The purpose of this systematic review is to analyze the published data on the efficacy and safety of doses higher than 180 mg/m2 of irinotecan recommended in the drug's summary of product characteristics in metastatic colorectal cancer patients with genotypes UGT1A1*1/*1 or *1/*28 who are treated with the FOLFIRI regimen.
MethodA systematic review of the literature was carried out in Medline and Embase searching for articles published up to December 2021. The methods used were based on the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The criteria for the inclusion of studies were previously defined based on the two secondary goals addressed in this review: 1) To analyze the magnitude of the differences in clinical responses and 2) To study the magnitude of the differences in adverse effects of irinotecan at high doses, as compared to the doses described in the summary of product characteristics corresponding to the FOLFIRI regimen in patients with metastatic colorectal cancer with genotypes UGT1A1*1/* 1 or *1/*28.
ResultsThe search yielded a total of 985 references, of which 13 were selected for analysis. Seven evaluated both efficacy and safety and six only safety. With regard to the studies that evaluated both efficacy and safety, six out of seven (85.7%) were in favor of increasing irinotecan dose according to the objective response rate and progression-free survival. Two of them even recommended dose increases based on overall survival. Irinotecan safety studies suggest that doses higher than 180 mg/m2 are tolerated by most UGT1A1*1/*1 and *1/*28 patients.
ConclusionsThe present systematic review shows the advisability of considering adjusting the dose of irinotecan when used as part of the FOLFIRI regimen based on the polymorphisms of the UGT1A1 gene as this may increase the likelihood of an adequate clinical response.
El objetivo de la presente revisión sistemática es analizar los datos publicados sobre la eficacia y seguridad de las dosis superiores a los 180 mg/m2 de irinotecán recomendadas en la ficha técnica en pacientes con cáncer colorrectal metastásico tratados con el esquema FOLFIRI y con genotipo UGT1A1*1/*1 y *1/*28.
MétodoSe realizó una revisión sistemática mediante una búsqueda bibliográfica en Medline y Embase de los artículos publicados hasta diciembre de 2021. Los métodos utilizados se basaron en los recomendados según Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Los criterios para la inclusión de los estudios se definieron previamente en base a los dos objetivos secundarios que aborda esta revisión: 1) Analizar la magnitud de la diferencia de la respuesta clínica y 2) estudiar la magnitud de la diferencia de los efectos adversos a irinotecán a dosis altas, en comparación con las dosis descritas en la ficha técnica para el esquema FOLFIRI en pacientes con cáncer colorrectal metastásico con el genotipo UGT1A1*1/*1 o *1/*28.
ResultadosLa estrategia de búsqueda reportó un total de 98 referencias, de las que 13 fueron seleccionadas para el análisis, 7 (53,8%) evaluando tanto eficacia como seguridad y 6 (46,2%) únicamente seguridad. En relación con los estudios que evaluaron eficacia y seguridad, 6 (85,7%) se mostraron favorables al aumento de dosis en términos de tasa de respuesta objetiva y supervivencia libre de progresión e, incluso, en 2 de ellos en supervivencia global. Los estudios que evaluaron seguridad apuntan a que dosis de irinotecán superiores a 180 mg/m2 son toleradas por la mayor parte de los pacientes UGT1A1*1/*1 y *1/*28.
ConclusionesLa presente revisión sistemática muestra la conveniencia de valorar el ajuste de dosis de irinotecán dentro del esquema FOLFIRI en función de los polimorfismos del gen UGT1A1, con un potencial aumento de las probabilidades de una adecuada respuesta clínica.
Irinotecan (CPT-11) is an antineoplastic agent used to treat several types of neoplasms such as gastric, pancreatic or colorectal cancer. It is a semi-synthetic derivative of camptothecin, which exerts its anti-tumor effect through the inhibition of topoisomerase I, an indispensable enzyme to cleave the double DNA strand during the cell replication process1. Specifically, irinotecan is a prodrug capable of converting to its active metabolite, 7-ethyl-10-hydroxy-camptothecin (SN-38), through the activity of liver carboxylesterases. This metabolite, SN-38, is responsible for the antineoplastic effect and toxicity of irinotecan. The latter has been reported to occur in a dose-limiting form in up to one-third of patients, with life-threatening diarrhea and neutropenia1.
Elimination of SN-38 requires its conjugation through the activity of uridine diphosphate glucuronyl transferase (UGT1A1), giving rise to a new inactive glucuronide metabolite, SN-38G, excreted through the kidneys or the biliary tract. Thus, several studies have shown that a decrease in this glucuronidation ability is correlated with increased toxicity of irinotecan2,3. It has been shown that a decrease in irinotecan's glucuronidation could be due to the presence of a polymorphism in the promoter region of the UGT1A1 gene (TA indel, rs8175347), which consists in a variation in the number of repeats of the TA dinucleotide and implies a decreased activity of the UGT1A1 enzyme.
