinical exacerbations, including those requiring hospitalization or emergency room visits and relevant clinical exacerbations by population subgroups according to plasma eosinophil count were estimated (Table 1). The drug efficacy was assessed by reducing the average of clinically relevant annual exacerbations for using mepolizumab against its therapeutic alternative. Conducting a cost minimization study requires clinical equivalence evidence of the tested drugs. Comparative clinical evidence is lacking quality between mepolizumab and omalizumab that shows clinical equivalence or difference between the two therapies. Therefore, a cost minimization study could not be performed, but a cost comparison study was carried out instead in analysis 1.
Cost estimateThe cost of medication (mepolizumab and omalizumab), of relevant clinical exacerbations, of emergency room visits and for hospitalization due to asthma exacerbations were included. Treatments were evaluated by laboratory sales price of drugs according to the Catalog of Medicinal Products of the General Council of Official Colleges of Pharmacists19. In regard to omalizumab, its notified and alternative price according to the routine clinical practice was collected, with a hypothetical price discount of 7.3%. Mepolizumab matches both the notified and alternative prices (Tables 1 and 2). The cost of a clinically significant exacerbation requiring hospitalization and/or emergency care, and hospitalization cost –assuming an average stay of nine days– were extracted from AsmaCost20 study, updated to euro currency in 2018. The analysis includes drugs’ direct costs and hospitalization and emergency care costs, due to its impact on the definition of clinically significant exacerbation. The study does not include costs arising from hospital medication management.
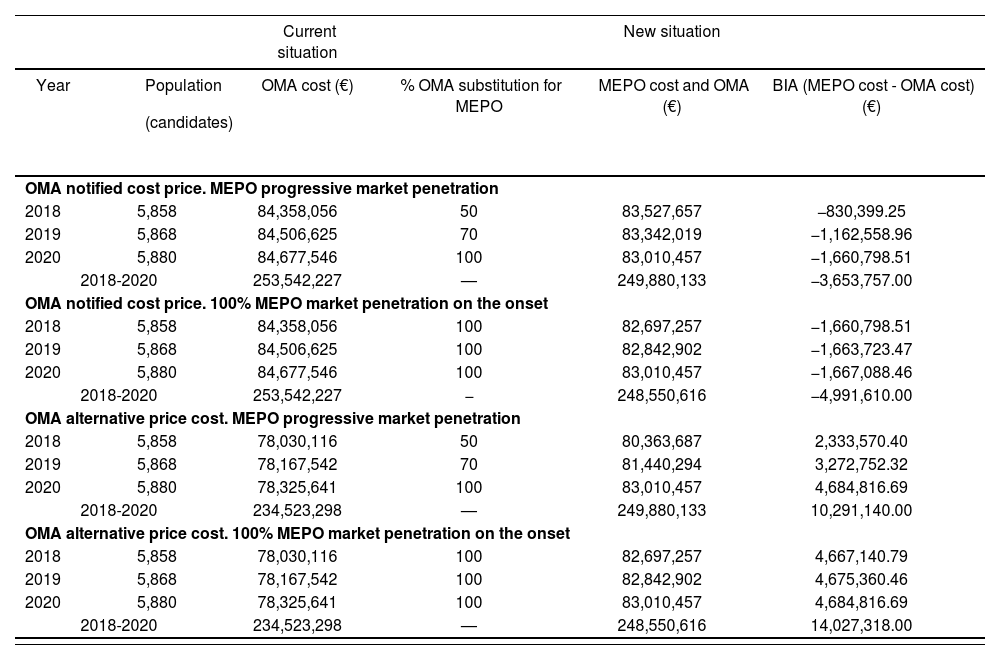
Budget impact analysis results in patients with allergic eosinophilic asthma and IgE mediated (year 2018-2020)
| Current situation | New situation | ||||
|---|---|---|---|---|---|
| Year |
| OMA cost (€) | % OMA substitution for MEPO | MEPO cost and OMA (€) | BIA (MEPO cost - OMA cost) (€) |
| OMA notified cost price. MEPO progressive market penetration | |||||
| 2018 | 5,858 | 84,358,056 | 50 | 83,527,657 | −830,399.25 |
| 2019 | 5,868 | 84,506,625 | 70 | 83,342,019 | −1,162,558.96 |
| 2020 | 5,880 | 84,677,546 | 100 | 83,010,457 | −1,660,798.51 |
| 2018-2020 | 253,542,227 | — | 249,880,133 | −3,653,757.00 | |
| OMA notified cost price. 100% MEPO market penetration on the onset | |||||
| 2018 | 5,858 | 84,358,056 | 100 | 82,697,257 | −1,660,798.51 |
| 2019 | 5,868 | 84,506,625 | 100 | 82,842,902 | −1,663,723.47 |
| 2020 | 5,880 | 84,677,546 | 100 | 83,010,457 | −1,667,088.46 |
| 2018-2020 | 253,542,227 | − | 248,550,616 | −4,991,610.00 | |