In Caucasians, the most common form (wild-type allele) of this promoter region is made up of six A(TA)6TAA repeats, which corresponds to normal activity levels of the enzyme. Nevertheless, the prevalence of the mutated allele (UGT1A1*28), which consists in seven repeats (A(TA)7TAA) and confers a decreased enzyme activity, is estimated to be from 30 to 40%4. Specifically, the incidence of the mutated homozygous genotype (UGT1A1*28/*28) (Gilbert's syndrome) in the Spanish population is approximately 9%, whereas the heterozygous genotype (UGT1A1*1/* 28) is approximately 51% and the wild-type genotype (UGT1A1 *1/*1) 40%5.
Several pharmacogenetic studies have shown a correlation between the presence of the UGT1A1*28 allele and the appearance of severe irinotecan-induced adverse events6–12. Drawing on this evidence, in 2005 the FDA reported the clinical usefulness of genotyping the UGT1A1 gene in patients who are candidates to receive irinotecan. However, the drug's summary of product characteristics (SmPC) does not recommend specific doses of irinotecan for different antineoplastic regimens according to the patients’ genetic profiles13. Moreover, no mention is made of the possibility of using higher than normal doses of irinotecan (180 mg/m2) as part of the FOLFIRI regimen (5-fluorouracil, folinic acid and irinotecan) in patients with the UGT1A1*1/*1 and *1/*28 genotypes14–17.
The purpose of the present systematic review is to analyze the data published on the effectiveness and safety of irinotecan at doses above the 180 mg/m2 recommended in the drug's SmPC in patients with metastatic colorectal cancer (mCRC) treated with the FOLFIRI regimen according to the UGT1A1 genotype.
MethodsLiterature searchA systematic literature search was conducted for articles published until December 2020 discussing the efficacy and safety of high irinotecan doses in wild-type patients or heterozygous for the UGT1A1*28 allele. The databases reviewed were Medline and Embase.
The MeSH and Emtree terms used are summarized in table 1. The methods used for the study were based on the recommendations of the PRISMA Statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)18, applying both the checklist and the flow diagram.
Search strategy on Pubmed
| Database | Search terms | Limits | Results | |
|---|---|---|---|---|
| Mesh terms | Pubmed |
| Adult: 19+ years, Adult: 19-44 years, Middle Aged + Aged: 45+ years, Middle Aged: 45-64 years, Aged: 65+ years, 80 and over: 80+ years, Young Adult: 19-24 years. | 82 |
| Emtree terms | Embase |
|
| 16 |
The inclusion criteria were defined in advance with consideration of the two secondary goals of this review, specifically:
- 1.
To analyze the magnitude of the differences between the clinical response to high doses of irinotecan and the response to the doses recommended in the product's SmPC for the FOLFIRI regimen in patients with mCRC with the UGT1A1*1/*1 or *1/*28 genotype.
- 2.
To study the magnitude of the differences between the adverse events resulting from high doses of irinotecan and those arising from the doses recommended in the product's SmPC in patients with mCRC with the UGT1A1*1/*1 or *1/*28 genotype.
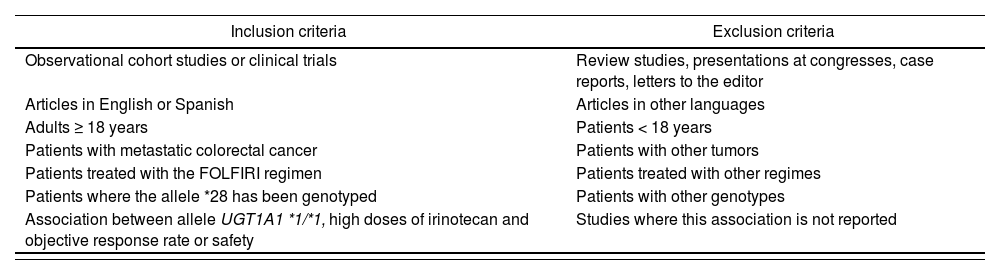
Studies inclusion was decided based on whether: (a) they were observational cohort studies or clinical trials; (b) patients with mCRC received higher doses of irinotecan than those currently recommended in the drug's SmPC; (c) patients were genotyped to identify the presence of the UGT1A1*28 allele and patients with the UGT1A1 *1/*1 genotype (wild-type) were included; and (d.1) the association between the UGT1A1 *1/*1 genotype, high doses of irinotecan and the objective response rate (ORR) was mentioned (patients were deemed to be responsive when a full or partial response was obtained), or (d.2) the association between the UGT1A1 *1/*1 genotype, high doses of irinotecan and safety was assessed. Studies not published in English or Spanish were excluded. The inclusion and exclusion criteria are detailed in table 2.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Observational cohort studies or clinical trials | Review studies, presentations at congresses, case reports, letters to the editor |
| Articles in English or Spanish | Articles in other languages |
| Adults ≥ 18 years | Patients < 18 years |
| Patients with metastatic colorectal cancer | Patients with other tumors |
| Patients treated with the FOLFIRI regimen | Patients treated with other regimes |
| Patients where the allele *28 has been genotyped | Patients with other genotypes |
| Association between allele UGT1A1 *1/*1, high doses of irinotecan and objective response rate or safety | Studies where this association is not reported |
The four authors evaluated independently the article abstracts to determine their eligibility in accordance with the inclusion/exclusion criteria outlined in table 2. All original peer-reviewed articles published in English that evaluated the efficacy and/or toxicity of high doses of irinotecan (> 180 mg/m2) according to the UGT1A1 genotype in adult patients with mCRC were considered for inclusion. Once the eligible abstracts were selected, the full-text versions of all potentially eligible texts were retrieved, and each of the four authors once again independently applied the inclusion/exclusion criteria. Finally, the references included in the study were accurately analyzed to find potentially relevant articles not identified in the initial systematic search. Articles were deemed to be worthy of inclusion when at least three of the four authors agreed with the pre-established criteria. The inter-class correlation (ICC) coefficient was calculated to determine the degree of concordance between the authors regarding article selection.
Data extractionThe reviewers created, tested and improved a data extraction template. The data extracted included: first author and year of publication; type of study and level of evidence; participants and type of cancer; irinotecan regimen and dosage; genotype and prevalence; clinical variables assessed [ORR, overall survival (OS) and progression-free survival (PFS)]; results and discussion (Tables 3 and 4).
Characteristics of the studies included with respect to the efficacy of high doses irinotecan
| First author and year of publication | Type of study | Participants and type of cancer | Regimen, dose and frequency of irinotecan administration | Genotypes and prevalence | Clinical variables analyzed | Results | Discussion |
|---|---|---|---|---|---|---|---|
| Efficacy and tolerance | |||||||
| Páez et al. 201916 | Phase II interventional clinical trial |
| FOLFIRI Control group: standard dose Experimental group: *1/*1: 300 mg/m2 and *1/*28: 260 mg/m2 | *1/*1: 37 (46.8%) (13, 32.5% experimental group, 24, 61.5% control group) *1/*28: 42 (53.2%) (27, 67.5% experimental group, 15, 38.5% control group) |
|
| Patients with *1/*1 and *1/*28 genotypes may receive irinotecan doses of > 180 mg/m2 as part of a FOLFIRI regimen and obtain better ORRs without significantly increases of toxicity. No significant differences were observed in PFS or OS, probably due to the small size of the sample. |
| Lu et al. 201419 | Observational retrospective cohort study |
| FOLFIRI + bevacizumab Two groups of patients: Genotyping and dose escalation vs no genotyping or dose escalation Initial dose *1/*1 and *1/*28: 180 mg/m2*28/*28: 120 mg/m2 Dose escalation every three cycles 30 mg/m2 | *1/*1: 61 (77.2%) *1/*28: 13 (16.5%) *28/*28: 5 (6.3%) |
|
|
|
| Tsai et al. 202017 | Phase III interventional clinical trial |
| FOLFIRI + bevacizumab Control group: standard dose Experimental group: increasing dose based on genotype: *1/*1: 260 mg/m2 and *1/*28: 240 mg/m2 | *1/*1: 1 97 (76.6%) *1/*28: 57 (21.5%) *28/*28: 5 (1.9%) |
|
| Regardless of the status of the KRAS gene, UGT1A1 gene genotyping allows patients to tolerate higher doses of irinotecan and potentially achieve a more favorable clinical results without significantly higher toxicity levels. |
| Phelip et al. 201620 | Phase II interventional clinical trial |
|
| *1/*1: 8 (34.6%) *1/*28: 13 (53.9%) *28/*28: 2 (11.5%) |
|
| Genotype-guided dose escalation resulted in better response rates, with ensuing higher resectability rates in the HD patients group, without a noticeable effect on toxicity. |
| Phase II interventional clinical trial |
| FOLFIRI + cetuximab *1/*1: 210 mg/m2, increasing 20% at every cycle up to 293 mg/m2*1/*28: 180 mg/m2, increasing 20% at every cycle up to 274 mg/m2*28/*28: 126 mg/m2, increasing 10% at every cycle up to 153 mg/m2 | *1/*1: 44 (51.8%) *1/*28: 34 (40.0%) *28/*28: 7 (8.2%) |
|
| No statistically significant differences were observed regarding efficacy and tolerance in any of the three patient groups. The incidence of AE was 33.3%, which is in line with that reported by other authors. |
| Efficacy and tolerance | |||||||
| Lu et al. 201522 | Observational retrospective cohort study |
| FOLFIRI + bevacizumab Two groups of patients: *1/*1; *1/*28 and *28/*28 Genotyping and dose escalation vs. no genotyping or escalation Initial dose *1/*1 and *1/*28: 180 mg/m228/*28: 120 mg/m2 Dose escalation every three cycles 30 mg/m2 | *1/*1 and *1/*28: 64 (92.86%) *28/*28: 6 (7.14%) |
|
| Irinotecan dose escalation in unmutated genotypes makes it possible to administer higher doses and achieve better response rates and more effective control of the disease. It also leads to a significantly higher PFS, without increasing AE. |