| OMA alternative price cost. MEPO progressive market penetration | |||||
| 2018 | 5,858 | 78,030,116 | 50 | 80,363,687 | 2,333,570.40 |
| 2019 | 5,868 | 78,167,542 | 70 | 81,440,294 | 3,272,752.32 |
| 2020 | 5,880 | 78,325,641 | 100 | 83,010,457 | 4,684,816.69 |
| 2018-2020 | 234,523,298 | — | 249,880,133 | 10,291,140.00 | |
| OMA alternative price cost. 100% MEPO market penetration on the onset | |||||
| 2018 | 5,858 | 78,030,116 | 100 | 82,697,257 | 4,667,140.79 |
| 2019 | 5,868 | 78,167,542 | 100 | 82,842,902 | 4,675,360.46 |
| 2020 | 5,880 | 78,325,641 | 100 | 83,010,457 | 4,684,816.69 |
| 2018-2020 | 234,523,298 | — | 248,550,616 | 14,027,318.00 | |
BIA: budget impact analysis; MEPO: mepolizumab; OMA: omalizumab.
This study evaluated the incremental cost and cost of treatment in the BIA analysis 1, and cost per avoided exacerbation and treatment cost in the study population in analysis 2.
Scenario analysis and uncertaintyIn analysis 1, several scenarios of gradual market penetration of mepolizumab replacing omalizumab (50, 70 and 100%) were carried out, and with different prices of omalizumab –notified and alternative prices–. In analysis 2, a sensitivity study was performed in order to assess the uncertainty about the minimum and maximum values of the confidence interval, 95% of relative risks (RR) of the variables (relevant clinical exacerbation, hospitalization and emergency care), as well as hospitalization costs and emergency care (Table 1). Analyses were performed using Microsoft Excel 2016®.
ResultsThe estimated study population is shown in table 1.
Analysis 1. Eosinophilic allergic asthma population and IgE-mediatedA mepolizumab average incremental cost opposed to omalizumab (alternative price) was estimated to be 797 euros per patient and year, although depending on each patient and dosage of omalizumab. In table 2, BIA data is shown according to its market penetration, notified or alternative price and year for IgE mediated eosinophilic asthma patients. Considering omalizumab's alternative discounted price, the scenario where mepolizumab could be included for patients with lgE mediated eosinophilic allergic asthma would cause an increase public spending from 2.3 to 4.6 million euros, according to the year and degree of mepolizumab's market penetration. The budgetary impact in three years would bring, either an increase of 10.3 million euros with gradual market penetration, or 14 million euros in a scenario where omalizumab would completely be replaced by mepolizumab. Considering omalizumab's notified price –which is greater than the alternative price–, the gradual introduction of mepolizumab in the NHS would save 3.6 million euros over three years, while the complete replacement of omalizumab for mepolizumab could reduce about 5 million of euros of public spending.