| Hebbar et al. (2013)23 |
|
| FOLFIRI Irinotecan dose: 260 mg/m2 |
|
|
| The HD-FOLFIRI regimen was well tolerated, with only 5 patients developing grade 3 toxicity. Use of these higher doses allows a more effective removal of metastases and an increase in OS. |
| Manfredi et al. 201524 |
|
| FOLFIRI + bevacizumab Irinotecan dose: 260 mg/m2 |
|
|
| The ORR of the patients (around 53-59%) did not appear to be higher than that observed for the FOLFIRI regimen by other authors (47-49%). As regards toxicity, the study had to be discontinued as one of the discontinuation criteria (≥ 3 severe toxicity events) was fulfilled. Despite these events, which occurred at the outset, toxicity was successfully address by reducing the dose administered. After this, most patients kept receiving their treatment with acceptable tolerability levels. |
| Tolerance | |||||||
| Kim et al. 201512 |
|
| FOLFIRI, with increasing doses of irinotecan | *1/*1: 31 (73.07%) *1/*28: 9 (23.07%) *28/*28: 1 (3.84%) |
|
| Irinotecan dose escalation as part of the FOLFIRI regimen was well tolerated by patients who were not mutated homozygotes or double heterozygotes for *28 and *6. |
| Kim et al. 201315 |
|
| Irinotecan with capecitabine. Dose escalation | *1/*1: 37 (74.19%) *1/*28: 12 (22.58%) *28/*28: 1 (3.22%) |
|
| The results of the study indicate that dose escalation is safe is patients who are not mutated homozygotes or double heterozygotes for *28 and *6. |
| Tolerance | |||||||
| Toffoli G et al. 201714 | Phase I interventional clinical trial |
| FOLFIRI + bevacizumab Only patients *!/*! or *l/*28 | *]/*]: 25 (52.0%) *1/*28: 23 (47.9%) | Threshold for dose escalation with grade 3/4 AEs |
| The MTD of irinotecan, used as part of FOLFIRI + bevacizumab, was de 310 mg/m2 for UGT1A1 *1/*1 patients and 260 mg/m2 for *1/*28 patients. |
| Toffoli G et al. 201011 | Phase I interventional clinical trial |
| FOLFIRI Only patients *1/*1 or *l/*28 Initial dose of irinotecan 215 mg/m2 | *1/*1: 32 (55.5%) *1/*28: 22 (38.0%) *28/*28: 5 (6.5%) | Threshold for dose escalation with grade 3/4 AEs |
| The recommended irinotecan dose of 180 mg/m2 as part of the FOLFIRI regimen is substantially lower than the dose that can be tolerated when patients with the UGT!A!*28/*28 genotype are excluded. Prospective genotype-based studies should test the efficacy of higher irinotecan doses as part of a FOLFIRI regimen. |
| Marcuello et al. 201110 | Phase I-IV interventional clinical trial |
| FOLFIRI Dose escalation of irinotecan |
|
|
| The recommended irinotecan dose of 180 mg/m2 in the FOLFIRI regimen is considerably lower than the tolerable doses when patients with the UGT!A!*28/*28 genotype are excluded. |
AE: adverse event; CRC: colorectal cancer; DCR: disease control ratio; HD: high dose; HR: hazard ratio; MTD: maximum tolerated dose; ORR: objective response rate; OS: overall survival; PFS: progression-free survival.
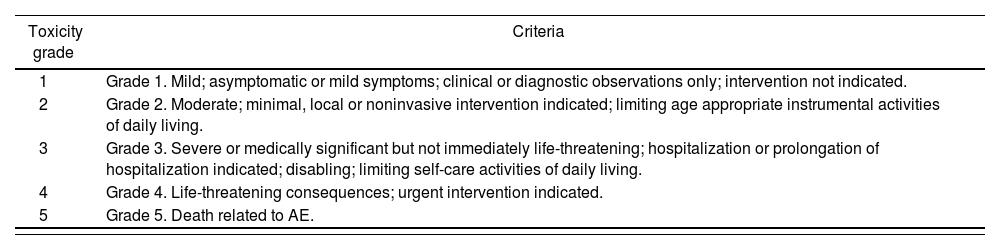
National Cancer Institute Common Terminology Criteria for adverse events25
| Toxicity grade | Criteria |
|---|---|
| 1 | Grade 1. Mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated. |
| 2 | Grade 2. Moderate; minimal, local or noninvasive intervention indicated; limiting age appropriate instrumental activities of daily living. |
| 3 | Grade 3. Severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care activities of daily living. |
| 4 | Grade 4. Life-threatening consequences; urgent intervention indicated. |
| 5 | Grade 5. Death related to AE. |
AE: adverse event.
The severity of adverse events was established in accordance with the Common Toxicity Criteria of the National Cancer Institute (now called the Common Terminology Criteria for Adverse Events; CTCAE), when they were present (Table 4)25.
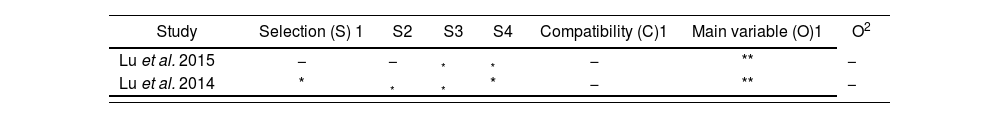
Level of evidenceThe methodological quality of eligible studies was evaluated based on discussions and consensus-building among all the authors of the study, with special focus on the use of objective evaluation criteria and a representative sample. The quality of the observational studies included was evaluated in accordance with the recommendations of the Newcastle-Ottawa (NOS) scale26,27. Eight items were selected for inclusion in the study, including patient selection, comparability between groups, and exposure factors. Studies with NOS scores from 0 to 3, 4 to 6 and 6 to 9 were considered of low, medium and high quality, respectively.
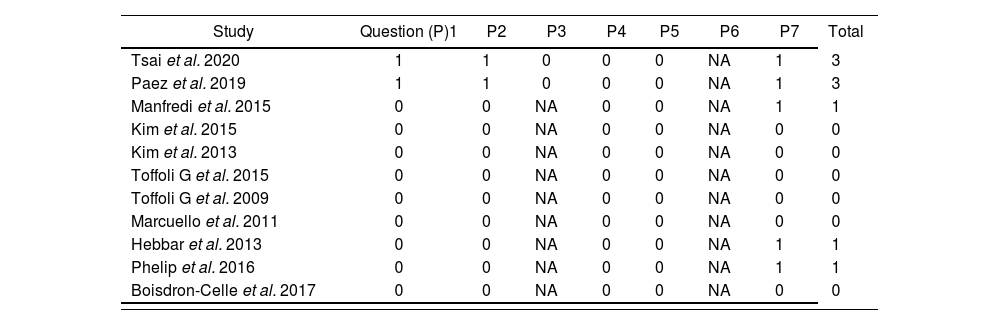
The methodological clinical trials quality was evaluated with Jadad,’s scale, also known as Jadad's score or Oxford quality scoring system, a procedure used to independently evaluate the methodological quality of a clinical trial. The scale assigns a score which rates study as low quality if the score < 3 points, and as rigorous in the case of randomized clinical trials (RCTs), if the score is > 5 points28.
ResultsStudy selectionThe search strategy yielded a total of 595 references, of which 13 were selected for the analysis of the effectiveness and safety of high doses of irinotecan (>180 mg/m2) when used as part of the FOLFIRI regimen. Specifically, 11 clinical trials and two observational studies were included. ICC was 0.90 (95% CI 0.84–0.94), for the total number of studies included; 1 (95% CI 1–1) for studies dealing with effectiveness; and 0.83 (95% CI 0.71–0.92) for studies dealing with safety.
Figure 1 shows the flow diagram (adapted from PRISMA) showing the process followed to include the 13 studies, 6 of them evaluating toxicity and 7 evaluating both effectiveness and toxicity, in patients treated for mCRC with high doses of irinotecan.
Table 3 shows the descriptive characteristics of the reviewed studies, including the type of study, participants, regimen, dose and rate of irinotecan administration, genotypes and prevalence, clinical variables evaluated, results and discussion.
ParticipantsA total of 992 patients treated with irinotecan with a FOLFIRI regimen for mCRC were included. In all cases, irinotecan was administered as first-line treatment (12, 92.3%) except for one study where it was administered as second-line treatment (1, 77%).
Level of evidence of the studiesThe assessment of the quality of the observational studies was carried out using the NOS scale, with all observational studies exhibiting a highquality level (Table 5). The evaluation of the quality of clinical trials, conducted using Jadad's scale, revealed that the phase I-II and IV clinical trials included in the present review, which analyzed the effectiveness and/or safety of high doses of irinotecan, were of low quality as all 11 (100%) clinical trials obtained a score ≤ 3 points, which means that none was assigned a score ≥ 5 points (Table 6).