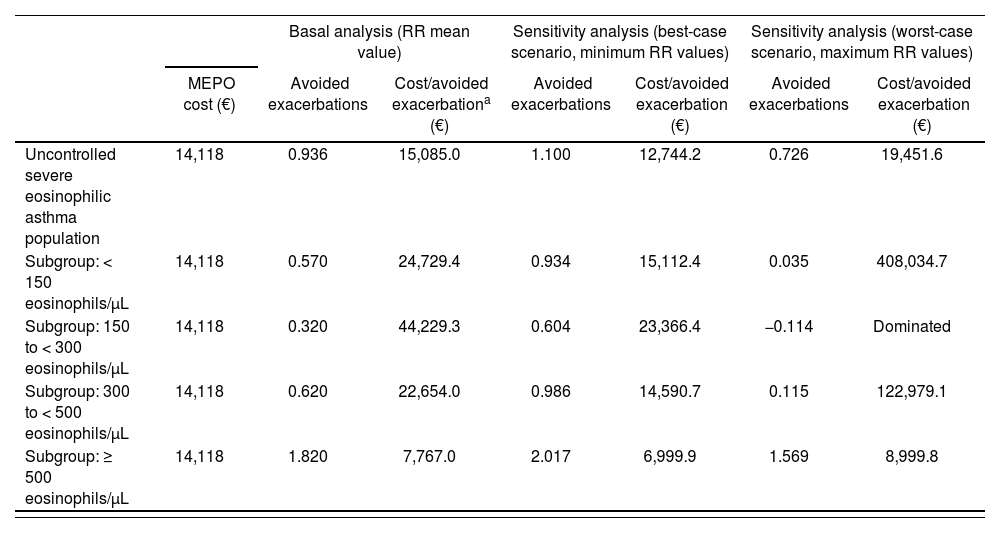
Analysis 2. Population with non lgE-mediated severe refractory asthmaTable 3 shows the data cost per avoided exacerbation applicable to people with non IgE-mediated eosinophilic severe refractory asthma, which constitutes 70% of the susceptible population of treatment for which the therapeutic alternative considered was ICS + LABA and/or OCS. The cost per avoided exacerbation by adding mepolizumab is 15,085 euros. Patients subgroups data according to their eosinophils plasma show a cost of 7,767 euros per avoided exacerbation (≥ 500 eosinophils/uL patients) for the group whose basal affectation is greater.
Cost per avoided exacerbation in population with severe refractory eosinophilic asthma (applicable to non IgE-mediated asthmatic patients) in 2018
| Basal analysis (RR mean value) | Sensitivity analysis (best-case scenario, minimum RR values) | Sensitivity analysis (worst-case scenario, maximum RR values) | |||||
|---|---|---|---|---|---|---|---|
| MEPO cost (€) | Avoided exacerbations | Cost/avoided exacerbationa (€) | Avoided exacerbations | Cost/avoided exacerbation (€) | Avoided exacerbations | Cost/avoided exacerbation (€) | |
| Uncontrolled severe eosinophilic asthma population | 14,118 | 0.936 | 15,085.0 | 1.100 | 12,744.2 | 0.726 | 19,451.6 |
| Subgroup: < 150 eosinophils/μL | 14,118 | 0.570 | 24,729.4 | 0.934 | 15,112.4 | 0.035 | 408,034.7 |
| Subgroup: 150 to < 300 eosinophils/μL | 14,118 | 0.320 | 44,229.3 | 0.604 | 23,366.4 | −0.114 | Dominated |
| Subgroup: 300 to < 500 eosinophils/μL | 14,118 | 0.620 | 22,654.0 | 0.986 | 14,590.7 | 0.115 | 122,979.1 |
| Subgroup: ≥ 500 eosinophils/μL | 14,118 | 1.820 | 7,767.0 | 2.017 | 6,999.9 | 1.569 | 8,999.8 |
MEPO: mepolizumab; RR: relative risk.
The sensitivity study shows that the RR is a very sensitive variable to the patients subgroups’ results. By taking maximum values of RR in < 500 eosinophils/µL subgroups, higher costs for avoided exacerbation are obtained, which are more than 100,000 additional euros opposed to the general population with uncontrolled eosinophilic asthma. In contrast, in a scenario of minimum RR values for the subgroup of patients with levels from 300 to < 500 eosinophils/µL, mepolizumab would cost 14,591 euros per avoided exacerbation.