Evaluation of the methodological quality of observational studies using the Newcastle-Ottawa (NOS) scale26,27
| Study | Selection (S) 1 | S2 | S3 | S4 | Compatibility (C)1 | Main variable (O)1 | O2 |
|---|---|---|---|---|---|---|---|
| Lu et al. 2015 | − | − | * | * | − | ** | − |
| Lu et al. 2014 | * | * | * | * | − | ** | − |
Evaluation of the methodological quality of clinical trials using Jadad's scale28
| Study | Question (P)1 | P2 | P3 | P4 | P5 | P6 | P7 | Total |
|---|---|---|---|---|---|---|---|---|
| Tsai et al. 2020 | 1 | 1 | 0 | 0 | 0 | NA | 1 | 3 |
| Paez et al. 2019 | 1 | 1 | 0 | 0 | 0 | NA | 1 | 3 |
| Manfredi et al. 2015 | 0 | 0 | NA | 0 | 0 | NA | 1 | 1 |
| Kim et al. 2015 | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 |
| Kim et al. 2013 | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 |
| Toffoli G et al. 2015 | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 |
| Toffoli G et al. 2009 | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 |
| Marcuello et al. 2011 | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 |
| Hebbar et al. 2013 | 0 | 0 | NA | 0 | 0 | NA | 1 | 1 |
| Phelip et al. 2016 | 0 | 0 | NA | 0 | 0 | NA | 1 | 1 |
| Boisdron-Celle et al. 2017 | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 |
NA: does not apply.
As for the studies evaluating the effectiveness and safety outcomes resulting from the use of high doses of irinotecan in patients not carrying the UGT1A1*28 allele, six out of the seven studies were in favor of a dose increase (Table 3). Páez D et al.16, who were among those in favor, randomized patients into a group receiving a high dose FOLFIRI regimen (HD-FOLFIRI) (experimental arm) and a group receiving FOLFIRI at the standard dose (control group). The patients in the experimental arm received 300 mg/m2 if they were wild-type and 260 mg/m2 if they harbored the UGTIA1 *1/*28 genotype, as compared with 180 mg/m2 in the control group. The patients’ response was measured in terms of ORR according to RECIST 1.1, PFS and OS criteria. Higher ORRs were obtained in patients on higher doses of irinotecan (67.5% vs 43.6%; p = 0.001), without a significant increase in associated toxicity (severe toxicity: 22.5% vs 20.5%), and without higher levels of PFS or OS.
A study by Lu et al.19, was mainly aimed at evaluating clinical response rate (CRR), PFS and OS, with statistically significant differences being found across all variables (Table 3). Patients were classified into two groups: one with and the other without dose escalation. In the first group, patients with UGT1A1 *1/*1 or *1/*28 genotypes received an initial dose of 180 mg/m2 of irinotecan and patients with an homozygous genotype for the allele *28 received an initial dose of 120 mg/m2. In the dose escalation group, the irinotecan dose was increased by 30 mg/m2 every three cycles. The authors found that the clinical response was higher is patients with dose escalation (69.4 vs 46.4%; p = 0.028), without any statistically significant difference with respect to grade 3/4 adverse events between the two groups (p = 0.189).
These results are in line with those reported by Tsai et al.17, who studied toxicity as well as effectiveness (in terms of ORR) of different irinotecan doses in patients treated with a FOLFIRI-bevacizumab regimen as first-line therapy. An experimental and a control group were established. The experimental group was treated with increasing doses of irinotecan depending on the nature of their genotype (wild types received 260 mg/m2 while heterozygotes received 240 mg/m2). The primary endpoint was evaluation of the PFS, while the secondary endpoints were measurement of the ORR, the disease control ratio (DCR) and OS. The results obtained were as follows: PFS: 14 vs 10 months (HR 0.539 [0.39–0.730], p < 0.001); ORR p < 0.001, DCR: p = 0.007, and OS: 30 vs 22 months (HR 0.693 [0.503–0.955], p < 0.025). The authors concluded that, in patients with mCRC, regardless of their KRAS status, UGT1A1 genotyping may increase the tolerability of higher doses of irinotecan, allowing favorable clinical results without significant increases in toxicity.
Another study to conduct a joint evaluation of increasing the irinotecan dose according to the patients’ UGT1A1 genotype and the safety profile was conducted by Phelip et al.20. This study included patients without previous treatment who presented with potentially resectable liver metastases, treated with six cycles of HD-FOLFIRI+cetuximab. Heterozygous patients (*1/*28) and those with a wild-type genotype (*1/*1) received a dose of irinotecan of 260 mg/m2, while patients with a mutated homozygous genotype (*28/*28) received 220 mg/m2. The primary endpoints were the resectability rate and the safety of the regimen administered. The results showed that high rates of tumor response (82.6% in patients receiving the six cycles) and of liver metastasis resection (80.7%) could be achieved, without intolerable levels of toxicity.
Boisdron-Celle et al.21 performed an evaluation of the efficacy and safety of the FOLFIRI-cetuximab regimen with high doses of irinotecan (*1/*1: 210 mg/m2, with a 20% increase at each cycle up to a maximum of 293 mg/m2; *1/*28: 180 mg/m2, with a 20%/increase at each cycle up to 274 mg/m2; and *28/*28: 126 mg/m2, with a 10% increase at each cycle up to a maximum of 153 mg/m2), without a control group. The primary endpoint of this study was ORR, with 25.8% of patients exhibiting a response, which allowed 43.5% of them to undergo a secondary resection of the metastasis. This dose escalation pattern did not result in significant ORR or PFS differences in the three groups analyzed, despite using a widely different irinotecan dose. Nor were there any differences observed with respect to safety.
The study carried out by Lu et al.22, which included patients with mCRC on first-line treatment with the FOLFIRI-bevacizumab regimen, stratified patients into two groups (group 1: *1/*1, *1/*28; and group 2: *28/*28). In the first group, patients with a wild-type or heterozygous genotype were started on a 180 mg/m2 dose of irinotecan, with dose escalation implemented every three cycles (with increases of 30 mg/m2 up to a maximum of 260 mg/m2 for wild-type genotypes and 240 mg/m2 for heterozygous genotypes). In group 2, patients were started on 120 mg/m2 of irinotecan (maximum dose following a 210 mg/m2 escalation). The primary endpoint was ORR and the DCR, as well as PFS, with a comparison being made between the wild-type/heterozygous genotype group (group 1) and the mutated homozygous genotype group (group 2). The following results were observed: ORR: 76.9% vs 20.0%, p < 0.001; DCR: 93.8% vs 40.0%, p < 0.001. No increase in adverse events was observed.
Hebbar et al.23 analyzed whether a combining chemotherapy strategy with FOLFIRI at high doses in UGT1A1 *1/*1 or *1/*28 genotype patients, together with radiofrequency ablation and surgery, could increase the clearance of liver metastases and survival rates in patients with mCRC. Based on their observation of complete clearance of metastasis in 33.3% of patients without any cases of grade 4 toxicity or death, as well as of an increase in OS, the authors concluded that FOLFIRI at high doses may represent an alternative for patients not amenable to targeted therapy, although the study had to be discontinued because of the small size of the sample.
Finally, and in contrast with other reports, Manfredi et al.24 used high doses of irinotecan (260 mg/m2) in heterozygous patients with a wild-type genotype (*1/*1 and *1/*28), and observed an ORR of 45.0% in the *1/*1 group and of 56.5% in the *1/*28 group, which was similar to the ORR observed in other studies without dose escalation. With regard to toxicity, the authors established the following criteria to discontinue the study: 1) ≥ 7 patients with an objective response (OR); and 2) ≥ 3 patients with severe toxicity. As during the preliminary studies, 26 patients in group 1 (UGT1A1*1) developed grade 4 neutropenia, 6 patients (4 in the *1/*1 group and 2 in the *1/*28 group) presented with grade 4 diarrhea, and 2 patients in the *1/*1 group exhibited febrile neutropenia, a decision was made to discontinue the study, even that a reduction of the dose resulted in an acceptable safety profile.
Results of studies evaluating the safety of irinotecan at doses above 180 mg/m2Several studies evaluated the maximum tolerated dose (MTD) of irinotecan when administered as part of the FOLFIRI regimen as a function of the patients’ UGT1A1 genotype. One of the first studies was carried out in 2010 by Toffoli et al.11 who reported that *1/*1 patients were able to tolerate a MTD of 370 mg/m2 and *1/*28 patients could tolerate 310 mg/m2. These results were subsequently corroborated by Marcuello et al.10, who found a MTD of 390 mg/m2 and 340 mg/m2, respectively. A more recent study, by Kim et al.12, published in 2015, reported MTDs of 330 mg/m2 and 300 mg/m2, respectively. In a study published in 2017, Toffoli et al.14 established the MTD for irinotecan in patients with mCRC treated with first-line FOLFIRI-bevacizumab. MTD was 310 mg/m2 and 260 mg/m2 for patients with the UGT1A1*1/*1 and *1/*28 genotypes, respectively. Another study, authored by Kim et al.15, evaluated the MTD in patients treated with the XELIRI (capecitabine-irinotecan) regimen and found a MTD of 380 mg/m2 in patients with a wild-type or heterozygous genotype, and of 240 mg/m2 for those with mutated homozygous genotypes. Finally, Kim et al.12 observed that the MTD of irinotecan when used as part of the FOLFIRI regimen in patients with an unmutated genotype was 330 mg/m2, for those with heterozygous genotypes of up to 300 mg/m2, and for those with homozygous genotypes of 150 mg/m2.