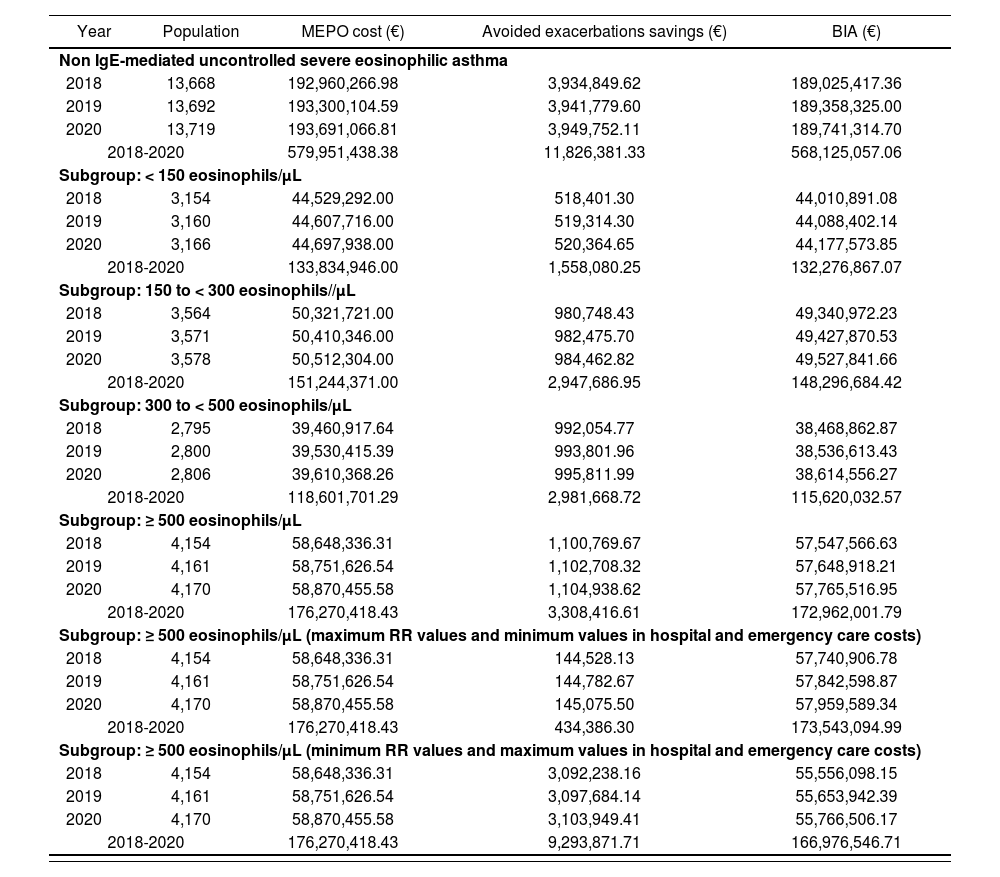
Table 4 provides BIA data for the non IgE-mediated refractory eosinophilic asthma population, as well as for subgroups according to plasma levels of eosinophils. The annual budgetary impact of population with non IgE-mediated eosinophilic asthma would reach 189 million euros (568.1 million over three years). If we add this amount to the result in three years of BIA for patients with IgE-mediated eosinophilic asthma (30% of the overall patients with eosinophilic asthma), and assuming a progressive market penetration of mepolizumab (10.3 million according to Table 2), a total BIA of 578.4 million euros for the population. The BIA for non IgE-mediated eosinophilic asthma population, which is divided into subgroups according to their eosinophils plasma levels (Table 4) gives us some estimates of annual 57.5 million in the subgroup with ≥ 500 cells/µL eosinophil count –which translates into 173 m in three years–. If we add the BIA result with a progressive introduction of mepolizumab in three years for IgE-mediated eosinophilic asthma patients to the use of mepolizumab's BIA, only in people with non IgE-mediated eosinophilic asthma and ≥ 500 eosinophils/µL levels, the BIA for all the population in three years would be 183.2 million euros.
Mepolizumab budget impact analysis for non lgE-mediated severe eosinophilic asthma population and subgroups during 2018-2020 (subgroup analysis sensitivity ≥ 500 eosinophils/μL)
| Year | Population | MEPO cost (€) | Avoided exacerbations savings (€) | BIA (€) |
|---|---|---|---|---|
| Non IgE-mediated uncontrolled severe eosinophilic asthma | ||||
| 2018 | 13,668 | 192,960,266.98 | 3,934,849.62 | 189,025,417.36 |
| 2019 | 13,692 | 193,300,104.59 | 3,941,779.60 | 189,358,325.00 |
| 2020 | 13,719 | 193,691,066.81 | 3,949,752.11 | 189,741,314.70 |
| 2018-2020 | 579,951,438.38 | 11,826,381.33 | 568,125,057.06 | |
| Subgroup: < 150 eosinophils/μL | ||||
| 2018 | 3,154 | 44,529,292.00 | 518,401.30 | 44,010,891.08 |
| 2019 | 3,160 | 44,607,716.00 | 519,314.30 | 44,088,402.14 |
| 2020 | 3,166 | 44,697,938.00 | 520,364.65 | 44,177,573.85 |
| 2018-2020 | 133,834,946.00 | 1,558,080.25 | 132,276,867.07 | |
| Subgroup: 150 to < 300 eosinophils//μL | ||||
| 2018 | 3,564 | 50,321,721.00 | 980,748.43 | 49,340,972.23 |
| 2019 | 3,571 | 50,410,346.00 | 982,475.70 | 49,427,870.53 |
| 2020 | 3,578 | 50,512,304.00 | 984,462.82 | 49,527,841.66 |
| 2018-2020 | 151,244,371.00 | 2,947,686.95 | 148,296,684.42 | |
| Subgroup: 300 to < 500 eosinophils/μL | ||||
| 2018 | 2,795 | 39,460,917.64 | 992,054.77 | 38,468,862.87 |
| 2019 | 2,800 | 39,530,415.39 | 993,801.96 | 38,536,613.43 |
| 2020 | 2,806 | 39,610,368.26 | 995,811.99 | 38,614,556.27 |
| 2018-2020 | 118,601,701.29 | 2,981,668.72 | 115,620,032.57 | |
| Subgroup: ≥ 500 eosinophils/μL | ||||
| 2018 | 4,154 | 58,648,336.31 | 1,100,769.67 | 57,547,566.63 |
| 2019 | 4,161 | 58,751,626.54 | 1,102,708.32 | 57,648,918.21 |
| 2020 | 4,170 | 58,870,455.58 | 1,104,938.62 | 57,765,516.95 |
| 2018-2020 | 176,270,418.43 | 3,308,416.61 | 172,962,001.79 | |
| Subgroup: ≥ 500 eosinophils/μL (maximum RR values and minimum values in hospital and emergency care costs) | ||||
| 2018 | 4,154 | 58,648,336.31 | 144,528.13 | 57,740,906.78 |
| 2019 | 4,161 | 58,751,626.54 | 144,782.67 | 57,842,598.87 |
| 2020 | 4,170 | 58,870,455.58 | 145,075.50 | 57,959,589.34 |
| 2018-2020 | 176,270,418.43 | 434,386.30 | 173,543,094.99 | |
| Subgroup: ≥ 500 eosinophils/μL (minimum RR values and maximum values in hospital and emergency care costs) | ||||
| 2018 | 4,154 | 58,648,336.31 | 3,092,238.16 | 55,556,098.15 |
| 2019 | 4,161 | 58,751,626.54 | 3,097,684.14 | 55,653,942.39 |
| 2020 | 4,170 | 58,870,455.58 | 3,103,949.41 | 55,766,506.17 |
| 2018-2020 | 176,270,418.43 | 9,293,871.71 | 166,976,546.71 | |
BIA: budget impact analysis; MEPO: mepolizumab; RR: relative risk.
Table 4 shows a sensitivity study on mepolizumab's budgetary impact for the subgroup of patients with ≥ 500 eosinophils/µL. It illustrates the variations in the BIA that could occur in mepolizumab's best and worst scenario, varying costs of emergency, hospitalization and RR of clinically relevant exacerbations. It is observed that the BIA of this subgroup in three years ranges from 166.9 to 173.5 million euros.
DiscussionThe emergence of high economic impact drugs makes economic studies necessary in order to favor the optimization of resources21. This economic evaluation compares two therapeutic alternatives in a group of patients diagnosed with eosinophilic asthma, showing signs that are consistent with the IgE-mediated persistent allergic asthma phenotype. The economic analysis design can help in clinical decision making to improve efficiency through price competition.
The health outcome was assessed by the number of avoided clinically relevant exacerbations with the use of mepolizumab. The selected variable is adequate to guide decision-making, as other studies assessed the decrease in hospital admissions, emergency room visits or primary care physicians22–24. On the other hand, the comparisons made regarding treatment alternatives (omalizumab and high dose of ICS + LABA and/or OCS) improve the validity of the study.