DiscussionThe antineoplastic activity of irinotecan, as well as its toxicity, depend on the activity of its metabolite SN-38 and, specifically, on its plasma concentration. SN-38 concentrations depend not only on the irinotecan dose administered, but also on its metabolism and clearance rate29. Thus, variants that reduce the transcription of the UGT1A1 gene, such as the allele *28, result in a slower clearance of SN-38 and, therefore, in higher toxicity levels. This means that depending on the genotype of the UGT1A1 gene, patients can be divided into two groups: those with adequate clearance of irinotecan (*1/*1 and *1/*28) and those with reduced irinotecan clearance (*28/*28). Nevertheless, this differentiation was not taken into consideration by the clinical trials evaluating the effectiveness of the FOLFIRI regimen in patients with mCRC1. Selecting patients as a function of the genotype they presented is likely to have allowed the use of higher doses in unmutated patients, with a potentially better clinical response.
Contrary to what occurs with mutations of the DPYD gene, which suppress the activity of the gene's protein and whose allele frequency is low, the allele frequency of *28 is approximately 30%4, which means that around 10% of the general population carries the mutation in an homozygous form (patients with Gilbert's syndrome). This is one of the reasons why the first clinical trials (phase 1 trials) that analyzed the safety profile of irinotecan were not able to document doses above 180 mg/m2. However, several post-authorization studies have analyzed the effectiveness and safety of high doses of irinotecan (> 180 mg/m2) administered as part of the FOLFIRI regimen in patients with mCRC as a function of the patients’ UGT1A1 genotype. Results indicate that doses above 180 mg/m2 are tolerated by non-homozygous patients (*28/*28) mutated for the UGT1A1 gene, and that these patients are not only able to tolerate higher doses but also attain higher efficacy levels when administered these higher doses, in terms of ORR16,19–21, PFS17,19,23 and, in some cases, OS19,23. Only one of the studies included in the present review did not show differences when higher doses of irinotecan were used in patients without a mutation in the UGT1A1 gene24.
Despite their heterogeneous design, the analyzed studies make it possible to establish that, in general terms, increasing the irinotecan dose to a maximum of 260 mg/m2 (in patients with a wild-type or heterozygous genotype of the UGT1A1 gene) results in a higher ORR16,19–21, more effective control of the disease17,22, resectability of liver metastases 20,23, and an increase of PFS17,19,23 and even OS19,23.
In addition, this increased efficacy is not overshadowed by higher toxicity levels. The MTD of irinotecan administered as part of the FOLFIRI regimen depends on the UGT1A1 genotype present. Patients with the *1/*1 and *1/*28 alleles are able to tolerate doses of up to 310–390 mg/m2 10–12,14,15,24.
Everything seems to indicate that patients without a homozygous mutation, i.e., 90% of the sample, could be receiving lower doses than required with the FOLFIRI regimen. With respect to the patients with the *28/*28 genotype, several studies have shown that this subgroup is the one exhibiting a higher risk of developing severe toxicity6,7,9,17,28. This is also stated in the Food and Drug Administration's (FDA) SmPC19. Moreover, studies by Marcuello and Kim have suggested that these patients present a MTD < 180 mg/m2.
This systematic review has some limitations. Firstly, only 11 RCTs and two observational studies were included because of the dearth of published data. Secondly, the evidence reviewed was highly heterogeneous as it includes different drug adjustment strategies based on genotype, chemotherapy agent combinations, and study variables. Thirdly, a statistical analysis to compare the data was not possible because of the heterogeneity of the sample, resulting most probably again from the dearth of evidence available. In the fourth place, most clinical trials were assigned as score ≤ 3 on Jadad's scale, which corroborates the lack of well-designed studies to provide more conclusive results and points out the need of conducting further research in this area. In the fifth place, our results must be interpreted with caution due to the publication bias associated with the possible underreporting of negative results. Finally, the fact that there was no comparator group, in which patients did not receive high doses of irinotecan, in some of the studies, and that one study could not be completed as it met one of the two discontinuation criteria defined before the start of the study is also an important limitation. However, the authors themselves declared that such overly strict criteria were not adapted to clinical practice.
Taking into consideration the limitations above-mentioned, the conclusions drawn in this study should be confirmed by randomized studies aimed basically at analyzing the efficacy of higher irinotecan doses than those recommended in the drug's SmPC in patients with mCRC treated with the FOLFIRI regimen as a function of their UGT1A genotype.
To conclude, the present systematic review confirmed the desirability of establishing high irinotecan dose FOLFIRI regimens according to the polymorphisms of the UGT1A1 gene carried by the patients, boosting the likelihood of obtaining a successful clinical response.
FundingNo funding.
AcknowledgmentsWe thank Míriam Basagaña (documentalist in Vall d'Hebron Hospital) for helping with the search strategy.
Conflict of interestNone of the authors declare any conflict of interest.