This study has limitations, such as the lack of effective comparative evidence and quality between mepolizumab and omalizumab in IgE-mediated eosinophilic asthma patients who are candidate population for both therapies, and specific intersection of the two sets, which lack empirical data. There have been two studies25,26 –one funded by GlaxoSmithKline laboratories– that performed an indirect comparison of mepolizumab against omalizumab in patients diagnosed with eosinophilic asthma and who show signs and symptoms that are consistent with the persistent allergic asthma phenotype. Although both describe no difference in efficacy between mepolizumab and omalizumab, they highlight the impossibility of making preferential use recommendations of one drug over another, due to its high heterogeneity between trials and different selection criteria for the use of both drugs. An indirect comparison analysis cannot be reliable, as mepolizumab was studied in eosinophilic component-mediated asthma, regardless of the IgE values, while omalizumab was studied in IgE-mediated asthma regardless of the eosinophilic component, and is used in patients with elevated IgE nonresponders to other treatments. These limitations were highlighted in reports evaluating mepolizumab in countries such as Canada27 and the United Kingdom28. Therefore, there has not been a cost minimization, but instead, it would be reasonable to select drugs by comparing costs, except for certain patients who, for any valid clinical reason, prefer one or avoid another.
Upon completion of the study, two other drugs with a similar mechanism of action to mepolizumab's were approved, although they were not yet sold in Spain, therefore, they were not the subject of this study14,15. Once marketed, and considering that they have not been compared to mepolizumab, an assessment on whether the possible indirect comparisons detect clinically relevant differences should be performed, taking into account the level of eosinophils in plasma. Its introduction in therapy could allow competition and reduce the budgetary impact of these agents. Its non inclusion in this study is a limitation that should be addressed in subsequent studies, which should be focused on these similar treatments’ potential competition once the first one –mepolizumab– is already marketed. Further comparison of these drugs in the same group would be appropriate, but also complex, because they have not been directly compared. These studies have different inclusion criteria and different subgroups definition, according to eosinophil count in blood.
Previous studies show that patients with elevated plasma eosinophil count benefit more patients, as opposed to low level patients8,9. It was observed in this economic analysis that patients with ≥ 500 eosinophils/µL showed a more favorable incremental cost-efficacy compared to those with lower counts. It should be stressed that subgroup analysis on pivotal trials meets the pre-specification, interaction, consistency in different studies7,12 and biological plausibility criteria. The published economic evaluation studies on mepolizumab with refractory eosinophilic asthma population, regardless of subgroup analysis, concluded that mepolizumab is not cost-effective, urging price discounts around 60-70% to become funding-recommended by the healthcare systems29,30. Bermejo I et al.28 described the assessment process on mepolizumab by the National Institute for Health and Care Excellence (NICE). In its economic assessment study, the target population was defined in terms of severity of asthma and ≥ 300 eosinophils/µL levels. They have shown to not be cost-effective for this subgroup of patients, and its use was recommended only when the laboratory provides an agreed and confidential price discount, so that it becomes cost-effective for said subgroup of patients.
To conclude, comparative clinical evidence is lacking quality between mepolizumab and omalizumab in eosinophilic component-mediated asthma and lgE mediated patients. Nor are there other economic evaluation studies comparing these two drugs. For this reason, a cost comparison in these patients was performed. From Spanish NHS perspective, and considering the high economic impact of mepolizumab, it would be reasonableto use the lower-cost drug and promote price competition. This strategy does not exclude the exceptional justified preference of a particular therapy by a patient. After this pharmacoeconomic analysis, prioritizing the use of mepolizumab in patients diagnosed with non lgE-mediated severe refractory eosinophilic asthma with high plasma levels of eosinophils (≥ 500 cells/µL), as indicated in Therapeutic Positioning Report on mepolizumab by the Spanish Agency of Medicines, would significantly improve the efficiency and reduce its budgetary impact12.
FundingNo funding.
Conference PresentationsPreliminary data as part of the work in the form of communication were presented under the name of: “Application of pharmacoeconomic evaluation by subgroups to severe refractory eosinophilic asthma”. 15th Andalusian Society of Hospital Pharmacy Congress, Almería, April 11-13, 2018.
Conflict of interestsNo conflict of interest.
Contribution to the scientific literatureStudy's contribution to existing knowledge: First published national data on mepolizumab's efficiency and budgetary impact for asthma patients.
Implications of the findings for practice, research, healthcare policies or general hospital pharmacy: Optimization of the use in the practice of mepolizumab by comparing costs and subgroup analysis.